Biochar stimulates the decomposition of simple organic matter and suppresses the decomposition of complex organic matter in a sandy loam soil
Abstract
Incorporating crop residues and biochar has received increasing attention as tools to mitigate atmospheric carbon dioxide (CO2) emissions and promote soil carbon (C) sequestration. However, direct comparisons between biochar, torrefied biomass, and straw on both labile and recalcitrant soil organic matter (SOM) remain poorly understood. In this study, we explored the impact of biochars produced at different temperatures and torrefied biomass on the simple C substrates (glucose, amino acids), plant residues (Lolium perenne L.), and native SOM breakdown in soil using a 14C labeling approach. Torrefied biomass and biochars produced from wheat straw at four contrasting pyrolysis temperatures (250, 350, 450, and 550 °C) were incorporated into a sandy loam soil and their impact on C turnover compared to an unamended soil or one amended with unprocessed straw. Biochar, torrefied biomass, and straw application induced a shift in the soil microbial community size, activity, and structure with the greatest effects in the straw-amended soil. In addition, they also resulted in changes in microbial carbon use efficiency (CUE) leading to more substrate C being partitioned into catabolic processes. While overall the biochar, torrefied biomass, and straw addition increased soil respiration, it reduced the turnover rate of the simple C substrates, plant residues, and native SOM and had no appreciable effect on the turnover rate of the microbial biomass. The negative SOM priming was positively correlated with biochar production temperature. We therefore ascribe the increase in soil CO2 efflux to biochar-derived C rather than that originating from SOM. In conclusion, the SOM priming magnitude is strongly influenced by both the soil organic C quality and the biochar properties. In comparison with straw, biochar has the greatest potential to promote soil C storage. However, straw and torrefied biomass may have other cobenefits which may make them more suitable as a CO2 abatement strategy.
Introduction
In recent years, conversion of plant biomass to biochar has received increasing attention based on its potential role in mitigating atmospheric CO2 emissions via sequestering carbon (C) in the soil (Lehmann et al., 2006; Lehmann, 2007). Because of its relative inertness, after amendment, biochar can remain in the soil for hundreds or thousands of years. This contrasts with crop residues (e.g., cereal straw) which turnover on a decadal timescale (Bruun et al., 2008). Thus, biochar created from cereal residues may act as a long-term C sink for offsetting CO2 emissions (Glaser et al., 2001; Marris, 2006; Lehmann, 2007; Mathews, 2008).
Although C-rich biochar may enhance soil C storage, it is important that it does not destabilize native soil organic matter (SOM) stores or have any other negative environmental consequences if it is to be adopted by policymakers and land owners as a climate change abatement strategy (Jones et al., 2012). This has led to extensive studies on the interactions between natural and anthropogenically derived biochar with both native SOM and plant/animal residues (Wardle et al., 2008; Jones et al., 2011; Luo et al., 2011). Many of these studies have suggested that if the magnitude of any priming effect was considerable (i.e., strong short-term changes in the turnover of SOM caused by comparatively moderate treatment of the soil; Kuzyakov et al., 2000), the benefits of C sequestration derived from biochar application into soil would be diminished (Cross & Sohi, 2011). Recent studies have shown both suppression and stimulation of soil organic C (SOC), plant residues, or root exudate decomposition induced by biochar application (Wardle et al., 2008; Liang et al., 2010; Cross & Sohi, 2011; Jones et al., 2011; Luo et al., 2011). For example, Wardle et al. (2008) reported that the application of black C stimulated SOC decomposition, while Jones et al. (2011) observed that it suppressed SOC turnover. It has been suggested that rapid microbial utilization of dissolved or volatile organic C contained in the biochar (Cross & Sohi, 2011), stimulation of microbial activity by changing the chemical environment, and improvements in soil structure and aeration status (Zimmerman, 2010; Jones et al., 2011; Smith et al., 2011) may account for the observed positive priming effects. In contrast, negative priming effects induced by biochar may be caused by adsorption and subsequent protection of dissolved organic C (DOC) on the surface of the biochar and changes in microbial diversity or in their rate of enzyme production or activity (Dudley & Churchill, 1995; Brändli et al., 2008; Koelamans et al., 2009). Based on the current uncertainty, we assume that biochar properties, which are controlled by the type of feedstock (Spokas & Reicosky, 2009) and pyrolysis/torrefaction conditions (Yuan et al., 2011; Al-Wabel et al., 2013; Méndez et al., 2013), play an important role in regulating SOM decomposition (Glaser et al., 2002; McClellan et al., 2007). Changes in pyrolysis temperature, for example, may lead to variations in ash content, porosity, and cation exchange capacity of biochars (Wang et al., 2013), which further affects the decomposition of SOM in biochar-amended soils (Yuan et al., 2014). However, the direction, magnitude, and temporal dynamics of biochar priming effects on the decomposition of soil C substrates are complex. Further the underlying mechanistic basis of the responses remains poorly understood.
Our objectives were to (i) explore the priming effect of biochar and torrefied biomass application on the decomposition of simple (glucose, amino acids) and complex C substrates (plant residues); (ii) evaluate whether biochar or torrefied biomass alters the turnover of native SOM; (iii) determine which biochar production conditions favor maximal C storage; and (iv) assess the advantages and disadvantages of using straw, and torrefied biomass or biochar as a soil C sequestration agent.
Materials and methods
Feedstock and biochar creation
Biochar was created by the thermal treatment of wheat (Triticum aestivum L.) straw, collected from the Henfaes Research Centre Wales, North Wales, UK (53°14′N; 4°10′W). The wheat straw was dried in an oven (80 °C, 24 h) and then cut into 10 cm pieces before being loaded into a glass pyrolysis vessel. The vessel was then placed in a muffle furnace for pyrolysis/torrefaction. The heating rate was 20 °C min−1, and the thermal treatment time was 1 h. Four peak torrefaction/pyrolysis temperatures were set (250, 350, 450, and 550 °C), and the corresponding biochar/torrefaction products were named B250, B350, B450, and B550, respectively. Here, torrefaction is referred to as the low temperature thermal treatment of biomass residues (250 °C) and pyrolysis to high temperature thermal treatment (350–550 °C; Gronnow et al., 2013; Bach et al., 2016). The main properties of the wheat straw are shown in Table 1.
| Soil | Straw | |
|---|---|---|
| pH | 6.44 ± 0.01 | 6.42 ± 0.16 |
| ECa (μS cm−1) | 40.9 ± 0.9 | 1026 ± 47 |
| Total C (g kg−1) | 21.6 ± 1.85 | 423 ± 1 |
| Total N (g kg−1) | 2.62 ± 0.12 | 5.45 ± 0.05 |
| DOC (mg C kg−1) | 99.1 ± 1.9 | 1666 ± 129 |
| K (mg kg−1) | 77.1 ± 13.1 | 7816 ± 35 |
| Ca (mg kg−1) | 735 ± 10 | 4524 ± 273 |
| Na (mg kg−1) | 30 ± 2 | 150 ± 2 |
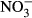 (mg N kg−1) (mg N kg−1) |
10.0 ± 0.4 | 0.66 ± 0.08 |
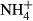 (mg N kg−1) (mg N kg−1) |
4.7 ± 0.4 | 14.8 ± 0.9 |
- Values represent means ± standard error of the mean (SEM), n = 4.
- a Electrical conductivity.
Soil was collected from the Ah horizon (0–15 cm, sandy loam) of a freely draining, grassland soil (Eutric Cambsiol soil type), which receives regular fertilization (120 kg N, 60 kg K, and 10 kg P annually) and was located at the Henfaes Research Centre. The site is used for both grassland and arable production and has a mean annual temperature of 11 °C (range −5 to 25 °C) and mean annual rainfall of 1060 mm (temperate climate regime). The soil was sieved to pass 5 mm to remove plant residues and stones and then dried at 20 °C prior to use. The major properties of the soil are shown in Table 1 with additional properties shown in Jones et al. (2011, 2012) and Farrar et al. (2012).
Analysis of soil, straw, and biochar
The ash content of the straw and biochar was measured by heating in a muffle furnace (575 °C, 3 h; Monti et al., 2008). Electrical conductivity (EC) and pH were determined in 1 : 5 (w/v) soil : distilled water and 1 : 20 (w/v) biochar : distilled water extracts with standard electrodes. Water holding capacity (WHC) of the biochar and straw was measured according to EBC (2012). Briefly, 2.0 g of biochar or straw was submersed in distilled water for 4 h, then placed on moist sand for 2 h, weighed and subsequently dried (105 °C, 24 h). Cation exchange capacity (CEC) of the biochar and straw was measured using the modified ammonium acetate method of Gaskin et al. (2008). Specific surface area (SSA) of the biochar and straw was measured using an Autosorb iQ/monosorb surface area analyzer (Quantachrome Instruments, Boynton Beach, FL, USA), with N2 absorption at 77 K, using the Brunauer–Emmett–Teller method. Available 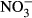 and
and 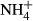 were determined in 0.5 m K2SO4 extracts (1 : 5 w/v) using the colorimetric methods of Mulvaney (1996) and Miranda et al. (2001). Exchangeable cations and available phosphorous (P) were extracted using 0.5 m acetic acid (1 : 5 w/v) and analyzed through a Model 410 Flame Photometer for Na, K, and Ca and the colorimetric molybdate blue method for P (Murphy & Riley, 1962).
were determined in 0.5 m K2SO4 extracts (1 : 5 w/v) using the colorimetric methods of Mulvaney (1996) and Miranda et al. (2001). Exchangeable cations and available phosphorous (P) were extracted using 0.5 m acetic acid (1 : 5 w/v) and analyzed through a Model 410 Flame Photometer for Na, K, and Ca and the colorimetric molybdate blue method for P (Murphy & Riley, 1962).
Experimental treatments
The experiments had six main treatments: (i) unamended soil (control), (ii) straw-amended soil, (iii) B250 amended soil, (iv) B350 amended soil, (v) B450 amended soil, and (vi) B550 amended soil. Biochar and straw were added to soil at a soil-to-residue ratio of 10 : 1 (w/w). The addition rates were based on the likely maximal addition rates of biochar in an agricultural topsoil (0–10 cm) and those used in previous field trials at the site (Jones et al., 2012). Wheat straw was chosen as it represents the major cereal waste produced in the UK and crop residue incorporated into soil (12.2 × 106 t yr−1 at ca. 3.5 t ha−1 yr−1; Defra, 2014). The straw addition rates are higher than those typically applied by farmers when averaged across a field, but reflect the hotspots of straw which frequently occur in topsoils after residue incorporation. All treatments were performed in quadruplicate.
Basal soil respiration
Briefly, 20 g of air-dried soil and 2 g of biochar or straw were mixed, the water content adjusted to 32% with distilled water and the samples placed in 50 cm3 sterile polypropylene tubes. Soil respiration was then measured over a 168-h period at 20 °C using an automated multichannel SR1-IRGA soil respirometer (PP Systems Inc., Hitchin, UK).
Mineralization of simple 14C-labeled C substrates
Two 14C-labeled simple C substrates and two 14C-labeled complex C substrates were used to determine the impact of biochar and straw on microbial SOC turnover. Glucose and free amino acids were chosen to simulate low molecular weight (MW) root exudates (simple substrates), and plant shoot residues (Lolium perenne L.) and aged SOM were chosen to simulate more complex high MW C substrates.
Soil (10 g, 32% moisture content) from each of the six experimental treatments was placed into sterile 50-cm3 polypropylene tubes. The tubes were then amended with 0.5 mL of either 14C-labeled glucose (36 mg C g−1 soil; 1.26 kBq mL−1) or an equimolar mixture of 16 amino acids (31.2 mg C g−1 soil; 1.38 kBq mL−1) (Jones et al., 2012). A vial containing 1 mL of 1 m NaOH was then placed above the samples to trap any 14CO2 evolved and the centrifuge tubes hermetically sealed. Samples were then placed in a climate-controlled room (20 °C) and the NaOH traps changed periodically over 21 days. The 14CO2 content in the NaOH traps was determined by liquid scintillation counting using Optiphase 3 scintillation fluid (PerkinElmer Corp., Waltham, MA, USA) and a Wallac 1404 liquid scintillation counter (PerkinElmer Corp.).
Mineralization of complex 14C-labeled C substrates
The 14C-labeled soil and plant residues were obtained from the same site used to collect the unlabeled soil. Briefly, steel frames were placed into the L. perenne L. grass swards. Acrylic chambers (15 × 30 cm, height 60 cm) were then clamped onto the frames and 7.4 MBq NaH14CO3 injected into a reaction vessel containing dilute HCl to generate 14CO2. The chambers were then sealed for 1 h and the headspace continuously mixed using a battery-powered fan (Hill et al., 2007). The chamber was then removed and the 14C-labeled shoot material harvested after 6 days, air-dried and stored at 20 °C in a sealed container. Six years after 14C labeling the swards, soil was recovered (0–10 cm depth) from the plots. This was considered to contain quasi-stable 14C-labeled SOM based on the C dynamics of this site (see Farrar et al., 2012; Hill et al., 2015).
As described for the simple C substrates, 14C-labeled L. perenne shoots (100 mg; 12.7 kBq g−1) were mixed with 10 g of soil for each of the six treatments. 14CO2 evolution was then determined over 21 days. Due to the lower specific activity of the 14C-labeled SOM, 100 g of 14C-labeled soil was mixed with biochar or straw in a 500 cm3 glass vessel similar to that described previously except that larger (4 mL) 1 m NaOH traps were used and 14CO2 evolution measured over 105 days.
Dissolved organic carbon dynamics
Soil (1 kg) from each of the six treatments was placed in plastic containers (135 × 102 × 283 mm) and incubated at 70% relative humidity and 20 °C for 60 days. During incubation, soil solutions were recovered nondestructively with 5-cm-long Rhizon® soil solution samplers (Rhizosphere Research Products B.V., Wageningen, the Netherlands). Dissolved organic C (DOC) and total dissolved N (TDN) in soil solution were determined using a Multi N/C 2100 analyzer (Analytik Jena, Jena, Germany). Dissolved organic and dissolved N (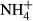 and
and 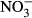 ) in soil solution were determined using a Multi N/C 2100 analyzer (Analytik Jena, Jena, Germany) and using the colorimetric methods of Mulvaney (1996) and Miranda et al. (2001), respectively.
) in soil solution were determined using a Multi N/C 2100 analyzer (Analytik Jena, Jena, Germany) and using the colorimetric methods of Mulvaney (1996) and Miranda et al. (2001), respectively.
Microbial biomass and community structure
At the end of the incubation experiment, soil (20 g) from the 1 kg containers was collected for phospholipid fatty acid (PLFA) profiling and for microbial biomass C and N determination. Following Buyer & Sasser (2012), soil from each treatment was freeze-dried (2 g) and 4 mL of Bligh-Dyer extractant containing an internal standard added. The samples were then sonicated (10 min, 20 °C), rotated end-over-end (2 h), and centrifuged (10 min). The liquid phase was transferred into clean screw-cap test tubes (13 × 100 mm) and 0.1 mL of chloroform and water added. The upper phase was discarded, whist the lower phase containing the extracted lipids was evaporated at 30 °C. Solid phase extraction using a 96-well SPE plate containing 50 mg of silica per well (Phenomenex Inc., Torrance, CA, USA) was used to separate lipids. Each sample was allowed to evaporate in a glass vial (30 min, 70 °C) with 0.5 mL of 5 : 5 : 1 methanol: chloroform: H2O; the latter process was performed for eluting phospholipids. After evaporation, a transesterification reagent (0.2 mL) was added to each vial, after which the vials were sealed and incubated (37 °C, 15 min). Acetic acid (0.075 m) and chloroform (0.4 mL) were added to each vial; chloroform was evaporated just to dryness and the samples dissolved in hexane. Measurements were performed on a 6890 gas chromatograph (Agilent Technologies, Wilmington, DE, USA) equipped with an autosampler, split–splitless inlet, and flame ionization detector. Fatty acid methyl esters (FAMEs) were separated on an Agilent Ultra 2 column, 25 m long × 0.2 mm internal diameter × 0.33 μm film thickness. Different taxonomic groups were classified as described in Frostegård et al. (1993) with acknowledgment of the caveats raised in Frostegård et al. (2011).
As for PLFA analysis, soil samples were collected after 60 days. Microbial biomass C and N (MBC and MBN) were determined based on the of CHCl3 fumigation-K2SO4 extraction method of Joergensen et al. (2011). After fumigation, DOC and TDN in the 0.5 m K2SO4 extracts were determined as described above. MBC was calculated using the standard conversion factor (Kec) of 0.45, while for MBN, a Ken value of 0.54 was used (Brookes et al., 1985; Wu et al., 1990).
Kinetic modeling of 14C-labeled glucose and amino acid mineralization in soil
Many earlier studies have indicated that the mineralization of simple 14C-labeled organic substrates such as those used here (e.g., amino acids and sugars) follow a biphasic kinetic pattern (Hill et al., 2008, 2012; Farrar et al., 2012; Oburger et al., 2012). A kinetic model was therefore fitted to the experimental data to provide information on the internal use of the 14C by the soil microbial community. Specifically, the model allows 14C taken up by the microbial biomass to be partitioned into that used for respiration (catabolic processes) and that used to make new cell biomass (anabolic processes) (Glanville et al., 2016).
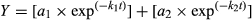 (1)
(1)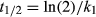 (2)
(2)However, the added C substrate to soil may be transformed by several microbial processes, and calculating the half-life period for the second phase (C pool 2, k2) is subject to uncertainty due to the complexity over the connectivity between pool C pool 1 and C pool 2 (Boddy et al., 2008; Glanville et al., 2016).
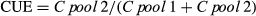 (3)
(3)A least sum of squares curve fitting algorithm in sigmaplot v12.3 (Systat Software Inc., San Jose, CA, USA) was used to fit the kinetic equation to the experimental data (Glanville et al., 2016).
Statistical analyses
Statistical analyses were carried out using spss v19.0 (SPSS Inc., Chicago, IL, USA). A one-way analysis of variance followed by a least significant difference test was used to determine significant differences (cutoff value of 95%) between treatments. Linear regression was undertaken in sigmaplot v12.5 (Systat Software Inc.).
Results
Analysis of biochar properties
As the torrefaction/pyrolysis temperature increased, biochar pH, specific surface area and water holding capacity significantly increased (Table 2). In contrast, biochar EC and CEC decreased with increasing temperature. Soluble C in the biochar exhibited a different pattern being maximal at an intermediate heating temperature (350 °C) and then declining markedly at the higher thermal regimes.
| B250 | B350 | B450 | B550 | |
|---|---|---|---|---|
| pH | 5.40 ± 0.08d | 8.84 ± 0.10c | 9.19 ± 0.10b | 9.74 ± 0.17a |
| EC (μS cm−1) | 3355 ± 125d | 1825 ± 158c | 1384 ± 39b | 1242 ± 77a |
| SSA (m2 g−1) | 2.70 ± 0.01d | 5.20 ± 0.01c | 4.55 ± 0.01b | 10.5 ± 0.01a |
| CEC (cmol kg−1) | 68.7 ± 0.5d | 58.6 ± 1.7c | 40.5 ± 1.2b | 22.0 ± 1.3a |
| WHC (%) | 275 ± 11b | 333 ± 23a | 355 ± 34a | 339 ± 12a |
| DOC (mg kg−1) | 1430 ± 90d | 1990 ± 140c | 1010 ± 20b | 560 ± 60a |
- EC, electrical conductivity; SSA, specific surface area; CEC, cation exchange capacity; WHC, water holding capacity; DOC, dissolved organic carbon.
- All values represent means ± SEM (n = 4). Different superscript letters represent significant differences between treatments at the P < 0.05 level.
Soil respiration
Compared to the unamended soil (79 μmol CO2 kg−1), soil respiration was significantly higher in the biochar or straw-amended soils (P < 0.001; Fig. 1). Overall, the straw-amended soil showed the highest CO2 emission being 5–10 times higher than in the different biochar treatments. In the biochar-amended soils, the highest pyrolytic temperatures had significantly lower CO2 emissions; however, these were still higher than in the soil-only control treatment.
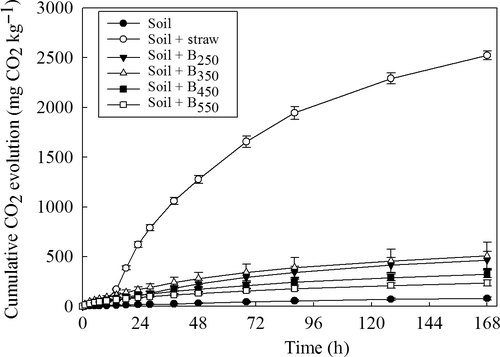
Mineralization of simple 14C-labeled substrates
The slowest 14C-glucose mineralization rate occurred in the unamended soil treatment (P < 0.001; Fig. 2a). Among the biochar-amended soils, the highest substrate mineralization rates were observed in the treatments containing biochar produced at lower temperatures. Biochar produced at 550 °C initially repressed glucose mineralization in comparison with the control; however, after 21 days, the amount of 14CO2 produced was not significantly different from the soil-only treatment. In comparison with biochar, the addition of straw greatly stimulated substrate mineralization particularly during the first 48 h; however, the subsequent rate of 14CO2 evolution after this initial mineralization phase was similar to the control.
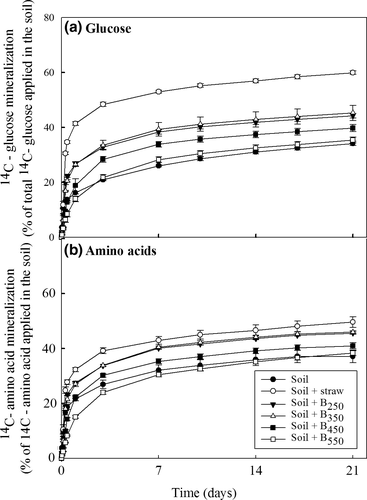
A similar trend to that observed for 14C-glucose was also seen for the effect of biochar and straw on the mineralization of amino acids in soil (Fig. 2b). The lowest amount of mineralization was seen in the unamended soil and the soil amended with biochar produced at the highest pyrolytic temperature. The greatest stimulation of mineralization of the amino acid mixture was again in the soil amended with straw and biochar produced at the lowest temperature.
Kinetic modeling of 14C-labeled glucose and amino acid mineralization in soil
Overall, the double exponential kinetic model fitted well to the experimental data. The average r2 value describing the closeness of fit of the model to the experimental data across all six treatments was 0.992 ± 0.002 for glucose and 0.993 ± 0.002 for the amino acid mixture (Tables 3 and 4). For 14C-glucose, most of the 14C was initially immobilized in the microbial biomass (C pool 2) with only a small amount immediately used in energy production (C pool 1). The presence of straw in the soil shifted the partitioning of C within the cell, with proportionally more 14C allocated to rapid energy production. Overall, the presence of biochar shifted C partitioning within the microbial community, particularly in the presence of torrefied biomass and low temperature biochars (B250, B350). This resulted in a significant alteration of microbial CUE. The presence of the chars produced at high temperatures also repressed the initial mineralization of glucose as evidenced by the increase in half-life associated with C pool 1. The lower rate constant (k2) values for C pool 2 may possibly suggest that biochars produced at high temperature marginally suppress the turnover of 14C immobilized in the microbial biomass. The turnover of this C pool equates to the turnover of the microbial biomass during both cell maintenance and also due to death of cells and subsequent extracellular (i.e., by exoenzymes) or intracellular (e.g., by protozoal ingestion) breakdown of the C in the microbial cells and use of the C released by other organisms (Glanville et al., 2016).
| Control | Straw | Biochar | ||||
|---|---|---|---|---|---|---|
| B250 | B350 | B450 | B550 | |||
| C pool 1 (% of total) | 16.6 ± 1.8 | 47.6 ± 1.9 | 31.6 ± 2.0 | 32.9 ± 1.9 | 28.7 ± 1.4 | 22.6 ± 1.2 |
| C pool 2 (% of total) | 82.7 ± 1.3 | 54.5 ± 2.4 | 69.3 ± 1.6 | 67.8 ± 1.6 | 71.1 ± 1.3 | 76.8 ± 1.1 |
| k1 (day−1) | 4.73 ± 1.32 | 3.31 ± 0.412 | 2.98 ± 0.47 | 2.20 ± 0.31 | 1.13 ± 0.14 | 0.83 ± 0.11 |
| k2 (day−1) | 0.012 ± 0.001 | 0.016 ± 0.003 | 0.012 ± 0.002 | 0.012 ± 0.002 | 0.009 ± 0.001 | 0.009 ± 0.001 |
| C pool 1 half-life (day) | 0.15 | 0.21 | 0.23 | 0.32 | 0.61 | 0.83 |
| C use efficiency (CUE) | 0.83 | 0.53 | 0.69 | 0.67 | 0.71 | 0.77 |
| Model r2 | 0.983 | 0.993 | 0.990 | 0.993 | 0.996 | 0.997 |
- The size of C pool 1 and C pool 2 represent the total amount of 14C initially assigned to catabolic and anabolic processes, respectively, within the cell. The decay constants k1 and k2 are the rates for pools C pool 1 and C pool 2, respectively. Values represent mean ± SEM. The model r2 value describes the fit of the kinetic model (Eqn 1) to the experimental data.
| Control | Straw | Biochar | ||||
|---|---|---|---|---|---|---|
| B250 | B350 | B450 | B550 | |||
| C pool 1 (% of total) | 24.2 ± 1.8 | 36.7 ± 1.9 | 32.2 ± 2.1 | 33.0 ± 2.2 | 30.5 ± 1.1 | 25.2 ± 0.8 |
| C pool 2 (% of total) | 75.3 ± 1.4 | 63.9 ± 1.4 | 67.9 ± 1.7 | 67.4 ± 1.8 | 69.7 ± 1.0 | 74.7 ± 0.8 |
| k1 (day−1) | 3.33 ± 0.63 | 3.37 ± 0.43 | 2.67 ± 0.43 | 2.31 ± 0.38 | 1.33 ± 0.12 | 0.79 ± 0.06 |
| k2 (day−1) | 0.010 ± 0.002 | 0.012 ± 0.002 | 0.012 ± 0.002 | 0.012 ± 0.002 | 0.009 ± 0.001 | 0.010 ± 0.001 |
| C pool 1 half-life (day) | 0.21 | 0.21 | 0.26 | 0.30 | 0.52 | 0.87 |
| C use efficiency (CUE) | 0.76 | 0.63 | 0.68 | 0.67 | 0.70 | 0.75 |
| Model r2 | 0.987 | 0.992 | 0.990 | 0.990 | 0.997 | 0.999 |
- The size of C pool 1 and C pool 2 represents the total amount of 14C initially assigned to catabolic and anabolic processes, respectively, within the cell. The decay constants k1 and k2 are the rates for pools C pool 1 and C pool 2, respectively. Values represent mean ± SEM. The model r2 value describes the fit of the kinetic model (Eqn 1) to the experimental data.
Kinetic modeling of the 14C-labeled amino acids through the microbial biomass revealed very similar results to those obtained for 14C-glucose. Overall, both straw and torrefied biomass promoted the allocation of more C toward catabolic processes resulting in lower CUE values in comparison with the unamended control. In addition, high temperature chars repressed the rate of amino acid-C flow through C pool 1 relative to the control. Biochar did not appear to alter the rate of amino acid-derived C processed through the microbial biomass (k2, C pool 2).
Mineralization of 14C-labeled native SOM and plant residues
The highest rate of 14C-SOM mineralization was seen in the straw-amended soil (Fig. 3a). However, although biochar produced at the two lowest pyrolysis temperatures initially stimulated SOC mineralization, after 60 days, all biochar amendments had significantly reduced SOC mineralization relative to the unamended soil (P < 0.001).
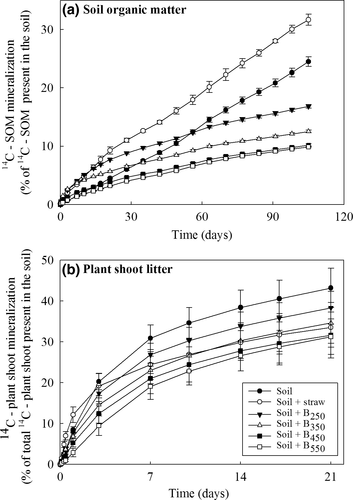
Although the results were variable, in contrast to other 14C-labeled substrates, the mineralization of the plant residues was suppressed by all amendments, including straw. However, in common with other substrates, the greatest suppression was seen in the treatment containing biochars produced at high temperatures (Fig. 3b).
Microbial biomass and community structure
Phospholipid fatty acid analysis, MBC and MBN were used to determine whether biochar or straw affected the microbial community structure and abundance. Compared to the unamended control treatment, the abundance of Gram-negative bacteria and fungi in the biochar-amended soils was higher, whereas the abundance of Gram-positive bacteria and anaerobes was significantly lower. In biochar-amended soils, an increase of pyrolytic temperatures was associated with a decrease of Gram-negative bacteria and fungi; in contrast, it was associated with an increase of Gram-positive bacteria and anaerobes (Table 5), and higher MBC and MBN (Table 6). Nevertheless, compared to the unamended soil, MBC and MBN were significantly higher in the biochar-amended soils. In addition, higher DOC concentrations were observed in the biochar-amended soil solutions, although these tended to decrease in the presence of chars produced at higher temperatures (Fig. 4).
| Group | Control | Straw | B250 | B350 | B450 | B550 |
|---|---|---|---|---|---|---|
| Gram+ bacteria | 27.3 ± 0.2d | 23.9 ± 0.5a | 25.1 ± 0.1b | 24.6 ± 0.3c | 24.1 ± 0.2c | 25.3 ± 0.3b |
| Gram− bacteria | 46.1 ± 0.2e | 50.8 ± 0.7a | 49.6 ± 0.1d | 48.2 ± 0.4c | 49.1 ± 0.8 cd | 47.6 ± 0.2b |
| Fungi | 1.6 ± 0.1c | 7.1 ± 0.2a | 2.8 ± 0.5b | 3.0 ± 0.4b | 3.3 ± 0.7b | 3.3 ± 0.6b |
| AMF | 4.6 ± 0.1b | 4.2 ± 0.1a | 3.9 ± 0.1d | 4.3 ± 0.1ac | 4.4 ± 0.1c | 4.7 ± 0.2b |
| Actinomycetes | 16.8 ± 0.2c | 9.6 ± 0.2a | 15.6 ± 0.2b | 16.5 ± 0.3c | 15.5 ± 0.2b | 15.2 ± 0.6b |
| Anaerobes | 1.3 ± 0.1c | 0.9 ± 0.0a | 1.0 ± 0.1b | 1.1 ± 0.1b | 1.0 ± 0.2ab | 0.9 ± 0.1ab |
| Eukaryotes | 2.4 ± 0.3bc | 3.4 ± 0.2a | 2.0 ± 0.2c | 2.4 ± 0.1b | 2.6 ± 0.4b | 2.9 ± 0.4ab |
- Gram+, Gram positive; Gram−, Gram negative; AMF, Putative arbuscular mycorrhizal fungi.
- Values represent means ± SEM (n = 4). Different superscript letters represent significant differences between treatments at the P < 0.05 level.
| Treatment | MBC (mg C kg−1) | MBN (mg N kg−1) |
|---|---|---|
| Soil | 635 ± 8e | 40 ± 2c |
| Straw | 1843 ± 127a | 160 ± 14a |
| B250 | 717 ± 17d | 31 ± 2d |
| B350 | 803 ± 45c | 44 ± 5c |
| B450 | 897 ± 20b | 43 ± 7bc |
| B550 | 921 ± 33b | 52 ± 3b |
- Values represent means ± SEM (n = 4). Different superscript letters represent significant differences between treatments at the P < 0.05 level.
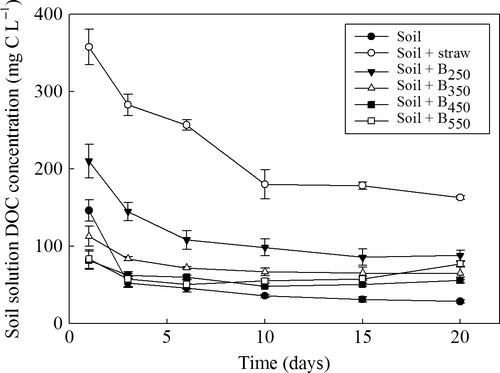
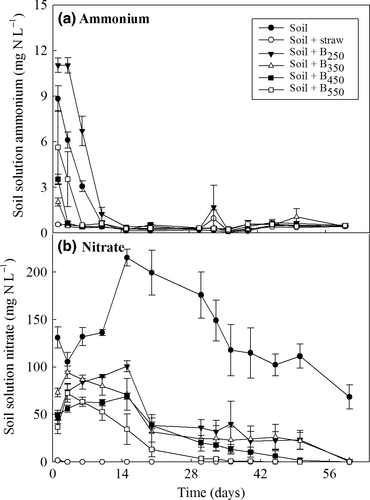
Soluble N concentrations were dominated by 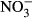 and decreased in all treatments over time (Fig. 5). Overall, the concentrations of
and decreased in all treatments over time (Fig. 5). Overall, the concentrations of 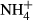 declined to very low levels in all treatments after 7 days although the most rapid decline was seen in the straw treatment. In contrast to the unamended control treatment,
declined to very low levels in all treatments after 7 days although the most rapid decline was seen in the straw treatment. In contrast to the unamended control treatment, 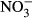 concentrations remained extremely, low in the presence of straw throughout the 60-day monitoring period. Generally, the presence of biochar resulted in an initial increase in
concentrations remained extremely, low in the presence of straw throughout the 60-day monitoring period. Generally, the presence of biochar resulted in an initial increase in 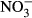 concentration; however, the concentration then progressively declined until almost no
concentration; however, the concentration then progressively declined until almost no 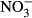 remained in solution by 60 days. The decline in
remained in solution by 60 days. The decline in 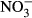 was most apparent in the high temperature chars. The average (±SEM) total soluble inorganic N concentrations in the different treatments over the 60-day incubation period were 136 ± 11 mg N L−1 (control), 0.4 ± 0.1 mg N L−1 (straw), 50 ± 9 mg N L−1 (B250), 45 ± 8 mg N L−1 (B350), 32 ± 7 mg N L−1 (B450), and 22 ± 8 mg N L−1 (B550).
was most apparent in the high temperature chars. The average (±SEM) total soluble inorganic N concentrations in the different treatments over the 60-day incubation period were 136 ± 11 mg N L−1 (control), 0.4 ± 0.1 mg N L−1 (straw), 50 ± 9 mg N L−1 (B250), 45 ± 8 mg N L−1 (B350), 32 ± 7 mg N L−1 (B450), and 22 ± 8 mg N L−1 (B550).
Discussion
Impact of straw and biochar on soil respiration
The results presented here clearly show that the addition of biochar to soil increased basal respiration, albeit to a much lesser extent than observed in the presence of straw. The addition of straw was characterized by a short lag phase in CO2 efflux, which presumably reflects the adaptation and growth of the microbial biomass in response to the addition of a large amount of labile C (Cayuela et al., 2009). This is supported by the observed increase in microbial biomass during the experiment. In contrast, no lag phase in soil respiration was apparent in the biochar treatments suggesting a lack of rapid microbial growth; however, all the biochars increased CO2 emissions relative to the control. While this response could be attributed to the positive priming of native SOM, it can also be attributable to the loss of C from the biochar itself. Using the same soil, Jones et al. (2011) showed that the increase in soil respiration after the addition of a wood-based biochar was partially due to the biotic breakdown of DOC contained in the biochar and from the abiotic release of CO2 from biochar minerals formed during pyrolysis. In our study, the stimulation in soil respiration was positively correlated with biochar DOC content (r2 = 0.935). The amount of DOC added to the soil in the biochar (56–199 mg C kg−1), however, was lower than the additional amount of CO2 produced from the biochar-soil mixtures over 7 days (154–386 mg C kg−1), relative to the control. It also cannot account for the increase in MBC in soil upon biochar addition (82–286 mg C kg−1). This suggests that biochar-derived DOC alone cannot account for the observed increase in CO2. It is also unlikely that abiotic CO2 release can explain this increase as the contribution from this source would be expected to increase with biochar production temperature (as the C-to-mineral ratio decreases and more metal oxides are formed; Angin, 2013). The additional CO2 could therefore originate from native SOC (i.e., positive priming) or from the microbial-induced solubilization and breakdown of the biochar (Jiang et al., 2016).
Positive priming effects of biochar
Based on our 14C-labeled SOM experiment, biochars produced at lower temperatures initially accelerated native SOM turnover by twofold to threefold within the first 7 days, suggesting that this may also account for some of the additional CO2 produced immediately after biochar addition. This positive priming, however, was short-lived and is unlikely to be of concern in terms of the net C balance of the soil in the longer term.
In this study, biochar application generally appeared to stimulate the mineralization of the simple C substrates, glucose, and amino acids. This was surprising as their mineralization is typically insensitive to major changes in soil management (Jones et al., 2005). A number of factors may explain the apparent stimulation of simple C substrate turnover including: (i) inputs of DOC from the biochar may promote general microbial activity in the soil leading to faster uptake rates and mineralization (De Nobili et al., 2001; Hamer et al., 2004), (ii) the biochar may absorb humic substances from soil solution which previously limited microbial activity (Ni et al., 2011), (iii) the biochar is inducing growth of the microbial community leading to a greater C sink, or (iv) biochar is influencing the internal partitioning of C within the microbial cell (immobilization-to-mineralization ratio) and thus microbial C use efficiency (CUE) (Farrell et al., 2015). Unlike previous studies (Riedel et al., 2014), our soil solution data do not lend support to hypothesis (ii). Although we present some evidence to support hypotheses (i) and (iii), the patterns of CO2 evolution suggest to us that hypothesis (iv) is the most likely explanation.
If biochar was alleviating stress in the microbial community (e.g., by absorbing toxic metals or xenobiotics), we would expect CUE to increase as less C is invested in energy-intensive stress avoidance strategies (Tiemann & Billings, 2011). However, the kinetic modeling undertaken here clearly suggests that biochar addition decreases microbial CUE, particularly in the presence of low temperature chars. This result contrasts with Jiang et al. (2016) where an increased CUE was observed in the presence of biochar. The consistently reduced CUE for the C substrates studied here could be attributable to shifts in microbial community structure, to reductions in N availability, or to shifts in available C within the soil. The largest reduction in CUE was observed in the straw treatment (C : N ratio = 77), consistent with N limitation within the microbial community and an increase in overflow respiration (Sinsabaugh et al., 2013; Spohn et al., 2016). In support of this N limitation hypothesis, biochar is known to readily adsorb soluble N (Jones et al., 2011; Subedi et al., 2015; Tian et al., 2016), potentially limiting its availability to the soil microbial community. This sorption was expected to be especially prevalent in biochars with a high CEC where the lowest CUE values were obtained. However, CEC was not correlated with the rate of disappearance of either 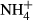 or
or 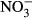 from soil solution, suggesting that this may not wholly explain our CUE results. Alternatively, DOC originating from the biochar may be preferred by the microbial community for catabolic processes, thereby freeing up a greater use of glucose and keto acids (produced from amino acid deamination) for use in respiratory pathways. Microbial community composition has also been hypothesized to influence CUE. In our study, we observed an increase in fungal biomass; however, increases in fungal-to-bacterial ratio are typically associated with an increase in CUE rather than a decrease as observed here (Keiblinger et al., 2010).
from soil solution, suggesting that this may not wholly explain our CUE results. Alternatively, DOC originating from the biochar may be preferred by the microbial community for catabolic processes, thereby freeing up a greater use of glucose and keto acids (produced from amino acid deamination) for use in respiratory pathways. Microbial community composition has also been hypothesized to influence CUE. In our study, we observed an increase in fungal biomass; however, increases in fungal-to-bacterial ratio are typically associated with an increase in CUE rather than a decrease as observed here (Keiblinger et al., 2010).
Overall, our results initially suggested that biochar accelerated low MW C turnover in soil. Closer examination of the results using kinetic modeling, however, actually revealed slower rates of turnover in the presence of biochar. This was associated with a major shift in microbial CUE which we attribute to the reduced availability of N and the increased presence of alternative C substrates in solution.
Negative priming effect of biochar on native SOM
Although biochar application initially stimulated native SOM turnover, in the longer term, it significantly reduced SOM and plant residue turnover, consistent with the findings of previous studies in the same soil (Jones et al., 2012) and in the meta-analyses undertaken by Maestrini et al. (2015) and Wang et al. (2016). Lu et al. (2014) also observed that biochar application suppressed SOC decomposition, whereas the co-addition of inorganic N stimulated it. Although Lu et al. (2014) hypothesized that the decrease in SOC decomposition was mainly due to the sorption of DOC by biochars, a range of factors could be responsible for the negative priming response observed here (Rittl et al., 2015a). While N limitation could repress microbial activity, it frequently leads to the positive priming of soil organic matter, suggesting that this is probably not the mechanism (Murphy et al., 2015). The results from the kinetic modeling presented here (C pool 2, k2) and in Jones et al. (2012) also suggest that biochar does not greatly alter the rate of turnover of C contained in the soil microbial biomass. Therefore, it is more likely that the negative priming is associated with the microbial community switching to an alternative source of C. This could be derived from the biochar itself or include a pool of SOM which was not heavily 14C-labeled. In support of the first theory, the observed reduction in soil solution DOC over time certainly suggests that the microbial community is utilizing an alternative C source. This is supported by the results of Jones et al. (2011) who showed that wood-derived biochar DOC could be rapidly respired by the soil microbial community.
Influence of biochar properties on the priming effect
Consistent with previous studies, our results showed that higher pyrolytic temperatures led to increases in specific surface area and moisture retention while decreasing CEC and DOC content (Mukherjee et al., 2011; Wang et al., 2013). The characteristics of these high temperature biochars promoted greater negative priming of SOM in our study, and they have recently been advocated as the best type of char for maximizing soil C storage (Yuan et al., 2014). However, increasing amounts of C are also volatilized during the production of biochar at higher pyrolysis temperatures (Lehmann et al., 2006), so ultimately less C per unit mass of feedstock is available for soil incorporation. In addition, some of the beneficial properties of the biochar may be lost (i.e., its ability to retain nutrients and moisture). Therefore, even though low temperature chars and torrefied biomass do not provide the optimal conditions for SOM stabilization, the greater volume of C available to add to the soil may offset this.
It has been proposed that the addition of crop residues to soil may promote the loss of SOM (Fontaine et al., 2004). In partial agreement with this, our study demonstrated that straw promoted the positive priming of native SOM; however, it also induced a large increase in microbial biomass C. As the specific activity of the 14C-labeled SOM is not known, we cannot calculate the overall net C balance of the system. However, a recent study by Cardinael et al. (2015) suggested that straw did not induce a net loss of SOC, while many studies have shown that cereal straw can replenish SOC reserves (Liu et al., 2003; Malhi et al., 2006). In practical terms, straw still represents the most widely available feedstock for farmers and from some perspectives could be seen as a better soil conditioning agent than biochar, particularly in the short term (as it actively promotes microbial activity and nutrient cycling, promotes better aggregation and structural stability, is less susceptible to wind/water erosion, and does not have to be transported away and processed prior to field application). Based on investigations of historical charcoal burial sites, however, biochar may also promote some of these attributes in the longer term (Borchard et al., 2014; Hernandez-Soriano et al., 2016). Further, ongoing legislative, economic, and social barriers are still likely to stifle widespread adoption of biochar in many cereal production areas (Jones et al., 2012; Rittl et al., 2015b). In conclusion, biochar had a much lesser effect on the size, activity, and structure of the soil microbial community in comparison with straw and resulted in greater protection of native SOM. It is also likely that the biochar-derived C will persist for longer in soil, particularly those chars produced at high temperatures. If the sole goal is to maximize C storage in soil, then biochars produced at higher temperatures have the greatest potential; however, if other soil quality cobenefits are required, then we will still advocate the use of straw and torrefied biomass produced at low temperatures.
Acknowledgements
This study was supported by the Welsh European Funding Office, under the SEREN program, the Key Agriculture R&D Program of Guizhou Province (NY [2012]3019), and the Opening Fund of the State Key Environmental Geochemistry (SKLEG15902).




