Evaluating two rechallenge strategies of immune checkpoint inhibitors: Durvalumab plus tremelimumab in advanced hepatocellular carcinoma
Takuya Yonemoto, Sadahisa Ogasawara, and Naoya Kanogawa contributed equally to this work.
Abstract
Aim
This study aimed to evaluate the safety and efficacy of durvalumab plus tremelimumab in patients with advanced hepatocellular carcinoma who have previously received atezolizumab plus bevacizumab (Atez/Bev). Additionally, it seeks to assess the feasibility of administering immunotherapy after the occurrence of immune-mediated adverse events (imAEs) in real-world clinical practice.
Methods
This retrospective study analyzed data from patients with advanced hepatocellular carcinoma treated with durvalumab plus tremelimumab at four Japanese institutions. Clinical outcomes, adverse events, tumor dynamics, and serum cytokine and chemokine levels were evaluated, with a focus on efficacy following prior Atez/Bev treatment.
Results
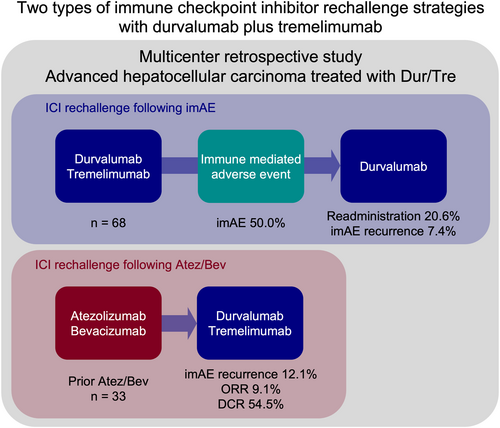
Durvalumab plus tremelimumab was administered to 68 patients. The objective response rate was 10.3%, and the disease control rate was 58.8%. Median progression-free survival was 3.1 months (95% confidence interval 2.0–4.9). imAEs occurred in 50.0% of patients, with colitis being the most common (22.1%). Durvalumab was safely readministered to 14 patients after imAE resolution, although five experienced recurrence. Among 33 patients (48.5%) previously treated with Atez/Bev, improved responses were noted, including two partial responses. Tumor growth dynamics decreased in 60.0% of patients receiving sequential therapy. Common adverse events included elevated liver enzymes (aspartate aminotransferase 50.0%, alanine aminotransferase 48.5%), pruritus (45.6%), and rash (44.1%).
Conclusions
Durvalumab plus tremelimumab therapy is feasible with proper imAE management and patient selection. Sequential treatment following Atez/Bev offers clinical benefit in advanced hepatocellular carcinoma, although some may experience rapid progression. Further biomarker research is needed to optimize immunotherapy strategies.
Graphical Abstract
This multicenter retrospective study evaluated durvalumab plus tremelimumab for advanced hepatocellular carcinoma in real-world practice. Results demonstrated that immune checkpoint inhibitors could be safely readministered after immune-mediated adverse events in carefully selected patients. The combination therapy showed clinical benefit in select patients previously treated with atezolizumab plus bevacizumab.
CONFLICT OF INTEREST STATEMENT
Sadahisa Ogasawara received honoraria from Bayer (Leverkusen, Germany), Eisai (Tokyo, Japan), Eli Lilly (Indianapolis, IN, USA), Chugai Pharma (Tokyo, Japan), AstraZeneca (Cambridge, UK), and Merck & Co., Inc. (Kenilworth, NJ, USA); consulting or advisory fees from Bayer, Eisai, Merck & Co., Inc., Chugai Pharma, Eli Lilly, and AstraZeneca; and research grants from Bayer, AstraZeneca, and Eisai. Masanori Atsukawa received a research grant from Eisai. Michihisa Moriguchi received honoraria from Bayer, Eisai, Eli Lilly, Chugai Pharma, and AstraZeneca. Naoya Kato received honoraria from Bayer, Eisai, Sumitomo Dainippon Pharma (Tokyo, Japan), and Merck & Co., Inc.; consulting or advisory fees from Bayer and Eisai; and research grants from Bayer and Eisai. Masanori Atsukawa and Naoya Kato are Editorial Board members of Hepatology Research. The remaining authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data supporting the findings of this study are available from the corresponding author upon reasonable request.