PTEN expression and morphological patterns in prostatic adenocarcinoma
Andrew J Spieker
Department of Biostatistics, Vanderbilt University, Nashville, TN, USA
These authors contributed equally to this work.
Search for more papers by this authorJennifer B Gordetsky
Department of Pathology, Microbiology and Immunology, Vanderbilt University Medical Center, Nashville, TN, USA
Department of Urology, Vanderbilt University Medical Center, Nashville, TN, USA
These authors contributed equally to this work.
Search for more papers by this authorAlexander S Maris
Department of Pathology, Microbiology and Immunology, Vanderbilt University Medical Center, Nashville, TN, USA
Search for more papers by this authorLauren M Dehan
Department of Pathology, Microbiology and Immunology, Vanderbilt University Medical Center, Nashville, TN, USA
Search for more papers by this authorJames E Denney
Department of Pathology, Microbiology and Immunology, Vanderbilt University Medical Center, Nashville, TN, USA
Search for more papers by this authorShanna A Arnold Egloff
Department of Pathology, Microbiology and Immunology, Vanderbilt University Medical Center, Nashville, TN, USA
Sarah Cannon Cancer Center, Nashville, TN, USA
Search for more papers by this authorKristen Scarpato
Department of Urology, Vanderbilt University Medical Center, Nashville, TN, USA
Search for more papers by this authorDaniel A Barocas
Department of Urology, Vanderbilt University Medical Center, Nashville, TN, USA
Search for more papers by this authorCorresponding Author
Giovanna A Giannico
Department of Pathology, Microbiology and Immunology, Vanderbilt University Medical Center, Nashville, TN, USA
Address for correspondence: Giovanna A Giannico, Department of Pathology, Microbiology, and Immunology, Vanderbilt University Medical Center, 1161 21st Avenue South, C-2104C Medical Center North, Nashville, TN 37232-2561, USA. e-mail: [email protected]
Search for more papers by this authorAndrew J Spieker
Department of Biostatistics, Vanderbilt University, Nashville, TN, USA
These authors contributed equally to this work.
Search for more papers by this authorJennifer B Gordetsky
Department of Pathology, Microbiology and Immunology, Vanderbilt University Medical Center, Nashville, TN, USA
Department of Urology, Vanderbilt University Medical Center, Nashville, TN, USA
These authors contributed equally to this work.
Search for more papers by this authorAlexander S Maris
Department of Pathology, Microbiology and Immunology, Vanderbilt University Medical Center, Nashville, TN, USA
Search for more papers by this authorLauren M Dehan
Department of Pathology, Microbiology and Immunology, Vanderbilt University Medical Center, Nashville, TN, USA
Search for more papers by this authorJames E Denney
Department of Pathology, Microbiology and Immunology, Vanderbilt University Medical Center, Nashville, TN, USA
Search for more papers by this authorShanna A Arnold Egloff
Department of Pathology, Microbiology and Immunology, Vanderbilt University Medical Center, Nashville, TN, USA
Sarah Cannon Cancer Center, Nashville, TN, USA
Search for more papers by this authorKristen Scarpato
Department of Urology, Vanderbilt University Medical Center, Nashville, TN, USA
Search for more papers by this authorDaniel A Barocas
Department of Urology, Vanderbilt University Medical Center, Nashville, TN, USA
Search for more papers by this authorCorresponding Author
Giovanna A Giannico
Department of Pathology, Microbiology and Immunology, Vanderbilt University Medical Center, Nashville, TN, USA
Address for correspondence: Giovanna A Giannico, Department of Pathology, Microbiology, and Immunology, Vanderbilt University Medical Center, 1161 21st Avenue South, C-2104C Medical Center North, Nashville, TN 37232-2561, USA. e-mail: [email protected]
Search for more papers by this authorAbstract
Aims
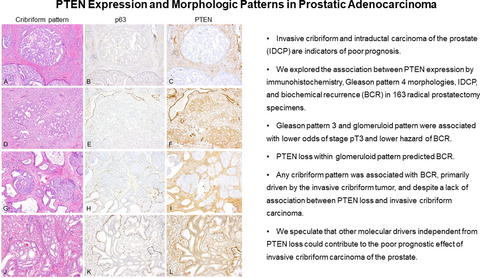
Cribriform morphology, which includes intraductal carcinoma (IDCP) and invasive cribriform carcinoma, is an indicator of poor prognosis in prostate cancer. Phosphatase and tensin homologue (PTEN) loss is a predictor of adverse clinical outcomes. The association between PTEN expression and morphological patterns of prostate cancer is unclear.
Methods and results
We explored the association between PTEN expression by immunohistochemistry, Gleason pattern 4 morphologies, IDCP and biochemical recurrence (BCR) in 163 radical prostatectomy specimens. IDCP was delineated from invasive cribriform carcinoma by p63 positive immunohistochemical staining in basal cells. Combined invasive cribriform carcinoma and IDCP were associated with a higher cumulative incidence of BCR [hazard ratio (HR) = 5.06; 2.21, 11.6, P < 0.001]. When including PTEN loss in the analysis, invasive cribriform carcinoma remained predictive of BCR (HR = 3.72; 1.75, 7.94, P = 0.001), while PTEN loss within invasive cribriform carcinoma did not. Glomeruloid morphology was associated with lower odds of cancer stage pT3 and lower cumulative incidence of BCR (HR = 0.27; 0.088, 0.796, P = 0.018), while PTEN loss within glomeruloid morphology was associated with a higher cumulative incidence of BCR (HR = 4.07; 1.04, 15.9, P = 0.043).
Conclusions
PTEN loss within glomeruloid pattern was associated with BCR. The presence of any cribriform pattern was associated with BCR, despite PTEN loss not significantly associated with invasive cribriform carcinoma. We speculate that other drivers independent from PTEN loss may contribute to poor prognostic features in cribriform carcinoma.
Graphical Abstract
Conflicts of interest
All authors report no conflicts of interest or financial disclosures that were pertinent to the following study.
Supporting Information
| Filename | Description |
|---|---|
| his14531-sup-0001-TableS1.docxWord document, 14.3 KB | Table S1. Logistic regression model comparing the odds of cancer stage pT3 relative to stage pT2 across Gleason patterns and morphologic patterns. |
| his14531-sup-0002-TableS2.docxWord document, 13.3 KB | Table S2. Generalized estimating equations to compare the odds of PTEN loss (homozygous or heterozygous) across different patterns. |
| his14531-sup-0003-TableS3.docxWord document, 13.9 KB | Table S3. Predictors of PTEN loss within Gleason pattern 3. |
| his14531-sup-0004-TableS4.docxWord document, 13.9 KB | Table S4. Predictors of PTEN loss within glomeruloid pattern. |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
References
- 1Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA: Cancer J. Clin. 2021; 71; 7–33. https://doi.org/10.3322/caac.21654
- 2Epstein JI, Amin MB, Fine SW et al. The 2019 Genitourinary Pathology Society (GUPS) White Paper on contemporary grading of prostate cancer. Arch. Pathol. Lab. Med. 2021; 145; 461–493.
- 3Offermann A, Hupe MC, Sailer V, Merseburger AS, Perner S. The new ISUP 2014/WHO 2016 prostate cancer grade group system: first resume 5 years after introduction and systemic review of the literature. World J. Urol 2020; 38; 657–662.
- 4Paner GP, Stadler WM, Hansel DE, Montironi R, Lin DW, Amin MB. Updates in the eighth edition of the tumour–node–metastasis staging classification for urologic cancers. Eur. Urol. 2018; 73; 560–569.
- 5Downes MR, Xu B, van der Kwast TH. Cribriform architecture prostatic adenocarcinoma in needle biopsies is a strong independent predictor for lymph node metastases in radical prostatectomy. Eur. J. Cancer. 2021; 148; 432–439.
- 6Keefe DT, Schieda N, El Hallani S et al. Cribriform morphology predicts upstaging after radical prostatectomy in patients with Gleason score 3 + 4 = 7 prostate cancer at transrectal ultrasound (TRUS)-guided needle biopsy. Virchows Arch. 2015; 467; 437–442.
- 7Kweldam CF, Kummerlin IP, Nieboer D et al. Presence of invasive cribriform or intraductal growth at biopsy outperforms percentage grade 4 in predicting outcome of Gleason score 3+4 = 7 prostate cancer. Mod. Pathol. 2017; 30; 1126–1132.
- 8Montironi R, Zhou M, Magi-Galluzzi C, Epstein JI. Features and prognostic significance of intraductal carcinoma of the prostate. Eur. Urol. Oncol. 2018; 1; 21–28.
- 9Choy B, Pearce SM, Anderson BB et al. Prognostic significance of percentage and architectural types of contemporary Gleason pattern 4 prostate cancer in radical prostatectomy. Am. J. Surg. Pathol. 2016; 40; 1400–1406.
- 10Dong F, Yang P, Wang C et al. Architectural heterogeneity and cribriform pattern predict adverse clinical outcome for Gleason grade 4 prostatic adenocarcinoma. Am. J. Surg. Pathol. 2013; 37; 1855–1861.
- 11Kweldam CF, Wildhagen MF, Steyerberg EW et al. Cribriform growth is highly predictive for postoperative metastasis and disease-specific death in Gleason score 7 prostate cancer. Mod. Pathol. 2015; 28; 457–464.
- 12McKenney JK, Wei W, Hawley S et al. Histologic grading of prostatic adenocarcinoma can be further optimized: analysis of the relative prognostic strength of individual architectural patterns in 1275 patients from the Canary Retrospective Cohort. Am. J. Surg. Pathol. 2016; 40; 1439–1456.
- 13Chua MLK, Lo W, Pintilie M et al. A prostate cancer ‘nimbosus’: genomic instability and SChLAP1 dysregulation underpin aggression of intraductal and cribriform subpathologies. Eur. Urol. 2017; 72; 665–674.
- 14Krohn A, Freudenthaler F, Harasimowicz S et al. Heterogeneity and chronology of PTEN deletion and ERG fusion in prostate cancer. Mod. Pathol. 2014; 27; 1612–1620.
- 15Ullman D, Dorn D, Rais-Bahrami S, Gordetsky J. Clinical utility and biologic implications of phosphatase and tensin homolog (PTEN) and ETS-related gene (ERG) in prostate cancer. Urology 2018; 113; 59–70.
- 16Krohn A, Diedler T, Burkhardt L et al. Genomic deletion of PTEN is associated with tumour progression and early PSA recurrence in ERG fusion-positive and fusion-negative prostate cancer. Am. J. Pathol. 2012; 181; 401–412.
- 17Jamaspishvili T, Berman DM, Ross AE et al. Clinical implications of PTEN loss in prostate cancer. Nat. Rev. Urol. 2018; 15; 222–234.
- 18Lotan TL, Heumann A, Rico SD et al. PTEN loss detection in prostate cancer: comparison of PTEN immunohistochemistry and PTEN FISH in a large retrospective prostatectomy cohort. Oncotarget 2017; 8; 65566–65576.
- 19Ronen S, Abbott DW, Kravtsov O et al. PTEN loss and p27 loss differ among morphologic patterns of prostate cancer, including cribriform. Hum. Pathol. 2017; 65; 85–91.
- 20Downes MR, Satturwar S, Trudel D, van der Kwast TH. Evaluation of ERG and PTEN protein expression in cribriform architecture prostate carcinomas. Pathol. Res. Pract. 2017; 213; 34–38.
- 21Morais CL, Han JS, Gordetsky J et al. Utility of PTEN and ERG immunostaining for distinguishing high-grade PIN from intraductal carcinoma of the prostate on needle biopsy. Am. J. Surg. Pathol. 2015; 39; 169–178.
- 22Pacelli A, Lopez-Beltran A, Egan AJ, Bostwick DG. Prostatic adenocarcinoma with glomeruloid features. Hum. Pathol. 1998; 29; 543–546.
- 23Epstein JI, Allsbrook WC Jr, Amin MB, Egevad LL, ISUP Grading Committee. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason grading of prostatic carcinoma. Am. J. Surg. Pathol. 2005; 29(9); 1228–1242.
- 24Guo CC, Epstein JI. Intraductal carcinoma of the prostate on needle biopsy: histologic features and clinical significance. Mod. Pathol. 2006; 19; 1528–1535.
- 25Moch HHP, Ulbright TM, Reuter VE. WHO Classification of tumours of the urinary system and male genital organs. Lyon, France: International Agency for Research on Cancer; 2016.
- 26Epstein JI, Egevad L, Amin MB et al. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason grading of prostatic carcinoma: definition of grading patterns and proposal for a new grading system. Am. J. Surg. Pathol. 2016; 40; 244–252.
- 27Goldstein J, Borowsky AD, Goyal R et al. MAGI-2 in prostate cancer: an immunohistochemical study. Hum. Pathol. 2016; 52; 83–91.
- 28Picanço-Albuquerque CG, Morais CL, Carvalho FLF et al. In prostate cancer needle biopsies, detections of PTEN loss by fluorescence in situ hybridization (FISH) and by immunohistochemistry (IHC) are concordant and show consistent association with upgrading. Virchows Arch. 2016; 468; 607–617.
- 29van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat. Methods Med. Res. 2007; 16; 219–242.
- 30Rubin DB. Multiple imputation for nonresponse in surveys. New York, NY: John Wiley & Sons; 1987.
10.1002/9780470316696 Google Scholar
- 31Fine J, Gray RJ. Proportional hazards model for the subdistribution of a competing risk. J. Am. Stat. Assoc. 1999; 94; 496–509.
- 32Diggle PJ, Heagerty P, Liang K, Zeger SL. Analysis of longitudinal data. 2nd edn. Oxford: Oxford University Press; 2014.
- 33Shah RB, Shore KT, Yoon J, Mendrinos S, McKenney JK, Tian W. PTEN loss in prostatic adenocarcinoma correlates with specific adverse histologic features (intraductal carcinoma, cribriform Gleason pattern 4 and stromogenic carcinoma). Prostate 2019; 79; 1267–1273.
- 34Tosoian JJ, Guedes LB, Morais CL et al. PTEN status assessment in the Johns Hopkins active surveillance cohort. Prostate Cancer Prostat. Dis. 2019; 22; 176–181.
- 35Lotan TL, Carvalho FLF, Peskoe SB et al. PTEN loss is associated with upgrading of prostate cancer from biopsy to radical prostatectomy. Mod. Pathol. 2015; 28; 128–137.
- 36Trock BJ, Fedor H, Gurel B et al. PTEN loss and chromosome 8 alterations in Gleason grade 3 prostate cancer cores predicts the presence of un-sampled grade 4 tumour: implications for active surveillance. Mod. Pathol. 2016; 29; 764–771.
- 37Kovtun IV, Cheville JC, Murphy SJ et al. Lineage relationship of Gleason patterns in Gleason score 7 prostate cancer. Cancer Res. 2013; 73; 3275–3284.
- 38Sowalsky AG, Ye H, Bubley GJ, Balk SP. Clonal progression of prostate cancers from Gleason grade 3 to grade 4. Cancer Res. 2013; 73; 1050–1055.
- 39Sowalsky AG, Kissick HT, Gerrin SJ et al. Gleason score 7 prostate cancers emerge through branched evolution of clonal Gleason pattern 3 and 4. Clin. Cancer Res. 2017; 23; 3823–3833.
- 40Hollemans E, Verhoef EI, Bangma CH et al. Clinicopathological characteristics of glomeruloid architecture in prostate cancer. Mod. Pathol. 2020; 33; 1618–1625.
- 41Böttcher R, Kweldam CF, Livingstone J et al. Cribriform and intraductal prostate cancer are associated with increased genomic instability and distinct genomic alterations. BMC Cancer 2018; 18; 8.