Nitrous oxide production by ammonia oxidizers: Physiological diversity, niche differentiation and potential mitigation strategies
Abstract
Oxidation of ammonia to nitrite by bacteria and archaea is responsible for global emissions of nitrous oxide directly and indirectly through provision of nitrite and, after further oxidation, nitrate to denitrifiers. Their contributions to increasing N2O emissions are greatest in terrestrial environments, due to the dramatic and continuing increases in use of ammonia-based fertilizers, which have been driven by requirement for increased food production, but which also provide a source of energy for ammonia oxidizers (AO), leading to an imbalance in the terrestrial nitrogen cycle. Direct N2O production by AO results from several metabolic processes, sometimes combined with abiotic reactions. Physiological characteristics, including mechanisms for N2O production, vary within and between ammonia-oxidizing archaea (AOA) and bacteria (AOB) and comammox bacteria and N2O yield of AOB is higher than in the other two groups. There is also strong evidence for niche differentiation between AOA and AOB with respect to environmental conditions in natural and engineered environments. In particular, AOA are favored by low soil pH and AOA and AOB are, respectively, favored by low rates of ammonium supply, equivalent to application of slow-release fertilizer, or high rates of supply, equivalent to addition of high concentrations of inorganic ammonium or urea. These differences between AOA and AOB provide the potential for better fertilization strategies that could both increase fertilizer use efficiency and reduce N2O emissions from agricultural soils. This article reviews research on the biochemistry, physiology and ecology of AO and discusses the consequences for AO communities subjected to different agricultural practices and the ways in which this knowledge, coupled with improved methods for characterizing communities, might lead to improved fertilizer use efficiency and mitigation of N2O emissions.
1 INTRODUCTION
Nitrous oxide (N2O) is an important greenhouse gas produced by ammonia oxidizers (AO) and denitrifiers. It ranks third behind carbon dioxide (CO2) and methane (CH4) in terms of radiative forcing, with estimated global N2O emission rates of 11 Tg/year (range 8.1–30.7 Tg/year; IPCC, 2013). However, N2O has a global warming potential that is 265- and 10-fold greater than those of CO2 and CH4, respectively, has a long atmospheric lifetime (109–125 years; Prather et al., 2015), contributed 17% to radiative forcing in 2005 (IPCC, 2013) and is predicted to be the primary contributor to ozone depletion in the stratosphere in the 21st Century (Ravishankara, Daniel, & Portmann, 2009). Atmospheric N2O levels are 20% greater than in pre-industrial times (MacFarling-Meure et al., 2006), largely through increases in reactive nitrogen, resulting from increased nitrogen fertilization as manure, inorganic nitrogen and urea (Smil, 1999). N2O levels are increasing by 0.73 ± 0.03 ppb/year (~0.3% year−1) and are projected to increase significantly through this century, due to food and animal feed demands of an increasing global population (Alexandratos & Bruinsma, 2012; Tilman et al., 2001).
Nitrification, the sequential oxidation of ammonia (NH3) to nitrite (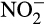 ) and nitrate (
) and nitrate (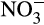 ) in the presence of oxygen, can contribute indirectly to N2O emissions, providing nitrogen oxides to denitrifying microorganisms for energy conservation under reduced oxygen conditions and anoxia (Kuypers, Marchant, & Kartal, 2018). Denitrifiers are traditionally considered major producers of N2O, but N2O is also emitted by organisms that oxidize NH3 to
) in the presence of oxygen, can contribute indirectly to N2O emissions, providing nitrogen oxides to denitrifying microorganisms for energy conservation under reduced oxygen conditions and anoxia (Kuypers, Marchant, & Kartal, 2018). Denitrifiers are traditionally considered major producers of N2O, but N2O is also emitted by organisms that oxidize NH3 to 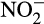 . This direct contribution to emissions is particularly important given projected increases in N fertilizer applications. Traditionally, ammonia oxidation was thought to be performed by bacterial ammonia oxidizers (AOB), but marine ammonia oxidation is now known to be dominated by archaeal ammonia oxidizers (AOA) and both groups are important in other ecosystems. Recently discovered complete ammonia oxidizers (comammox bacteria) oxidize both NH3 and
. This direct contribution to emissions is particularly important given projected increases in N fertilizer applications. Traditionally, ammonia oxidation was thought to be performed by bacterial ammonia oxidizers (AOB), but marine ammonia oxidation is now known to be dominated by archaeal ammonia oxidizers (AOA) and both groups are important in other ecosystems. Recently discovered complete ammonia oxidizers (comammox bacteria) oxidize both NH3 and 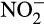 within the same cell (Daims et al., 2015; van Kessel et al., 2015), but little is currently known of their ecology. Cellular rates of N2O production by AOA and AOB differ, leading to different contributions to emissions, and there is evidence for niche differentiation between AOA and AOB with respect to environmental factors and land use strategies. This article reviews evidence for differences in N2O emissions by AO in culture and in natural ecosystems and assesses potential links between niche differentiation, N2O emissions and agricultural systems and potential mitigation strategies.
within the same cell (Daims et al., 2015; van Kessel et al., 2015), but little is currently known of their ecology. Cellular rates of N2O production by AOA and AOB differ, leading to different contributions to emissions, and there is evidence for niche differentiation between AOA and AOB with respect to environmental factors and land use strategies. This article reviews evidence for differences in N2O emissions by AO in culture and in natural ecosystems and assesses potential links between niche differentiation, N2O emissions and agricultural systems and potential mitigation strategies.
2 THE ROLE OF NITRIFIERS IN BIOGEOCHEMICAL CYCLING
In both terrestrial and aquatic ecosystems, the major source of bioavailable nitrogen is fixation of atmospheric dinitrogen (N2) by free-living or plant-associated N2-fixing bacteria, photosynthetic cyanobacteria and hydrogenotrophic methanogenic archaea (Boyd & Peters, 2013). Following consumption by higher trophic levels, excretion and death, mineralization of organic N by microbes releases inorganic ammonium (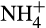 ), which can be assimilated by plants and microorganisms. Ammonium also provides an essential source of energy for obligate aerobic, lithotrophic microorganisms, the ammonia oxidizers. These organisms oxidize NH3 to
), which can be assimilated by plants and microorganisms. Ammonium also provides an essential source of energy for obligate aerobic, lithotrophic microorganisms, the ammonia oxidizers. These organisms oxidize NH3 to 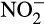 , which is then oxidized to
, which is then oxidized to 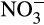 , before dissimilatory reduction under anaerobic/microaerobic conditions. Facultative denitrifiers can return N2 to the atmosphere (Kuypers et al., 2018). These nitrogen cycling processes are supplemented by a network of other biological and physicochemical processes.
, before dissimilatory reduction under anaerobic/microaerobic conditions. Facultative denitrifiers can return N2 to the atmosphere (Kuypers et al., 2018). These nitrogen cycling processes are supplemented by a network of other biological and physicochemical processes.
In aerobic marine ecosystems, nitrification is highly efficient and results in concentrations of 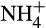 in the nanomolar range (Horak et al., 2013), but can be limited under reduced oxygen concentration in oxygen minimum zones (OMZ; Ward, 2011) and within decaying particulate organic matter or polluted environments, where oxygen demand can be high. These conditions favor denitrification.
in the nanomolar range (Horak et al., 2013), but can be limited under reduced oxygen concentration in oxygen minimum zones (OMZ; Ward, 2011) and within decaying particulate organic matter or polluted environments, where oxygen demand can be high. These conditions favor denitrification.
In natural terrestrial ecosystems, in which 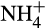 is provided via mineralization and
is provided via mineralization and 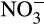 is available, the balance between nitrification and denitrification is again controlled largely by oxygen concentration, which is itself controlled by soil moisture content. At high moisture content, the potential for aerobic nitrification will be low and denitrification will dominate. The situation reverses as soil moisture decreases, and oxygen concentration increases (Bateman & Baggs, 2005). These potential rates are moderated by other factors, for example, rates of NH3 supply from mineralization or supply of organic carbon and
is available, the balance between nitrification and denitrification is again controlled largely by oxygen concentration, which is itself controlled by soil moisture content. At high moisture content, the potential for aerobic nitrification will be low and denitrification will dominate. The situation reverses as soil moisture decreases, and oxygen concentration increases (Bateman & Baggs, 2005). These potential rates are moderated by other factors, for example, rates of NH3 supply from mineralization or supply of organic carbon and 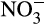 for denitrification (Booth, Stark, & Rastetter, 2005). This leads to variability in relative concentrations of
for denitrification (Booth, Stark, & Rastetter, 2005). This leads to variability in relative concentrations of 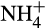 and
and 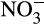 and in relative activities of nitrifiers and denitrifiers at both ‘bulk’ and microscale levels. For example, even in ‘aerobic’ soils, denitrification may occur in microenvironments, where oxygen diffusion is limited, or in regions of high decomposition, where oxygen demand is high.
and in relative activities of nitrifiers and denitrifiers at both ‘bulk’ and microscale levels. For example, even in ‘aerobic’ soils, denitrification may occur in microenvironments, where oxygen diffusion is limited, or in regions of high decomposition, where oxygen demand is high.
Despite spatial heterogeneity, and resultant complexity, soils that have not been subjected to modern agricultural developments have a ‘closed’ nitrogen cycle in which most  generated from mineralization is assimilated by plants and soil microbes and <10% is oxidized to
generated from mineralization is assimilated by plants and soil microbes and <10% is oxidized to 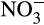 (Haynes, 1986). The closed nature of this cycle is facilitated further by the production of biological nitrification inhibitors (BNI) by some plants and NH3 concentrations in such soils are relatively high and
(Haynes, 1986). The closed nature of this cycle is facilitated further by the production of biological nitrification inhibitors (BNI) by some plants and NH3 concentrations in such soils are relatively high and 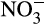 concentrations low (Subbarao et al., 2012).
concentrations low (Subbarao et al., 2012).
This situation is reversed in managed, agricultural soils due to application of inorganic, 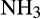 - and urea-based fertilizers. Over the past 50 years, N fertilizer use increased in Western Europe until stabilization in the 1990s, but continues to accelerate globally through increased usage elsewhere, with further increases projected to support future increases in world population (FAO, 2017; Lassaletta, Billen, Grizzetti, Anglade, & Garnier, 2014; Tilman et al., 2001). N entering terrestrial ecosystems produced via the Haber–Bosch process (170 Tg annually; FAO, 2017) exceeds that produced naturally and this energy-intensive process consumes >1% of the global energy demand (IFA/UNEP, 1998). For AO, this represents an injection of energy and has created an imbalance in the nitrogen cycle, with high
- and urea-based fertilizers. Over the past 50 years, N fertilizer use increased in Western Europe until stabilization in the 1990s, but continues to accelerate globally through increased usage elsewhere, with further increases projected to support future increases in world population (FAO, 2017; Lassaletta, Billen, Grizzetti, Anglade, & Garnier, 2014; Tilman et al., 2001). N entering terrestrial ecosystems produced via the Haber–Bosch process (170 Tg annually; FAO, 2017) exceeds that produced naturally and this energy-intensive process consumes >1% of the global energy demand (IFA/UNEP, 1998). For AO, this represents an injection of energy and has created an imbalance in the nitrogen cycle, with high 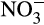 production due to high nitrification rates. These effects appear to have been exacerbated by crop breeding programmes developed on the assumption of high levels of inorganic nitrogen fertilization, decreasing NH3 competition between nitrifiers and plants and reducing the requirement for BNI, such that these are not produced by modern crop varieties (Subbarao et al., 2017). This has significantly reduced N fertilizer use efficiency to approximately 47% (Lassaletta et al., 2014), through leaching of anionic
production due to high nitrification rates. These effects appear to have been exacerbated by crop breeding programmes developed on the assumption of high levels of inorganic nitrogen fertilization, decreasing NH3 competition between nitrifiers and plants and reducing the requirement for BNI, such that these are not produced by modern crop varieties (Subbarao et al., 2017). This has significantly reduced N fertilizer use efficiency to approximately 47% (Lassaletta et al., 2014), through leaching of anionic 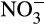 , in contrast to cationic
, in contrast to cationic 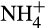 that is bound to negatively charged particles. It has also led to greatly elevated N2O emissions.
that is bound to negatively charged particles. It has also led to greatly elevated N2O emissions.
3 DIVERSITY OF AMMONIA-OXIDIZING MICROORGANISMS
Three main groups of aerobic ammonia-oxidizing prokaryotes have been described: AOB, AOA and comammox bacteria. AOB belong to beta- and gammaproteobacteria classes, with two (Nitrosomonas and Nitrosospira) and one (Nitrosococcus) genera, respectively (Purkhold et al., 2000). These genera display different environmental distributions indicating distinct physiological characteristics. Nitrosococcus is mainly found in marine environments and salt lakes (Campbell et al., 2011), although one strain was recently enriched from an acidic soil (Hayatsu et al., 2017). Nitrosomonas has been found in similar environments but also in engineered systems, such as wastewater treatment systems (Mobarry, Wagner, Urbain, Rittmann, & Stahl, 1996; Schramm et al., 1996). Nitrosospira organisms dominate soil AOB but are found in a range of ecosystems and are genetically diverse, in terms of both 16S rRNA and amoA gene markers (Aigle, Prosser, & Gubry-Rangin, 2019; Purkhold et al., 2000; Purkhold, Wagner, Timmermann, Pommerening-Röser, & Koops, 2003).
AOA belong to the class Nitrososphaeria within the phylum Thaumarchaeota and known AOA diversity is represented by four order-level phylogenetic lineages: Nitrososphaerales, Nitrosopumilales, Ca. Nitrosotaleales and thermophilic Ca. Nitrosocaldales (Brochier-Armanet, Boussau, Gribaldo, & Forterre, 2008; Kerou, Alves, & Schleper, 2016; Stieglmeier, Alves, & Schleper, 2014). AOA are ubiquitous and mesophilic lineages and are genetically diverse, based on amoA and 16S rRNA gene phylogenies, with higher diversity described for terrestrial than marine lineages (Alves, Minh, Urich, Haeseler, & Schleper, 2018; Gubry-Rangin et al., 2011; Pester, Schleper, & Wagner, 2011). Thermophilic AOA diversity is lower, possibly reflecting sampling bias (Alves et al., 2018; Gubry-Rangin, Williams, & Prosser, 2018).
All comammox bacteria belong to the genus Nitrospira of the class Nitrospira, which also includes canonical nitrite-oxidizing bacteria (Daims, Lücker, & Wagner, 2016), and have been detected in a range of natural and engineered environments (Fowler, Palomo, Dechesne, Mines, & Smets, 2018; Palomo et al., 2018; Pjevac et al., 2017; Wang et al., 2019; Zheng et al., 2019), with the possible exception of oceanic environments. Available amoA gene sequences suggest two phylogenetic groups (clades A and B) of similar diversity with potential environmental specializations. Currently cultivated representatives belong to clade A (Daims et al., 2015; van Kessel et al., 2015) and while both clades A and B are found in soil, conditions under which comammox bacteria are active in soil have only been demonstrated for clade B (Shi et al., 2018; Wang et al., 2019).
4 MECHANISMS OF AMMONIA OXIDATION
4.1 Ammonia oxidation to hydroxylamine
The first step in ammonia oxidation in all currently characterized aerobic, autotrophic AO is oxidation of NH3 to hydroxylamine by NH3 monooxygenase (AMO), a copper-based, broad-range, membrane-bound oxygenase. Although studied in detail in relatively few organisms (Arp & Stein, 2003; Kozlowski, Stieglmeier, Schleper, Klotz, & Stein, 2016), AMO has similar characteristics in AOA and AOB. Both contain the same homolgues (amoA, -B and -C) encoding three structural subunits and AOA possess an additional gene (amoX) likely encoding a fourth sub-unit (Tolar et al., 2017). Although the active enzyme complex has not been identified, comparison with the particulate methane monooxygenase suggests the β-subunit as the active site (Tolar et al., 2017). The structural gene for the α-subunit, amoA, has become the target gene for detection and distinction of AOA and AOB in natural environments (e.g. Francis, O'Mullan, & Ward, 2003; Rotthauwe, Witzel, & Liesack, 1997; Tourna, Freitag, Nicol, & Prosser, 2008) and, with 16S rRNA genes, for phylogenetic analysis (Alves et al., 2018; Gubry-Rangin et al., 2011; Pester et al., 2011; Purkhold et al., 2000; Stephen, McCaig, Smith, Prosser, & Embley, 1996). Comparative genomic analysis of comammox Nitrospira indicates that their ammonia oxidation genomic repertoire is more similar to AOB than AOA, with homologues of amoA genes found in all autotrophic AO and of AOB-like hydroxylamine dehydrogenase and c-type cytochromes, which are responsible for transferring electrons to the quinone pool (Daims et al., 2015).
A potential difference between AO is substrate affinity. A report of high NH3 affinity in Nitrosopumilus maritimus provided a convincing explanation for the dominance of AOA in marine environments, where NH3 is present at nanomolar concentrations, and led to the suggestion that all AOA had higher affinity than AOB (Martens-Habbena, Berube, Urakawa, José, & Stahl, 2009). The generality of this finding was challenged by two studies (Hink, Lycus, et al., 2017; Kits et al., 2017), which questioned the high affinity in N. maritimus and the clear distinction between AOA and AOB. The comammox strain, Nitrospira inopinata, also has high NH3 affinity, leading to the view that comammox bacteria are oligotrophs with a competitive advantage in low NH3 environments (Kits et al., 2017).
These studies raise a number of general points. Caution is required in generalizing from results obtained from a small number (often one) laboratory cultures whose properties will differ from those of the ‘natural’ strain, through uncharacterized genetic and physiological changes during selective isolation. Km values for whole cells, rather than individual enzymes, do not identify the processes (e.g. ammonia oxidation vs. ammonia uptake) that are limiting activity and affinity may be determined by non-enzymatic ‘characteristics’, for example, cell surface area:volume ratio. In addition, affinity constants for activity (Km) will differ from those for growth (Ks), which are more relevant for outcomes of competition (see Prosser, 2012). The relevance and significance of substrate affinity for organisms in spatially complex and heterogeneous environments such as biofilms and soil, in which 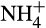 exchanges with surfaces on which cells are attached, are also unclear.
exchanges with surfaces on which cells are attached, are also unclear.
4.2 Hydroxylamine oxidation
The conversion of hydroxylamine to 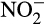 is much less understood and leads to many of the difficulties in assessing contributions of different processes to N2O production in natural environments. Until recently, it was commonly accepted that hydroxylamine is oxidized to
is much less understood and leads to many of the difficulties in assessing contributions of different processes to N2O production in natural environments. Until recently, it was commonly accepted that hydroxylamine is oxidized to 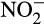 by hydroxylamine dehydrogenase (previously referred to as hydroxylamine oxidoreductase) in AOB (Arp & Stein, 2003). This reaction generates four electrons, two of which fuel ammonia oxidation, while two enter the electron transport chain to generate ATP and reducing equivalents. This mechanism failed to explain fully a number of experimental observations and is now challenged by enzymatic studies that suggest that the direct product of the hydroxylamine dehydrogenase reaction is not
by hydroxylamine dehydrogenase (previously referred to as hydroxylamine oxidoreductase) in AOB (Arp & Stein, 2003). This reaction generates four electrons, two of which fuel ammonia oxidation, while two enter the electron transport chain to generate ATP and reducing equivalents. This mechanism failed to explain fully a number of experimental observations and is now challenged by enzymatic studies that suggest that the direct product of the hydroxylamine dehydrogenase reaction is not 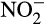 but nitric oxide (NO; Caranto & Lancaster, 2017). This reaction generates only three electrons and NO may then be converted abiotically to
but nitric oxide (NO; Caranto & Lancaster, 2017). This reaction generates only three electrons and NO may then be converted abiotically to 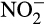 . Alternatively, generation of a fourth electron is possible, and likely, through enzymatic oxidation to
. Alternatively, generation of a fourth electron is possible, and likely, through enzymatic oxidation to 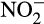 . Caranto, Vilbert, and Lancaster (2016) suggested that under aerobic conditions, NO could be rapidly oxidized to
. Caranto, Vilbert, and Lancaster (2016) suggested that under aerobic conditions, NO could be rapidly oxidized to 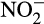 by
by 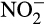 reductase, encoded by nirK, as this enzyme can act reversibly. However, nirK expression is not high in AOB, expression is not coordinate with other ammonia oxidation genes, nirK is not found in genomes of all AOB and growth of Nitrosomonas europaea was unaffected by deletion of nirK (Cantera & Stein, 2007; Kozlowski, Price, & Stein, 2014). Caranto et al. (2016) also proposed oxidation of NO by an as-yet uncharacterized NO oxidoreductase. A potential candidate is a red Cu protein, nitrosocyanin, encoded by ncyA, which is co-ordinately expressed with other ammonia oxidation genes. Although this may fulfill this role in some AOB, nycA homologues are not present in all AOB genomes or in comammox bacteria (Kits et al., 2019).
reductase, encoded by nirK, as this enzyme can act reversibly. However, nirK expression is not high in AOB, expression is not coordinate with other ammonia oxidation genes, nirK is not found in genomes of all AOB and growth of Nitrosomonas europaea was unaffected by deletion of nirK (Cantera & Stein, 2007; Kozlowski, Price, & Stein, 2014). Caranto et al. (2016) also proposed oxidation of NO by an as-yet uncharacterized NO oxidoreductase. A potential candidate is a red Cu protein, nitrosocyanin, encoded by ncyA, which is co-ordinately expressed with other ammonia oxidation genes. Although this may fulfill this role in some AOB, nycA homologues are not present in all AOB genomes or in comammox bacteria (Kits et al., 2019).
Hydroxylamine oxidation in AOA is less well characterized. AOA (N. maritimus) can grow on hydroxylamine, producing 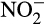 (Vajrala et al., 2013), and ammonia oxidation is inhibited by the NO-quenching agent PTIO (2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide; Jung et al., 2014; Kozlowski, Stieglmeier, et al., 2016; Shen, Stieglmeier, Dai, Urich, & Schleper, 2013; Yan et al., 2012). There is currently no obvious candidate enzyme for hydroxylamine oxidation to NO in AOA and genomes contain no gene homologous to those encoding haem-based enzymes. The current model for hydroxylamine oxidation proposes hydroxylamine and NO as co-substrates for a currently unidentified, putative hydroxylamine dehydrogenase enzyme complex that produces two molecules of
(Vajrala et al., 2013), and ammonia oxidation is inhibited by the NO-quenching agent PTIO (2-phenyl-4,4,5,5-tetramethylimidazoline-1-oxyl 3-oxide; Jung et al., 2014; Kozlowski, Stieglmeier, et al., 2016; Shen, Stieglmeier, Dai, Urich, & Schleper, 2013; Yan et al., 2012). There is currently no obvious candidate enzyme for hydroxylamine oxidation to NO in AOA and genomes contain no gene homologous to those encoding haem-based enzymes. The current model for hydroxylamine oxidation proposes hydroxylamine and NO as co-substrates for a currently unidentified, putative hydroxylamine dehydrogenase enzyme complex that produces two molecules of 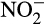 , generating five electrons, and that nirK (which is expressed highly in AOA) reduces one of these molecules to NO, for further oxidation (Kozlowski, Stieglmeier, et al., 2016). The absence of heme-based enzymes in AOA suggests that the enzyme may be copper-based.
, generating five electrons, and that nirK (which is expressed highly in AOA) reduces one of these molecules to NO, for further oxidation (Kozlowski, Stieglmeier, et al., 2016). The absence of heme-based enzymes in AOA suggests that the enzyme may be copper-based.
Comammox bacteria contain hydroxylamine dehydrogenase and produce NO, but lack ncyA gene homologues. PTIO inhibition is similar to that in AOA, suggesting similar tight regulation of NO production, although a NO reductase has yet to be identified (Kits et al., 2019).
5 MECHANISMS OF NITROUS OXIDE PRODUCTION DURING NITRIFICATION
5.1 Nitrifier denitrification
Classical heterotrophic denitrifiers rely on nitrifiers for 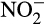 or
or 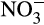 and are responsible for N2O emissions under anaerobic conditions (coupled nitrification–denitrification). Ammonia oxidizers also produce N2O directly through a number of partially understood mechanisms. Traditionally, the most important was considered to be nitrifier denitrification, the reduction of
and are responsible for N2O emissions under anaerobic conditions (coupled nitrification–denitrification). Ammonia oxidizers also produce N2O directly through a number of partially understood mechanisms. Traditionally, the most important was considered to be nitrifier denitrification, the reduction of 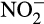 to N2O via NO, mainly under reduced oxygen concentrations (Goreau et al., 1980; Kozlowski et al., 2014; Poth & Focht, 1985; Shaw et al., 2006; Wrage-Mönnig et al., 2018; Zhu, Burger, Doane, & Horwath, 2013). There is currently no evidence of N2O reductase gene homologues in AO genomes (e.g. Campbell et al., 2011; Chain et al., 2003; Norton et al., 2008; Spang et al., 2012; Tourna et al., 2011; Walker et al., 2010) and reports of reduction of N2O to N2 by AO are rare, although nitrosocyanin may have this function (Arciero, Pierce, Hendrich, & Hooper, 2002; Beyer, Gilch, Meyer, & Schmidt, 2009; Schmidt, 2009; Todt & Dörsch, 2016; Whittaker, Bergmann, Arciero, & Hooper, 2000).
to N2O via NO, mainly under reduced oxygen concentrations (Goreau et al., 1980; Kozlowski et al., 2014; Poth & Focht, 1985; Shaw et al., 2006; Wrage-Mönnig et al., 2018; Zhu, Burger, Doane, & Horwath, 2013). There is currently no evidence of N2O reductase gene homologues in AO genomes (e.g. Campbell et al., 2011; Chain et al., 2003; Norton et al., 2008; Spang et al., 2012; Tourna et al., 2011; Walker et al., 2010) and reports of reduction of N2O to N2 by AO are rare, although nitrosocyanin may have this function (Arciero, Pierce, Hendrich, & Hooper, 2002; Beyer, Gilch, Meyer, & Schmidt, 2009; Schmidt, 2009; Todt & Dörsch, 2016; Whittaker, Bergmann, Arciero, & Hooper, 2000).
In denitrifiers, 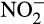 or
or 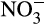 acts as a terminal electron acceptor and is linked to growth and energy production from organic carbon (Kuypers et al., 2018). In AO, the most likely role of denitrification enzymes is the removal of excess electrons at high NH3 concentration (Hink, Lycus, et al., 2017) and possibly detoxification of any accumulated
acts as a terminal electron acceptor and is linked to growth and energy production from organic carbon (Kuypers et al., 2018). In AO, the most likely role of denitrification enzymes is the removal of excess electrons at high NH3 concentration (Hink, Lycus, et al., 2017) and possibly detoxification of any accumulated 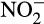 (Beaumont et al., 2002; Stein & Arp, 1998).
(Beaumont et al., 2002; Stein & Arp, 1998).
Nitrifier denitrification has been studied most in N. europaea, which possesses genes homologous to those of classical heterotrophic denitrifiers: nirK, encoding a Cu-containing nitrite reductase, and norB, encoding NO reductase (Chain et al., 2003; Kozlowski et al., 2014). While NorB is obligatory for nitrifier denitrification, NirK may be involved in oxidation of hydroxylamine rather than 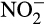 reduction, possibly involving an alternative nitrite reductase (Kozlowski et al., 2014). While studies have focused on N. europaea, there is evidence for genetic and physiological diversity within AOB, for example, differences in gene content and responses to anoxia of oligotrophic and non-oligotrophic strains (Kozlowski, Kits, & Stein, 2016).
reduction, possibly involving an alternative nitrite reductase (Kozlowski et al., 2014). While studies have focused on N. europaea, there is evidence for genetic and physiological diversity within AOB, for example, differences in gene content and responses to anoxia of oligotrophic and non-oligotrophic strains (Kozlowski, Kits, & Stein, 2016).
There is currently no evidence for nitrifier denitrification in AOA or comammox bacteria as both groups lack NO reductase genes (e.g. Daims et al., 2015; Kim et al., 2011; Kits et al., 2017; Spang et al., 2012; Tourna et al., 2011; Walker et al., 2010), although the neutrophilic soil AOA Nitrosocosmicus oleophilus may be capable of enzymatically denitrifying 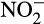 to N2O at low pH (Jung et al., 2019). Furthermore, N2O produced by both AOA and comammox bacteria possesses a site preference (difference in δ15N of the α and β N atoms of N2O) which is distinct from that produced via nitrifier denitrification (Jung et al., 2014; Kits et al., 2019; Löscher et al., 2012; Santoro, Buchwald, McIlvin, & Casciotti, 2011) and production is independent of oxygen availability (Hink, Nicol, & Prosser, 2017; Stieglmeier, Mooshammer, et al., 2014).
to N2O at low pH (Jung et al., 2019). Furthermore, N2O produced by both AOA and comammox bacteria possesses a site preference (difference in δ15N of the α and β N atoms of N2O) which is distinct from that produced via nitrifier denitrification (Jung et al., 2014; Kits et al., 2019; Löscher et al., 2012; Santoro, Buchwald, McIlvin, & Casciotti, 2011) and production is independent of oxygen availability (Hink, Nicol, & Prosser, 2017; Stieglmeier, Mooshammer, et al., 2014).
5.2 N2O production through hydroxylamine oxidation
Hooper and Terry (1979) provided evidence that NO and N2O are also produced through incomplete oxidation of hydroxylamine. NO was considered to result from incomplete oxidation of hydroxylamine, with N2O produced through the activity of NO reductase (Kozlowski et al., 2014; Pacheco, McGarry, Kostera, & Corona, 2011). This mechanism is compatible with the revised, 2-intermediate model for ammonia oxidation (Caranto & Lancaster, 2017). Furthermore, there is evidence that cytochrome P460 converts hydroxylamine to N2O with NO as an intermediate under anaerobic conditions, enabling detoxification of hydroxylamine and NO and, in the presence of oxygen, NO would then abiotically dissociate to N2O (Caranto et al., 2016).
Hydroxylamine oxidation in AOA differs from that in AOB with cycling of NO, which reacts with hydroxylamine to form 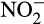 (Kozlowski, Stieglmeier, et al., 2016), and there is currently no evidence for enzymatic N2O production from either hydroxylamine or NO. However, N2O may be produced through abiotic reactions linked to hydroxylamine oxidation (see below). Production mechanisms in N. inopinata are suggested to be similar to those of AOA, associated with abiotic conversion of hydroxylamine only, with no evidence for denitrification activity producing N2O with decreasing oxygen availability (Kits et al., 2019).
(Kozlowski, Stieglmeier, et al., 2016), and there is currently no evidence for enzymatic N2O production from either hydroxylamine or NO. However, N2O may be produced through abiotic reactions linked to hydroxylamine oxidation (see below). Production mechanisms in N. inopinata are suggested to be similar to those of AOA, associated with abiotic conversion of hydroxylamine only, with no evidence for denitrification activity producing N2O with decreasing oxygen availability (Kits et al., 2019).
5.3 Linked biotic and abiotic processes
A range of abiotic processes exists by which hydroxylamine,  and NO can be converted to N2O. The chemical reactions responsible, and their consequences for global emissions, have recently been reviewed (Heil, Vereecken, & Brüggemann, 2016; Zhu-Barker, Cavazos, Ostrom, Horwath, & Glass, 2015). They involve non-enzymatic conversions of products and intermediates of the nitrification process and therefore combine abiotic and biotic processes.
and NO can be converted to N2O. The chemical reactions responsible, and their consequences for global emissions, have recently been reviewed (Heil, Vereecken, & Brüggemann, 2016; Zhu-Barker, Cavazos, Ostrom, Horwath, & Glass, 2015). They involve non-enzymatic conversions of products and intermediates of the nitrification process and therefore combine abiotic and biotic processes. 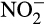 may be reduced, by a range of reducing agents, and fixed by soil organic matter (SOM). The significance of abiotic processes is rarely considered, through difficulties in detection and measurement in natural environments, and measurement of
may be reduced, by a range of reducing agents, and fixed by soil organic matter (SOM). The significance of abiotic processes is rarely considered, through difficulties in detection and measurement in natural environments, and measurement of 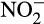 concentration, rather than flux, but there is some evidence for
concentration, rather than flux, but there is some evidence for 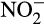 accumulation in terrestrial and aquatic ecosystems (Shen, Ran, & Cao, 2003; van Cleemput & Samater, 1995).
accumulation in terrestrial and aquatic ecosystems (Shen, Ran, & Cao, 2003; van Cleemput & Samater, 1995).
Chemical decomposition of hydroxylamine generates N2O and small amounts of N2 (Bremner, Blackmer, & Waring, 1980). More importantly, hydroxylamine and nitrous acid (rather than 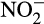 ) react to generate N2O, particularly at low pH (Nelson, 1977). Detailed mechanisms are poorly understood and hydroxylamine can also be oxidized and, like
) react to generate N2O, particularly at low pH (Nelson, 1977). Detailed mechanisms are poorly understood and hydroxylamine can also be oxidized and, like 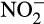 , may form complexes with SOM (Heil et al., 2016). Abiotic production of N2O from hydroxylamine has rarely been considered seriously because of lack of evidence for release of hydroxylamine from AO cells and low or undetectable levels in natural environments. Concentrations in marine environments are in the nM range, and highest when nitrification is active, and improved techniques have now led to its detection in soil at 0.3–35 µg N/kg dry soil (Liu, Vereecken, & Brüggemann, 2014). Low environmental concentrations may, in fact, reflect the high and diverse reactivity of hydroxylamine rather than low flux and Liu et al. (2017) demonstrated release of hydroxylamine from several AO in laboratory culture. Within AOB, production was greatest in Nitrosospira multiformis and N. europaea, but undetectable in Nitrosomonas nitrosa or Nitrosomonas communis, with low levels produced by the comammox strain N. inopinata. Within AOA, hydroxylamine was produced by Nitrososphaera gargensis and Nitrosotenuis uzonensis, but not by Nitrososphaera viennensis or Nitrosotalea devanaterra.
, may form complexes with SOM (Heil et al., 2016). Abiotic production of N2O from hydroxylamine has rarely been considered seriously because of lack of evidence for release of hydroxylamine from AO cells and low or undetectable levels in natural environments. Concentrations in marine environments are in the nM range, and highest when nitrification is active, and improved techniques have now led to its detection in soil at 0.3–35 µg N/kg dry soil (Liu, Vereecken, & Brüggemann, 2014). Low environmental concentrations may, in fact, reflect the high and diverse reactivity of hydroxylamine rather than low flux and Liu et al. (2017) demonstrated release of hydroxylamine from several AO in laboratory culture. Within AOB, production was greatest in Nitrosospira multiformis and N. europaea, but undetectable in Nitrosomonas nitrosa or Nitrosomonas communis, with low levels produced by the comammox strain N. inopinata. Within AOA, hydroxylamine was produced by Nitrososphaera gargensis and Nitrosotenuis uzonensis, but not by Nitrososphaera viennensis or Nitrosotalea devanaterra. 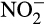 reduced chemical decomposition of hydroxylamine and proportions of NH3 converted to N2O were similar to those found previously in AOA and AOB.
reduced chemical decomposition of hydroxylamine and proportions of NH3 converted to N2O were similar to those found previously in AOA and AOB.
Although AOB produce N2O enzymatically, smaller contributions via abiotic reactions with hydroxylamine, NO or 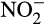 , and the observed influence of NO-quenching agents on AOA and comammox bacteria activity suggest leakage of NO (Jung et al., 2014; Kits et al., 2019; Kozlowski, Stieglmeier, et al., 2016; Shen et al., 2013; Yan et al., 2012). In the presence of oxygen, NO and hydroxylamine can be rapidly converted abiotically to N2O (Kozlowski, Stieglmeier, et al., 2016; Stieglmeier, Mooshammer, et al., 2014). Although it has implicitly been assumed that abiotic conversion is intracellular, direct evidence is lacking and it is possible that some or all abiotic production occurs after export or leakage of hydroxylamine from AO.
, and the observed influence of NO-quenching agents on AOA and comammox bacteria activity suggest leakage of NO (Jung et al., 2014; Kits et al., 2019; Kozlowski, Stieglmeier, et al., 2016; Shen et al., 2013; Yan et al., 2012). In the presence of oxygen, NO and hydroxylamine can be rapidly converted abiotically to N2O (Kozlowski, Stieglmeier, et al., 2016; Stieglmeier, Mooshammer, et al., 2014). Although it has implicitly been assumed that abiotic conversion is intracellular, direct evidence is lacking and it is possible that some or all abiotic production occurs after export or leakage of hydroxylamine from AO.
The situation is therefore complex. The two enzymatic pathways for N2O production in AOB, from hydroxylamine and 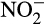 , are treated as distinct pathways but share NO as an intermediate.
, are treated as distinct pathways but share NO as an intermediate. 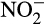 , NO and hydroxylamine are also intermediates in abiotic processes, which may require inorganic reducing agents, in solution or in solid form. Other factors can also influence production, for example, storage compounds, including intracellular hydroxylamine, may be responsible for apparent anoxic production. In addition, physiological studies are generally performed in well-mixed systems in which environmental conditions are constant. In natural environments, conditions will be transient, potentially leading to metabolic imbalance that may result in accumulation of intermediates involved in N2O production. It is difficult to assess the specific roles of abiotic reactions, but there is sufficient evidence to merit further research into abiotic conversion of hydroxylamine and its relative contribution to emissions in comparison with nitrifier denitrification.
, NO and hydroxylamine are also intermediates in abiotic processes, which may require inorganic reducing agents, in solution or in solid form. Other factors can also influence production, for example, storage compounds, including intracellular hydroxylamine, may be responsible for apparent anoxic production. In addition, physiological studies are generally performed in well-mixed systems in which environmental conditions are constant. In natural environments, conditions will be transient, potentially leading to metabolic imbalance that may result in accumulation of intermediates involved in N2O production. It is difficult to assess the specific roles of abiotic reactions, but there is sufficient evidence to merit further research into abiotic conversion of hydroxylamine and its relative contribution to emissions in comparison with nitrifier denitrification.
Assessment of global rates and contributions of abiotic processes is technically difficult and requires development and improvement of approaches for application of stable isotope tracing methods, including analysis of 15N site preferences of N2O for the different processes. Estimates for the formation of N2O in sterile soils were suggested to be 31%–75% of total N2O production in non-sterile agricultural soils (Venterea, 2007). However, there are difficulties in studying sterilized soil including incomplete sterilization, alteration of soil properties and an absence of biotic production of hydroxylamine or 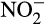 , which are precursors for abiotic production (Lotrario et al., 1995; McNamara, Black, Beresford, & Parekh, 2003; Nowak & Wronkowska, 1987).
, which are precursors for abiotic production (Lotrario et al., 1995; McNamara, Black, Beresford, & Parekh, 2003; Nowak & Wronkowska, 1987).
6 DISTINCTION OF ARCHAEAL AND BACTERIAL N2O PRODUCTION
Biochemical and physiological studies of individual organisms provide a basis for the assessment of differences between AO and have been used in developing techniques to distinguish growth and activity of different groups in natural communities, for example, differential inhibitors or isotopic methods. 15N- and 18O-enriched substrates can distinguish N2O production from nitrification and denitrification processes in the environment (see Ostrom & Ostrom, 2017; Wrage-Mönnig et al., 2018), although their ability to distinguish autotrophic and heterotrophic processes has been questioned (Bakken & Frostegård, 2017). Importantly, however, these techniques cannot differentiate emissions associated with AOA, AOB and comammox bacteria activities, which have common substrates and products. Attempts to distinguish these activities involve correlation of nitrification activity and N2O production with growth or transcriptional activity of different AO, usually via quantification of temporal changes in group-specific amoA genes or transcripts, respectively. There are significant limitations to this approach, but their value is increased when used in conjunction with inhibitors targeting specific groups in short-term assays. Alkynes can inhibit monooxygenases through irreversible covalent binding of the active site. Acetylene is a potent inhibitor of AMO of all AO, but 1-octyne has recently been established as a specific inhibitor of AOB (Hink, Nicol, et al., 2017; Taylor et al., 2013). The NO-scavenger PTIO has been used as a specific inhibitor of AOA (Kozlowski, Stieglmeier, et al., 2016; Shen et al., 2013; Yan et al., 2012), but also inhibits comammox bacteria (Kits et al., 2019), and simvastatin can specifically inhibit AOA (Zhao, Bello, Meng, Prosser, & Gubry-Rangin, in press).
6.1 N2O yield
N2O production associated with nitrification is quantified as the ratio of N2O to 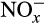 (
(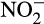 and/or
and/or 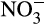 ) produced or NH3 consumed, and is termed N2O yield. Yields vary significantly within AO through differences in the physiology, and comparison with yields in environmental samples provides information on likely sources of production. N2O yield of AOB cultures ranges from 0.1% to 8% (Anderson, Poth, Homstead, & Burdige, 1993; Hink, Lycus, et al., 2017; Jiang & Bakken, 1999; Shaw et al., 2006), due to differences in available
) produced or NH3 consumed, and is termed N2O yield. Yields vary significantly within AO through differences in the physiology, and comparison with yields in environmental samples provides information on likely sources of production. N2O yield of AOB cultures ranges from 0.1% to 8% (Anderson, Poth, Homstead, & Burdige, 1993; Hink, Lycus, et al., 2017; Jiang & Bakken, 1999; Shaw et al., 2006), due to differences in available  and oxygen concentrations that affect enzymatic reactions. Higher yields are observed with increasing NH4+ concentration, potentially due to a lower reaction rate of hydroxylamine dehydrogenase than AMO, leading to accumulation of hydroxylamine, which is subsequently transformed abiotically to N2O (Hink, Lycus, et al., 2017). An alternative explanation is redox balancing, in which electrons generated by hydroxylamine dehydrogenase are shuttled to denitrification enzymes when they exceed the capacity of terminal oxidases (Hink, Lycus, et al., 2017). N2O yields from AOA cultures are below or in the lower range of those observed for AOB, that is, 0.04%–0.3%, and
and oxygen concentrations that affect enzymatic reactions. Higher yields are observed with increasing NH4+ concentration, potentially due to a lower reaction rate of hydroxylamine dehydrogenase than AMO, leading to accumulation of hydroxylamine, which is subsequently transformed abiotically to N2O (Hink, Lycus, et al., 2017). An alternative explanation is redox balancing, in which electrons generated by hydroxylamine dehydrogenase are shuttled to denitrification enzymes when they exceed the capacity of terminal oxidases (Hink, Lycus, et al., 2017). N2O yields from AOA cultures are below or in the lower range of those observed for AOB, that is, 0.04%–0.3%, and  and oxygen concentration have no or little effect on yield (Jung et al., 2011; Hink, Lycus, et al., 2017; Kim et al., 2012; Löscher et al., 2012; Santoro et al., 2011; Stieglmeier, Mooshammer, et al., 2014; Qin et al., 2017). This is consistent with the assumption that N2O is produced only from abiotic reactions of intermediate compounds (Kozlowski, Stieglmeier, et al., 2016), although enzymatic production has been reported in N. oleophilus (Jung et al., 2019). Comammox bacteria and AOA lack homologues of AOB NO reductase, suggesting low yields of N2O, and abiotic production with a yield of 0.07% has been reported in N. inopinata (Kits et al., 2019).
and oxygen concentration have no or little effect on yield (Jung et al., 2011; Hink, Lycus, et al., 2017; Kim et al., 2012; Löscher et al., 2012; Santoro et al., 2011; Stieglmeier, Mooshammer, et al., 2014; Qin et al., 2017). This is consistent with the assumption that N2O is produced only from abiotic reactions of intermediate compounds (Kozlowski, Stieglmeier, et al., 2016), although enzymatic production has been reported in N. oleophilus (Jung et al., 2019). Comammox bacteria and AOA lack homologues of AOB NO reductase, suggesting low yields of N2O, and abiotic production with a yield of 0.07% has been reported in N. inopinata (Kits et al., 2019).
7 NICHE SPECIALIZATION AND DIFFERENTIATION IN AMMONIA OXIDIZERS
AOA and AOB belong to different domains of life and differ considerably in cellular structure and fundamental aspects of metabolism and physiology. This suggests the potential for niche differentiation associated with different physiologies and has fuelled the search for associations between environmental characteristics and AOA and AOB abundance and community composition. Comammox studies are in their infancy but all current evidence indicates that they are oligotrophs (Kits et al., 2017).
Marine AO communities are dominated by AOA (Beman, Popp, & Francis, 2008), AOA are often absent in wastewater treatment plants (Mussmann et al., 2011) and investigation of niche differentiation between AO has focussed on terrestrial environments, with two factors attracting particular attention: pH and  . In laboratory culture, many AO can grow at pH ≥ 6.5 (Hatzenpichler et al., 2008; Lehtovirta-Morley et al., 2016; Tourna et al., 2011), but obligate autotrophic growth in acidic liquid batch culture is restricted to acidophilic AOA belonging to the Nitrosotalea group, which grow in the pH range 4–6 (Jung et al., 2014; Lehtovirta-Morley et al., 2014; Lehtovirta-Morley, Stoecker, Vilcinskas, Prosser, & Nicol, 2011). This is reflected in their global presence and activity in acidic soils (Gubry-Rangin et al., 2011), which contain AOA clades (particularly Nitrososphaera clade C11) that are currently not represented in culture, restricting physiological studies (Gubry-Rangin et al., 2018). AOA dominate ammonia oxidation in acidic soils, which comprise 30% of soils globally, some of which are heavily fertilized arable soils (von Uexküll & Mutert, 1995). Betaproteobacterial AOB activity has been observed at pH as low as 5.5 in biofilms (Allison & Prosser, 1993), but AOA and AOB activities are found in soils down to pH 4.5. AOB activity may be linked to an uncharacterized Nitrosospira clade active in acidic soils (Aigle et al., 2019) and growth of ureolytic AO at low pH is also possible, if NH3 is supplied as urea, as ureolytic activity is not pH-dependent (Burton & Prosser, 2001). Evidence for this mechanism in soil is lacking and the role of AOB and Nitrososphaera clade C11 in acid soils, and potential metabolisms, require further study.
. In laboratory culture, many AO can grow at pH ≥ 6.5 (Hatzenpichler et al., 2008; Lehtovirta-Morley et al., 2016; Tourna et al., 2011), but obligate autotrophic growth in acidic liquid batch culture is restricted to acidophilic AOA belonging to the Nitrosotalea group, which grow in the pH range 4–6 (Jung et al., 2014; Lehtovirta-Morley et al., 2014; Lehtovirta-Morley, Stoecker, Vilcinskas, Prosser, & Nicol, 2011). This is reflected in their global presence and activity in acidic soils (Gubry-Rangin et al., 2011), which contain AOA clades (particularly Nitrososphaera clade C11) that are currently not represented in culture, restricting physiological studies (Gubry-Rangin et al., 2018). AOA dominate ammonia oxidation in acidic soils, which comprise 30% of soils globally, some of which are heavily fertilized arable soils (von Uexküll & Mutert, 1995). Betaproteobacterial AOB activity has been observed at pH as low as 5.5 in biofilms (Allison & Prosser, 1993), but AOA and AOB activities are found in soils down to pH 4.5. AOB activity may be linked to an uncharacterized Nitrosospira clade active in acidic soils (Aigle et al., 2019) and growth of ureolytic AO at low pH is also possible, if NH3 is supplied as urea, as ureolytic activity is not pH-dependent (Burton & Prosser, 2001). Evidence for this mechanism in soil is lacking and the role of AOB and Nitrososphaera clade C11 in acid soils, and potential metabolisms, require further study.
There is also evidence for niche differentiation associated with NH3 supply. AOB growth is favored in soils fertilized by single additions of high levels of inorganic NH3, while AOA grow preferentially when NH3 is produced through mineralization of organic N (Hink, Gubry-Rangin, Nicol, & Prosser, 2018; Hink, Nicol, et al., 2017; Verhamme, Prosser, & Nicol, 2011). AOA and AOB relative activities may therefore be influenced by different N fertilization strategies, with subsequent effects on N2O yields.
8 TERRESTRIAL ECOSYSTEMS
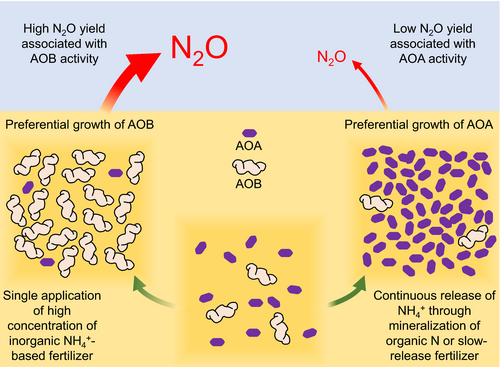
Terrestrial environments contribute 56%–70% of N2O emissions globally with agricultural systems contributing ~40% of that derived from soils (Davidson, 2009; Syakila & Kroeze, 2011). As described above, AO activity contributes directly to N2O emissions through both biotic and linked abiotic processes. However, coupled with the activity of NOB, they also contribute indirectly to N2O emissions by producing 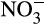 for heterotrophic denitrifiers and subsequent reduction of NO to N2O as part of denitrification. The application of AO inhibitors has therefore been considered as a method to inhibit N2O emissions directly or indirectly (Ruser & Schulz, 2015). Although NH3 can be lost through volatilization, reduction of ammonia oxidation is generally considered beneficial for plant uptake and decreased fertilization loss, and niche differentiation of AOA and AOB suggests that controlling the activities of each group may also have substantial impacts on reducing N2O emissions. This leads to the hypothesis that inorganic fertilization will benefit AOB, and high N2O yield and emissions, while slow release of ammonia from native organic N, organic fertilizer or slow-release fertilizer will favor AOA and lower emissions (Figure 1).
for heterotrophic denitrifiers and subsequent reduction of NO to N2O as part of denitrification. The application of AO inhibitors has therefore been considered as a method to inhibit N2O emissions directly or indirectly (Ruser & Schulz, 2015). Although NH3 can be lost through volatilization, reduction of ammonia oxidation is generally considered beneficial for plant uptake and decreased fertilization loss, and niche differentiation of AOA and AOB suggests that controlling the activities of each group may also have substantial impacts on reducing N2O emissions. This leads to the hypothesis that inorganic fertilization will benefit AOB, and high N2O yield and emissions, while slow release of ammonia from native organic N, organic fertilizer or slow-release fertilizer will favor AOA and lower emissions (Figure 1).
 -based fertilizer or with slow release of
-based fertilizer or with slow release of  from soil organic nitrogen or a slow-release fertilizer. The initial AO community, prior to incubation, is dominated by AOA, which are generally assumed to be smaller than AOB
from soil organic nitrogen or a slow-release fertilizer. The initial AO community, prior to incubation, is dominated by AOA, which are generally assumed to be smaller than AOBThis hypothesis has been tested most critically in microcosms in which AOA and AOB N2O production is distinguished using differential inhibitors or comparison of isotopic signatures with those obtained in AOA or AOB cultures. The inhibitor approach was first used by Hink, Nicol, et al. (2017) in soil microcosms in which N2O production was dominated by AO and N2O emissions by AOA and AOB were discriminated by differential inhibition of AOB using 1-octyne. Inorganic N fertilization stimulated N2O production, which was reduced in the presence of 1-octyne, and estimated N2O yields associated with AOA and AOB activity were comparable to those obtained in pure cultures, that is, the N2O yield of AOA was approximately half that of AOB. AOA-associated N2O emission was also determined in unfertilized soils, in which NH3 is supplied through mineralization of organic N, and N2O yields were again similar to those reported for AOA cultures. These results therefore indicate that the relative contribution to ammonia oxidation and N2O generation by AOB may be greatly reduced by controlling the rate of  supply.
supply.
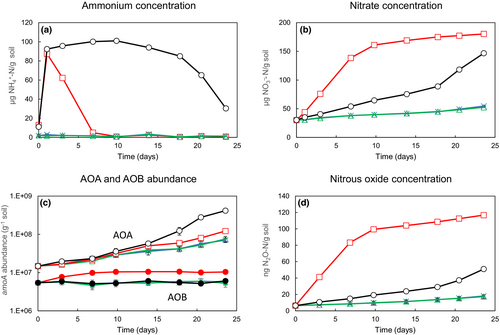
To test the potential for reduction in N2O through use of different fertilizer strategies, a similar approach compared single addition of urea-N at high concentration and continuous low production from a slow-release urea-N fertilizer (polymethylene urea) or organic N mineralization (Hink et al., 2018). When fertilizer was supplied at high concentration, AOB-dominated activity and N2O production, with high yield (Figure 2).
 and (b)
and (b) 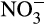 concentrations, (c) AOA and AOB amoA gene abundances and (d) N2O emissions from soil microcosms incubated after addition of water (green triangle, blue cross) or under conditions of high
concentrations, (c) AOA and AOB amoA gene abundances and (d) N2O emissions from soil microcosms incubated after addition of water (green triangle, blue cross) or under conditions of high  supply (0–10 days) or low
supply (0–10 days) or low  supply (10–25 days; black circle, red square). Ammonium was supplied as a slow-release, urea-based fertilizer that contained residual free urea. (Urea was converted rapidly to
supply (10–25 days; black circle, red square). Ammonium was supplied as a slow-release, urea-based fertilizer that contained residual free urea. (Urea was converted rapidly to  ). This generated high initial concentrations of available
). This generated high initial concentrations of available  , which was oxidized within 10 days. Thereafter,
, which was oxidized within 10 days. Thereafter,  was generated by mineralization of native organic nitrogen or of the slow-release fertilizer. In addition, microcosms were incubated with (black circle, green triangle) or without (red square, blue cross) 1-octyne, a specific inhibitor of AOB. AO growth in the absence of fertilizer (green triangle, blue cross) was due to mineralization of native organic nitrogen. Under these conditions, AOB growth was not detectable, AOA grew slowly and N2O emissions were low and unaffected by the 1-octyne. Initial high inorganic
was generated by mineralization of native organic nitrogen or of the slow-release fertilizer. In addition, microcosms were incubated with (black circle, green triangle) or without (red square, blue cross) 1-octyne, a specific inhibitor of AOB. AO growth in the absence of fertilizer (green triangle, blue cross) was due to mineralization of native organic nitrogen. Under these conditions, AOB growth was not detectable, AOA grew slowly and N2O emissions were low and unaffected by the 1-octyne. Initial high inorganic  after fertilizer addition (1–10 days) resulted in greater growth of AOB, increases in the AOB:AOA ratio and substantial N2O emissions that were significantly reduced, almost to those of control microcosms, in the presence of 1-octyne. During slow release of
after fertilizer addition (1–10 days) resulted in greater growth of AOB, increases in the AOB:AOA ratio and substantial N2O emissions that were significantly reduced, almost to those of control microcosms, in the presence of 1-octyne. During slow release of  (10–25 days), growth was dominated by AOA, N2O emissions were significantly reduced and emissions were unaffected by the AOB inhibitor, 1-octyne. AOA growth was stimulated, consistent with continuous slow release from the fertiliser and endogenous organic nitrgoen. Note, however, that stimulation of AOA growth was greater when AOB were inhibited and inorganic
(10–25 days), growth was dominated by AOA, N2O emissions were significantly reduced and emissions were unaffected by the AOB inhibitor, 1-octyne. AOA growth was stimulated, consistent with continuous slow release from the fertiliser and endogenous organic nitrgoen. Note, however, that stimulation of AOA growth was greater when AOB were inhibited and inorganic  remained high. (See Hink et al., 2018 for experimental details and further discussion of results.)
remained high. (See Hink et al., 2018 for experimental details and further discussion of results.)Inhibition of AOB activity by 1-octyne reduced N2O production and yield but AOA activity increased, demonstrating the ability of AOA to grow at high  concentration in the absence of AOB activity. Low
concentration in the absence of AOB activity. Low  supply, through slow release, led to dominance of activity by AOA and low N2O yield.
supply, through slow release, led to dominance of activity by AOA and low N2O yield.
AOB-dominated nitrification and high AOB-associated N2O production have also been observed after N fertilization of alluvial (pH 8) and red (pH 6) soils (Wang et al., 2016). Fertilization did not result in AOA growth and, in fact, led to a decrease in AOA abundance in the red soil. AOA did not grow in the alluvial soil but grew in control and 1-octyne-treated, unfertilized red soils, but not following acetylene inhibition. AOA growth was not associated with detectable N2O production. In fertilized soils, proportions of emissions associated with AOB and AOA were similar (70.5%–78.1% and 18.7%–19.7%, respectively) to those observed by Hink, Nicol, et al. (2017). Meinhardt et al. (2018) complemented this approach by additional use of PTIO, to inhibit AOA, and isotopic analysis, to distinguish N2O arising from hydroxylamine oxidation and nitrifier denitrification in an alkaline switch-grass soil. Nitrification, AOA and AOB growth and activity and N2O production were low in control soil microcosms. Inorganic N fertilization favored AOB growth and increased N2O production, and isotopic analysis indicated that N2O production was associated with AOB activity, while production in unfertilized soil was similar to that from AOA cultures. These results were consistent with field data, in which AOB abundance correlated with N2O emissions.
While 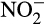 concentration in soil is typically low, accumulation of
concentration in soil is typically low, accumulation of 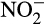 and its influence on N2O emissions has also been investigated in microcosms. Venterea et al. (2015) and Breuillin-Sessoms et al. (2017) reported
and its influence on N2O emissions has also been investigated in microcosms. Venterea et al. (2015) and Breuillin-Sessoms et al. (2017) reported 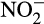 accumulation and associated N2O production in bovine urine- and urea-fertilized soil. Increased N2O production after fertilization was thought to be due to nitrifier denitrification of accumulated
accumulation and associated N2O production in bovine urine- and urea-fertilized soil. Increased N2O production after fertilization was thought to be due to nitrifier denitrification of accumulated 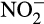 . Interestingly, Giguere, Taylor, Suwa, Myrold, and Bottomley (2017) reported
. Interestingly, Giguere, Taylor, Suwa, Myrold, and Bottomley (2017) reported 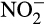 accumulation in three non-cropped soils that was not associated with increased N fertilization. This led to increased N2O production in soil in which ammonia oxidation was dominated by AOA, or shared between AOB and AOA, and production was stimulated by addition of
accumulation in three non-cropped soils that was not associated with increased N fertilization. This led to increased N2O production in soil in which ammonia oxidation was dominated by AOA, or shared between AOB and AOA, and production was stimulated by addition of 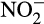 . While this can be explained by nitrifier denitrification by AOB, some of the activity was associated with AOA. The mechanism for this is unclear, but could be through abiotic reaction between
. While this can be explained by nitrifier denitrification by AOB, some of the activity was associated with AOA. The mechanism for this is unclear, but could be through abiotic reaction between 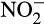 and leaked hydroxylamine or currently uncharacterized AOA nitrifier denitrification. Regardless of the mechanism, the authors highlight the potential for nitrite accumulation to influence N2O yields in soil studies.
and leaked hydroxylamine or currently uncharacterized AOA nitrifier denitrification. Regardless of the mechanism, the authors highlight the potential for nitrite accumulation to influence N2O yields in soil studies.
Duan, Fan, Zhang, and Xiong (2019) used 1-octyne to distinguish AOA- and AOB-associated N2O emissions in urea-amended microcosms containing several Chinese soils cultivated with vegetables in greenhouses and previously treated with different levels of urea fertilizer. AOA-dominated N2O production in microcosms containing previously unfertilized soil and AOA and AOB contributed to production in soil with a history of intermediate fertilization. AOA abundance increased with historically high urea fertilization levels and dominated nitrification and N2O production in the two most heavily fertilized soils. The authors also reported a correlation between N2O production and accumulated 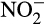 , suggesting differential inhibition of NOB at high ammonium concentration. These, and other studies of complex soil ecosystems, indicate that the potential for localized depletion of oxygen and
, suggesting differential inhibition of NOB at high ammonium concentration. These, and other studies of complex soil ecosystems, indicate that the potential for localized depletion of oxygen and 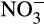 accumulation must also be considered, through ammonia oxidation following high levels of fertilization, that will provide conditions favorable for N2O production by heterotrophic nitrifiers.
accumulation must also be considered, through ammonia oxidation following high levels of fertilization, that will provide conditions favorable for N2O production by heterotrophic nitrifiers.
In addition, an increasing number of microcosm and field studies have generated data on correlations between N2O and AOA or AOB amoA gene or transcript abundance. These studies have generally been carried out to assess the effects of fertilizer on production rates and AO abundances, rather than to test the above hypotheses, and suffer from the fundamental limitations of correlation-based studies and use of gene abundance as a measure of activity. They frequently report AOB growth only, following fertilization, and therefore find correlations between N2O production and AOB, rather than AOA abundance, for example, studying fertilized paddy soil subjected to wetting and drying (Abid, Gu, Zhang, Wang, & Di, 2018), biochar-treated wheat–maize soil (Liu et al., 2019), arable soil (Song et al., 2018) and forest soil (Martins et al., 2017). Several studies, however, reported a greater role for AOA in nitrification and correlation between AOA and N2O, for example, in tropical rainforest soil (Soper et al., 2018) and Tibetan alpine soil (Peng et al., 2018).
Directed microcosm studies, using inhibitor and isotopic approaches to distinguish AOA and AOB activities, therefore support proposed links between niche specialization and the consequences for N2O production and yield. Care must be taken in interpreting results, inhibitor specificity may require more rigorous testing and additional inhibitors may be required to differentiate comammox bacteria. PTIO inhibits N. inopinata and N. maritimus (Martens-Habbena et al., 2015; Kits et al., 2019) and is therefore not specific for AOA activity, and both PTIO and 1-octyne can inhibit both AOA and AOB at sufficiently high concentrations (Shen et al., 2013; Taylor et al., 2013). They must therefore be used with care with complex natural communities. In addition, greater activity of AOA when AOB are inhibited may lead to overestimation of N2O production by AOA when both groups are present and potentially active. Studies based only on AO abundances produce a range of effects with results that are difficult to interpret because of a number of limitations, including lack of control, inability to distinguish effects of different factors and difficulty in relating abundance to activity.
9 AQUATIC ECOSYSTEMS
The major source of nitrogen in marine and freshwater ecosystems is biological nitrogen fixation. AO therefore obtain NH3 through mineralization of organic N and N2O is produced by both ammonia oxidizers and traditional denitrifiers, the latter also providing a sink for N2O. Production is greatest in regions of equatorial and coastal upwelling (transporting N2O produced in deep sediments), OMZ and areas of high productivity, with hotspots in the Arabian Sea and East tropical South Pacific (ETSP; Nevison, Weiss, & Erickson, 1995). Marine systems are estimated to contribute ~3.8 Tg N2O/year, equivalent to 21% of global emissions, although the range of estimates is large (1.8–9.4 Tg/N; IPCC, 2013). This high range is due to uncertainties in methodology, reliance on assumed correlations between oxygen utilization and N2O production and lack of information for parametrization of simulation models, in addition to uncertainties regarding biophysical processes, such as transfer functions from surface waters to atmosphere, wind dispersal and hydrogeography.
AOA dominate AO communities in the open ocean and are the major contributors to marine ammonia oxidation and AO-associated N2O production (Santoro et al., 2011), although a minority of studies report similar abundances for AOA and AOB. AOA abundance is low in surface waters, except in the Arctic (Müller et al., 2018), through photoinhibition and low NH3 flux, but increases between ~100 and 1,000 m, before decreasing at greater depths. AOA communities are dominated by two phylogenetic groups within the Nitrosopumilales (Santoro, Richter, & Dupont, 2019). The water column A (WCA) or high ammonium concentration group is found in polar regions, contains the cultivated AOA Nitrosopelagicus brevis and dominates when total AOA abundance is high. The water column B (WCB) or low ammonium concentration group dominates at depths >300 m and currently has no cultivated representative. Correlations of gene and transcript abundances and nitrification rates with environmental conditions suggest that WCA has a broad environmental niche, but WCB genes correlate with lower temperature, higher nutrients and low chlorophyll (Smith, Casciotti, Chavez, & Francis, 2014). The basis of niche differentiation between these two groups, however, remains uncertain.
A role for ammonia oxidation and N2O production by AOA is based on correlations between gene and transcript abundances and N2O production rates and isotopic methods. For example, Löscher et al. (2012) reported N2O production in the East Tropical North Atlantic (ETNA; oxygen concentration >40 µmol/L) and the OMZ of ETSP waters. In the ETSP, N2O emissions correlated with both archaeal amoA gene abundance and those involved in denitrification, suggesting a mixed origin for N2O, while ETNA production correlated with archaeal amoA gene abundance only. AOA outnumbered AOB by one to two orders of magnitude. N2O production was also inhibited when sea water was incubated with the inhibitor N1-guanyl-1,7-diaminoheptane, which inhibits synthesis of hypusine, required for protein synthesis in archaea. Peng et al. (2015) used on-board incubations of ETNP (East tropical North Pacific) water to assess depth-related nitrification potential, amoA gene abundance, N2O production and selective inhibition of AOB and AOA by ATU and PTIO respectively. AOA outnumbered AOB by approximately one order of magnitude and estimated Km values were similar to those of N. maritimus, confirming the potential for activity in the OMZ. Horak et al. (2013) also measured a Km of 98 nmol/L in incubations of Puget Sound sea water where, again, AOA outnumbered AOB by one to two orders of magnitude. In contrast, Ji, Babbin, Jayakumar, Oleynik, and Ward (2015) measured rates and gene abundances in ETSP water and suggested nitrification and associated N2O production were greatest between the euphotic zone and the OMZ, but that denitrification dominates production in the OMZ. Effects of climate change have also been investigated by Rees, Brown, Jayakumar, and Ward (2016), who found that acidification (pH reduction by 0.06–0.4) of polar and subpolar Atlantic Ocean waters did not affect AOA community composition but reduced N2O production in proportion to the reduction in NH3 availability (vs.  ) due to the pH reduction.
) due to the pH reduction.
Many simulation models estimate N2O production rate from measurements of oxygen concentration, assuming a linear negative relationship. Trimmer et al. (2016) tested this assumption by determining relationships between AOA amoA and nirK gene abundances (AOB were below the detection limit), oxygen concentration and N2O production in an ETNP oxycline. Correlations between oxygen and molecular data were similar to those from low-oxygen regions of the Baltic Sea (Berg, Vandieken, Thamdrup, & Jürgens, 2015), supporting AOA activity at low oxygen concentration. N2O production increased exponentially as oxygen concentration decreased from 30 to 1 µmol O2/L and there was no evidence for denitrification or nitrifier denitrification. This confirmed early work of Goreau et al. (1980) and the improved information on dynamics improved modelling of N2O emissions in OMZs. This led to estimates of 17 µmol N2O m−2 day−1, which agree with previous estimates, and predictions that ETNP and global OMZ production generates 2.1 and 5.1 Tg N/year as N2O, respectively.
9.1 Lakes and coastal regions
AOB appear to dominate ammonia oxidation in freshwater environments and Wenk et al. (2016) used isotope measurements to distinguish different sources of N2O production in two basins (North and South) of Lake Lugano. Production was higher in the holomictic lake and was associated with denitrification and bacterial (rather than archaeal) ammonia oxidation in the redox transition zone. N2O production in the meromictic North Basin was lower and was due to nitrifier denitrification. Frame, Lau, Nolan, Goepfert, and Lehmann (2017) compared N2O production in Lake Lugano and Namibian coastal waters. In the former, AOB outnumbered AOA and isotope methods indicated AOA or AOB production by abiotic conversion of hydroxylamine and insignificant nitrifier denitrification. In Namibian seawater, AOA outnumbered AOB, but the mechanism of production was not clear. Angell et al. (2018) correlated abundance of functional genes associated with N2O production with process data in salt marshes subjected to different levels of N fertilization. N fertilization increased rates of production and consumption of N2O, increased the contribution of denitrification, had no effect on AOA composition but changed AOB community composition, which contributed more than AOA to N2O production.
9.2 Wastewater treatment systems
Wastewater treatment encompasses a wide range of aerobic and anaerobic processes in which N2O is generated by ammonia oxidizers and/or denitrifiers, contributing to 1.3% of global anthropogenic emissions (Kampschreur et al., 2009). AO will generate N2O in aerobic processes, but their contributions will be influenced by spatial heterogeneity, with anoxic conditions within flocs of activated sludge processes and biofilms formed in trickling filters. The impact of these conditions and of pH are discussed in recent reviews (Blum et al., 2018; Sabba, Terada, Wells, Smets, & Nerenberg, 2018; Todt & Dörsch, 2016). AOB are generally considered to dominate AO communities, but Yin, Bi, and Xu (2018) found that AOB dominated 13 of 23 wastewater treatment plants, including 3 in which AOA were not detectable. AOA outnumbered AOB by ~1–2 orders of magnitude in 10 plants but, if cellular ammonia oxidation and N2O rates are lower for AOA, AOA will not necessarily dominate activity. These findings do, however, suggest that AOA should not be considered insignificant, but it is currently not clear which conditions influence relative abundances of AOA and AOB. Mitigation of N2O emissions may therefore be possible, but requires investigation of niche specialization and sources of emissions.
10 POTENTIAL STRATEGIES FOR MITIGATION OF N2O EMISSIONS
N2O emissions in open oceans are from natural, rather than anthropogenic sources, and mitigation in coastal regions and freshwater environments is achieved by reducing N run-off. Soils are the main source of N2O globally and advances in the understanding of the ecology of the organisms involved presents an opportunity to influence agricultural practices and decrease global emissions.
Ammonia oxidation directly and indirectly leads to all microbially mediated N2O production. Ammonia oxidation can reduce volatilization of NH3 in alkaline soils but reduction of AO activity in non-alkaline agricultural soils will not only improve fertilizer use efficiency, by increasing the residence time of  in soil and the opportunity for plant uptake, but will also decrease AO-associated N2O emissions. This can be achieved by use of synthetic nitrification inhibitors, whose benefits and limitations have been reviewed elsewhere (Coskun, Britto, Shi, & Kronzucker, 2017). BNIs associated with arable crops may provide a more efficient approach to nitrification inhibition (Subbarao et al., 2012) and Byrnes et al. (2017) demonstrated the use of forage grasses with high BNI activity in decreasing N2O emissions in urine bovine pasture. Both synthetic inhibitors and BNIs have been developed against AOB and, while some also inhibit AOA, others have not been tested. Future studies should therefore assess inhibition of a range of both AOB and AOA, given increasing appreciation of diversity within these groups.
in soil and the opportunity for plant uptake, but will also decrease AO-associated N2O emissions. This can be achieved by use of synthetic nitrification inhibitors, whose benefits and limitations have been reviewed elsewhere (Coskun, Britto, Shi, & Kronzucker, 2017). BNIs associated with arable crops may provide a more efficient approach to nitrification inhibition (Subbarao et al., 2012) and Byrnes et al. (2017) demonstrated the use of forage grasses with high BNI activity in decreasing N2O emissions in urine bovine pasture. Both synthetic inhibitors and BNIs have been developed against AOB and, while some also inhibit AOA, others have not been tested. Future studies should therefore assess inhibition of a range of both AOB and AOA, given increasing appreciation of diversity within these groups.
Increased understanding of niche differentiation within AO, and within AOA and AOB, and significant improvements in the ability to quantify these organisms, adds a further dimension and presents new opportunities. For example, fertilizer use efficiency will be greater in soils dominated by AOA or AOB, following application of inorganic or slow-release fertilizers, respectively, given their different NH3 supply preferences. Ammonia oxidation by either group will result in N2O emissions, but management strategies that favor AOA would have an additive effect in reducing N2O emissions, as N2O yield from AOB activity is double that of AOA (Hink et al., 2018; Hink, Nicol, et al., 2017). In contrast, liming of acidic soil is likely to increase AOB, rather than AOA, increasing N2O emissions associated with AOB activity. This highlights the need to consider the impact on relevant and important microbial communities, in addition to benefits for plant productivity, and is consistent with calls for greater consideration of sustainability in agricultural practice (Rockström et al., 2017).
Realization of this potential requires a broader range of microcosm studies with a greater range of soil types and conditions and fertilization strategies to confirm the relatively limited data currently available. Importantly, it also requires well-designed field experiments to translate laboratory findings to agricultural soils and to assess impacts of mitigation strategies on microbial communities, plant productivity, fertilizer use efficiency and N2O emissions under field conditions. To date, field studies have focused largely on inorganic N fertilizers and few have considered diversity within AO and the consequences for N2O emissions.
11 CONCLUSION
Research over the last 25 years has dramatically increased our understanding of AO diversity and the importance of AO community composition in determining rates of ammonia oxidation, the influence of environmental factors and important consequences for N2O emissions. This provides the basis for better quantitative information required for parameterization of biogeochemical models to improve predictions and assess impacts of different management strategies. Physiological studies should consider these requirements but care should also be taken in generalizing and extrapolating findings from single isolates, single genomes and single studies and information is required to determine the degree to which isolates represent active members of natural communities. Ecological studies require greater focus on structured experiments that identify the combinations of factors that determine the activity of different AO groups based on niche specialization, with less emphasis on descriptive studies. Research would also benefit from the development of better techniques to distinguish the many different biotic and abiotic processes contributing to N2O production and to distinguish activity of different functional microbial groups in situ. A critical requirement, however, is for studies that test the predictions of physiological and microcosm experiments ultimately to inform mitigation strategies under field conditions.
ACKNOWLEDGEMENTS
This work was financially supported by the AXA Research Fund (GWN), a Royal Society University Research Fellowship UF150571 (CGR) and all authors are members of the Nitrous Oxide Research Alliance (NORA), a Marie Skłodowska-Curie ITN and research project under the EU's seventh framework programme (FP7).
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest.



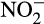 and N2O by nitrifying bacteria at reduced concentrations of oxygen
and N2O by nitrifying bacteria at reduced concentrations of oxygen
