Enzymes for dermatological use
Abstract
Skin is the ultimate barrier between body and environment and prevents water loss and penetration of pathogens and toxins. Internal and external stressors, such as ultraviolet radiation (UVR), can damage skin integrity and lead to disorders. Therefore, skin health and skin ageing are important concerns and increased research from cosmetic and pharmaceutical sectors aims to improve skin conditions and provide new anti-ageing treatments. Biomolecules, compared to low molecular weight drugs and cosmetic ingredients, can offer high levels of specificity. Topically applied enzymes have been investigated to treat the adverse effects of sunlight, pollution and other external agents. Enzymes, with a diverse range of targets, present potential for dermatological use such as antioxidant enzymes, proteases and repairing enzymes. In this review, we discuss enzymes for dermatological applications and the challenges associated in this growing field.
1 INTRODUCTION
The skin is the main external organ of the human body, the primary function of which is to prevent water loss and avoid penetration of microorganisms and potentially toxic substances.1 However, it has many other roles including temperature regulation, vitamin D production, sensory perception and immunoregulation. Skin comprises three main layers: the epidermis (most external and avascular), the dermis (rich in blood vessels, lymphatic vessels, nerve endings, fibroblasts, and macrophages and extracellular matrix (ECM)) and the subcutaneous tissue composed of fat cells (lipocytes). Despite its robustness, external (ultraviolet radiation (UVR), pollution, drugs, etc.) and physiological (altered composition and localization of lipids and proteins in the epidermis, self-renewal dysfunctions, etc.) stressors can lead to skin disorders. Topical products can deliver active ingredients to specific skin layers to prevent and treat skin disorders. Cosmetic products can be used to prevent or even treat the effect of external stressors on the skin.2
According to the United States Food and Drug Administration (FDA), cosmetic products are defined as products intended to be rubbed, poured, sprinkled, sprayed on, introduced into or otherwise applied to the human body for cleansing, beautifying or altering the appearance.3 Similar definitions are given by other agencies and commissions worldwide such as the European Union (EU) Cosmetic Regulation 1223/2009 and the Brazilian National Health Surveillance Agency (ANVISA). In 1984, Dr Albert Kligman proposed the term ‘cosmeceutical’ to define a category of products intended to improve the health of the skin and therefore placed between cosmetics and pharmaceutical products. However, up-to-date regulatory agencies have not accepted this category. The term cosmeceutical has no meaning under the law, except for Korean and Japanese legislations that distinguish cosmetic products, functional (quasi-drug) cosmetics and drugs. Nonetheless, it is widely used in the dermatology and skincare market.3-6
On the other hand, pharmaceutical products are well defined as ‘articles intended for the diagnosis, cure, mitigation, treatment, or prevention of diseases’ and ‘articles (other than food) intended to affect the structure or any function of the body of man or other animals’.3 Topical products for skin application may be in a grey area in which a product can meet criteria of a cosmetic and a drug, since they may have more than one intended use. However, the requirements for drug products of good manufacturing practices, registration, quality control and labelling are more challenging than those for cosmetics. In addition, a cosmetic ingredient must come from a list of substances generally recognized as safe at a given concentration; otherwise, the product could be classified as a drug.4, 5, 7
Among the pharmaceutical and cosmetic forms intended for topical use, gels, ointments and emulsions (creams) stand out. A gel is a semi-solid formulation composed by a gelling agent to provide firmness to a colloidal dispersion. It can be based on water (hydrogel) or organic liquids (organogel). Ointments are highly emollient vehicles consisting of a solution or dispersion of one or more active ingredients in a non-aqueous base (hydrocarbons, waxes or polyols), intended for skin or mucosal application. Emulsions or creams are formed by a lipophilic phase and a hydrophilic phase, one dispersed in the other. They contain one or more active ingredients dissolved or dispersed in a proper base and are typically used for external application to the skin or mucous membranes. After cutaneous application, the active substance must go through several steps to reach the target site. The formulation is spread over the skin surface and the active substance is released from the matrix at its application site.2, 8, 9 The extent to which the active ingredient is released from the formulation depends on its composition, physical and physicochemical properties, and the integrity and hydration status of the skin. After release, the active ingredient is free to diffuse through the skin layers. Depending on its physicochemical characteristics the active substance can accumulate on the external layers of the skin (stratum corneum and viable epidermis), corresponding to topical delivery, or can diffuse across the different layers of the skin and reach circulation, corresponding to a transdermal delivery.2
As an efficient outermost barrier, the skin has important metabolic properties that must be preserved. Self-renewal relies on a complex framework of biologic proteins as enzymes. Skin homeostasis is important for product development. For example, the integrity of the stratum corneum, the outermost layer, is highly sensitive to formulation pH when it differs significantly from that of skin (pH ~ 5). The activity of enzymes involved in cornification, such as β-glucocerebrosidase, sphingomyelinase and phospholipases, decreases with increased pH in the stratum corneum, highlighting the importance of ingredients selection for topical formulations.6, 10, 11
Recently, cosmetic and pharmaceutical companies have been investing in proteomics, genomics, and molecular research to improve skin conditions and/or provide new anti-ageing treatments. Biotechnological products are the fastest expanding segment in the personal care market and enzymes are of particular interest. In comparison with small molecule drugs and cosmetic agents, biological products can offer better specificity, and enzymes have been investigated for topical application to treat the damaging effects of pollution, solar UVR and other external agents.11 For example, some new sunscreens claim to contain photolyase, an enzyme that repairs DNA damage caused by UVR.12 According to the report published by the Grand View Research (2021), the global enzyme market was valued at USD 10.69 billion in 2020 and it is expected to grow 6.5% until 2028. Enzymes in Personal Care & Cosmetics is one of the industry trends.13
Enzymes for topical application can be classified into three main categories: antioxidants, proteases and DNA repair enzymes.11, 14 In this review, we will discuss the enzymes of each category commonly used for dermatological applications, as well the main challenges and perspectives associated to the delivery of these proteins into the skin. A summary of the enzymes applications is presented in Table 1. It is important to mention that for collagenase, at least 153 clinical trials were found in databases at the time of writing this article and not all are mentioned here.
| Enzyme | Approved and under approval uses | Potential uses | Limitations |
|---|---|---|---|
| Antioxidant enzymes | |||
| Catalase |
|
|
|
| Superoxide dismutases |
|
||
| Peroxiredoxin |
|
|
|
| Wound proteases | |||
| Keratinase | NCT | ||
| Papain | |||
| Collagenase* |
|
||
| DNA damage-repairing enzymes | |||
| T4 endonuclease |
|
||
| Photolyase |
|
||
- Note: NCT is ‘no clinical trials found’.
2 ANTIOXIDANT ENZYMES
Aerobic organisms typically contain antioxidant enzymes as natural and complex defence mechanisms against reactive oxygen species (ROS), such as the superoxide anion radical (O2•−), singlet oxygen (O2)1 and hydroxyl radicals (OH•).15 This class of enzymes is present in mammals,16, 17 some plants,18, 19 algae,20, 21 fungi22, 23 and other organisms. ROS are generated intrinsically from physiological processes of metabolic homeostasis and inflammatory signalling.24, 25 High levels of ROS can lead ‘oxidative eustress’ to ‘oxidative stress’, disrupting homeostasis25, 26 and acting as cytotoxic and pro-tumorigenic molecules.27
Antioxidant enzymes have been widely studied to treat skin diseases as skin cancer,28 vitiligo,29 ageing-related injuries,30 psoriasis,31 rosacea32 and acne,33 among others. Figure 1 illustrates a schematic representation of the general mechanism of action of antioxidant enzymes with dermatological potential.
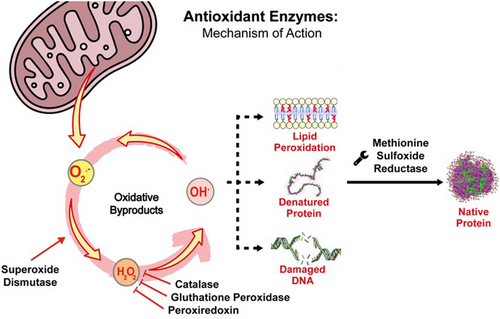
2.1 Superoxide dismutase
Some SODs have copper (Cu) and zinc (Zn),35, 36 iron (Fe),37 manganese (Mn)36, 38 or other metals as cofactors. They are ubiquitous in eukaryotic organisms38-40 and found in prokaryotic organisms.37, 41 Cu-Zn-SOD can be found in peroxisomes, cytosol42, 43 and, to a lesser degree, in the intermembrane space of mitochondria43; MnSOD predominantly in the mitochondrial matrix,43 while FeSOD exists in prokaryotic cells and some plants.44 Cu-Zn-SOD is present in human epidermis, with higher activity in younger people and tends to decrease with age, especially after 60 years45 and has a role in wound healing.46 SOD antioxidant\activity appears to be higher in males than in females.45
Higher levels of SOD are usually found in skin habitually exposed to UVR such as the face, to inhibit UVR-induced melanogenesis owing to ROS.47 However, solar exposure appears to decrease the enzyme levels in the stratum corneum, making it more vulnerable to oxidative stress.48 In vivo studies showed no significant variations in SOD levels between photoaged and intrinsically aged skin.49 Similar activity levels were found in young and old murine skin. There may be a spectral effect as UVA radiation (320–400 nm) seems to inhibit SOD activity in reconstructed epidermis,50 though earlier studies showed no effect in cultured human skin fibroblasts from healthy donors.51 UVB (290–320 nm) irradiation does not seem to affect SOD.52
MnSOD is naturally evoked by cytokines in psoriatic epidermis53 and modulates the inhibition of tumour growth.54 This enzyme may accelerate wound healing55 and its deficiency promotes cellular senescence and ageing phenotypes in skin.56 SOD activity has also been correlated to a decrease in acne severity.57 Grange et al. (2009) found that ROS are rapidly produced by keratinocytes upon stimulation by Cutibacterium acnes (the aetiological agent of acne, previously named Propionibacterium acnes) surface proteins and that SOD has a role in decreasing this effect.58 In this sense, some adjuvant therapies that aim to increase SOD serum levels through coenzyme Q10 supplementation for example have been used to treat acne.33 More specifically, SOD3 was shown to suppress toll-like receptor-2 (TLR-2), nuclear factor κβ (NF-κβ) and p38 expression in C. acnes. It also has an anti-inflammatory role reducing the expression of caspase-1 and inhibiting pro-inflammatory cytokines such as tumour necrosis factor-α (TNF-α), interleukin-1β (IL-1β), IL-6 and IL-8. SOD is correlated with reduced lipid accumulation and expression of lipogenic regulators in sebocytes affected by C. acnes.59 Its anti-inflammatory potential was also shown in vitro to treat atopic dermatitis in mice using mesenchymal stem cells overexpressing SOD3, as well as human keratinocytes.47 According to Jankovic et al. (2016), SOD3 can reset the ratio between the superoxide anion and L-arginine in the skin of diabetic rats. Based on this, molecules with SOD mimetic activity have been investigated at least since 1983.60
There is no consensus on skin and blood SOD levels in vitiligo patients. A group in India reported that significantly high SOD levels were observed in active vitiligo patients compared with stable vitiligo and healthy controls.61 Some authors have confirmed these results.62 Sravani et al. (2009) showed that vitiligo patients present higher SOD levels than the controls,62 while others have not.63
An ointment formulation of SOD and interleukin-1β was found to be effective on reparative processes of burn wounds, decreasing inflammation and accelerating wound-healing.64 This was supported in a study of experimentally scalded rats in which recombinant human CuZnSOD in three different formulations—native enzyme, enzyme-loaded gel and enzyme-loaded liposomes—reduced oedema, wound size and tissue necrosis. The liposome formulation was also efficient in ROS scavenging, accelerating wound-healing processes.65 SOD seems to ameliorate the healing process in cold burns.66 Similarly, hydrogels containing CuZnSOD enhanced repair of chronic wounds.67 Other drug-delivery systems were investigated as alternatives for SOD. For example, SOD and catalase (which will be further discussed) immobilized in silver and gold nanoparticles were found to reduce UV radiation-induced oxidative stress in rat skin. Nonetheless, the immobilized enzymes had the same effect of free enzymes on the tested parameters (DNA and lipids oxidation products, total antioxidant capacity and expression of antioxidant enzymes).68 More recently, the same enzymes immobilized on gold and silver nanoparticles were shown to enhance DNA repairing systems of rat skin after exposure to UV radiation.69 Three-day application of free or immobilized SOD and catalase decreased the levels of the most common DNA damage products generated by UV irradiation, namely cyclobutane pyrimidine dimers (CPDs) and pyrimidine-pyrimidone (6–4) photoproducts (6–4PP). The enzymes were more effective when immobilized on gold nanoparticles than on silver nanoparticles.
The use of SODs in dermocosmetic products is limited by its loss of activity in the external environment. Therefore, researchers have investigated different organisms as sources of enzymes with improved stability.70 MnSOD from Deinococcus radiodurans expressed in E. coli, for example, presents higher resistance to UVR.71 SOD expressed by different organisms and proteoforms show variation in molecular weight, kinetics and stability. Saccharomyces cerevisiae CuZnSOD, for example, has a molecular weight of 32 kDa, similar to Bos taurus CuZnSOD (32.5 kDa).72, 73 B. taurus CuZnSOD has a kM value of 3.55 ± 0.08 x 10−4 M,74 with optimal temperature and pH at 25°C and 7.8–8.0, respectively.75, 76 The isoelectric point (pI) also varies with origin and has a wide range of values from 1.0 to 10.5. Similarly, pH stability varies from 1 to 12.77
2.2 Catalase
This enzyme is common in aerobic organisms with a cytochrome system (with some exceptions) and has a major role in the prevention and repairing of cell damage by hydrogen peroxide excess.78-80 Studies have shown that catalase can prevent skin ageing by reverting alterations related to MAP kinases.81 Usually, it is isolated from bacteria and mammalian tissue and/or organs where it occurs at higher concentrations, such as the liver.79, 80, 82 Catalase from Aspergillus niger, suggested for dermatological use, has a molecular weight of 385 kDa83 and pI of 6.5.84 Optimal temperature and pH are 60°C and 7.0, respectively, but the enzyme is stable at pHs from 4.0 to 8.3 and a temperature range from 20°C to 60°C.85 Catalase has an important role in aged skin. Jankovic et al. (2019)86 detected increased catalase in the skin of elderly rats, accompanied by degenerative structural changes in epidermal and dermal compartments along with low skin renewal capacity. Its activity abruptly declined through adulthood and in middle-aged rat skin but increased in the oldest groups (18-month and 21-month-old groups). Also, exposure to UVA decreases catalase activity in the stratum corneum, leading to vulnerability to oxidative stress.48 In a more recent publication, UVB exposure was also associated with lower levels of catalase activity in the skin that may contribute to carcinogenesis.87
Catalase has been associated with several skin conditions. In vitiligo patients, increased oxidative stress is observed in skin because of lower levels of the enzyme.62 Significantly down expression of catalase was also observed in fibroblasts of Hutchinson-Gilford Progeria Syndrome patients88 and low levels were found in piebaldism patients.89 One congenital disorder, acatalasemia or Takahara's disease, is specifically related to the lack or major reduction in catalase expression.90
Despite this potential, only products containing pseudocatalase are currently available in the market to treat vitiligo. This is a transition metal bicarbonate complex activated by UVB radiation.91, 92 However, pseudocatalase formulations have not been proved sufficiently effective owing to the limited activity in comparison with the natural enzyme. According to the Braunschweig Enzyme Database (BRENDA), one catalase molecule catalyses the decomposition of 2.8 million of H2O2 molecules per second; corresponding to one of the highest turnover numbers (Kcat) of all enzymes.93
Recently, our group showed that catalase-loaded polymersomes of Pluronic L-121 provided antioxidant skin protection in vitro (pig ear skin model), especially in the deepest layers of the skin compared to free catalase, with the added benefit of low cytotoxicity for topical application.94 Further, enzymatic radical scavenger activity with catalase encapsulation in sugar ester vesicles was observed in in vivo experiments performed in post-burn skin, with enhanced kinetic parameters, thermal stability, reusability and protection against trypsin digestion.95 Our group also investigated the bioconjugation of catalase with polyethylene glycol (PEG) as a potential treatment for vitiligo and UVR-induced skin damag.e96 We showed that PEGylation increased enzyme stability and provided extra safety since the PEGylated enzyme concentrates in the external layers of the skin, preventing permeation.
2.3 Peroxiredoxin
Prx is found in plants,100 bacteria,101 mammals102 and other organisms. It presents several isoforms such as Prx I, II, III,103 IV,99 V104 and VI.98 According to the literature, purified Prx from several species,—for example, yeast and bacteria—does not contain common redox centres such as metal ions.97, 105, 106 The mechanism of action is correlated to the disulphide linkage in Cys47, which can be oxidized by hydrogen peroxide.105, 107 Despite the higher expression in mammal tissues, Prx presence in skin was only detected in 1999, more precisely different isoforms were found in rat skin and hair follicles.108, 109 Since then, it has been associated with protection against UVB-induced apoptosis of epidermal keratinocytes.110 Increased levels of Prx II were detected in psoriasis and associated to biological regulation, defence and inflammatory responses, and ROS regulation.111
Experiments in mice indicate Prx II has an important role in promoting the growth of primary mesenchymal stem cells, which are important to dermal thickness and wound healing.101, 112 Also, in vivo and in vitro studies suggest that Prx I deficiency in mice is correlated with skin cancer.113 Another study with Prx VI indicates a dual role in skin cancer development: a tumour-preventive effect, but acceleration of tumour progression at higher levels of expression.114 Transgenic mice overexpressing the human Prx IV gene have shown fibroblast proliferation and migration, especially in adult and aged mice, also more resistant to H2O2 cytotoxicity.115 In addition, Prx IV can protect the skin during skin cancer treatment.116 It improves the recovery after membrane lipid peroxidation.98
Human PrX is about 34 kDa and optimal temperature is 37°C.117, 118 This enzyme is stable to 60°C117 while stability pH can vary significantly from pH = 4.5 for Mycobacterium tuberculosis119 to 7.8–10.2 for Taiwanofungus camphoratus.120
2.4 Methionine sulfoxide reductase
Methionine sulfoxide reductases (Msr) (EC 1.8.4.11) are part of mammalian antioxidant defence121 and can be divided in two monomeric classes: MsrA and MsrB.122, 123 They catalyse the reduction of free methionine and methionine residues in peptides and proteins, repairing methionine oxidation by H2O2.124 Therefore, Msr are important not only for antioxidant defence but also for cellular regulation.125 MsrA is found in epidermis, hair follicles and eccrine glands as sebaceous glands; it is related to melanogenesis, DNA repair systems and photoprotection.126 MSRB2 expression is found in melanocytes, while both MSRB1 and MSRB3 are found to be expressed by vascular endothelial cells.127
According to the literature, MsrA knockout mice presented enhanced sensitivity to oxidative stress, shorter lifespan, atypical walking pattern and higher tissue levels of oxidized protein, indicating that MsrA plays an important role in ageing.121, 128 On the contrary, MsrA gene overexpression in Drosophila sp. increased lifespan.125 Vitiligo patients were observed to have low levels of MsrA and MsrB,129 and research indicates that both enzymes are deactivated at high concentrations of hydrogen peroxide.130
Msr activity may influence cutaneous ageing and carcinogenesis,127 and the topical application of MsrA has been shown to protect against UVB-induced damage in skin models.131 Despite the dermatological potential, studies in the literature about Msr are scarce.122, 132 The optimal pH is 7.4 for the enzyme from B. taurus and H. sapiens.133 Its isoeletric point is 5.7 in Nicotiana tabacum134; temperature stability is 53.6°C for MsrA from Treponema denticola135 and 85°C from Thermococcus kodakarensis.136 The H. sapiens MsrA has a molecular weight of approximately 23–25 kDa,124, 137 while the enzyme from M. musculus is 32 kDa138 and from B. taurus is 25 kDa.137
3 PROTEASES
Proteases or proteinases are proteolytic enzymes extensively used in clinical practice for the debridement of wounds. They are widely present in all living organisms139, 140 and offer several applications in cosmetic, pharmaceutical, food and textile products.141 Proteases are classified as hydrolases and catalyse the hydrolytic cleavage of peptide bonds by nucleophilic attack.142 According to the catalytic mechanism, proteases are divided into four groups: aspartic proteases, metalloproteases, serine proteases and cysteine proteases.143, 144 Papain, keratinase and collagenases are examples of important proteases with dermatological applications, as depicted in Figure 2.
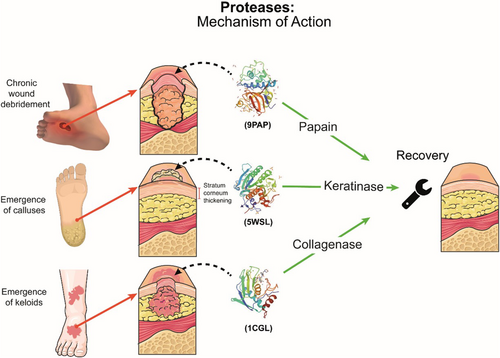
3.1 Keratinase
Keratin is a sulphur-containing fibrous and structural polypeptide and an intrinsic part of the epidermis and appendages including hair, nails, feathers, wool, as well as animal parts like horns, hooves and squama.145 It presents a rigid structure owing to the disulphide crosslinks along the polypeptide chain.146 Keratin is an abundant biomass in nature, only surpassed by chitin and cellulose.147 It is considered a major environmental problem due industrial waste.148 Microbial proteolytic enzymes responsible for degradation of keratin degradation and other insoluble substrates are called keratinases (EC 3.4.4.25).149 Common sources of these extracellular enzymes are actinomycetes, bacteria and fungi.150
Microbial keratinases have invaluable potential in different processes including the production of food, organic fertilizers, detergents, cosmetics, as well as medicine. Keratinases can be used to treat skin conditions involving hyperkeratinization such as acne, nail disorders, and removal of corns and calluses.151 Hyperkeratinization is common in acne patients, in which keratinized cells accumulate in the sebaceous canal and develop into comedones or acne lesions.152 Corns and calluses are common on toes and feet, due to repetitive friction and pressure, they are characterized as hyperkeratotic lesions.153 This process is a natural and protective response from the body to stressors; however, it usually causes discomfort.154 Keratinases could replace the salicylic acid, commonly used in the treatment of calluses, in a sustainable manner.155 Hyperkeratosis is also present in epidermolysis bullosa simplex with mottled pigmentation—a disease characterized by punctate hyperkeratosis of the palms, soles and dystrophic nails156—and other conditions such as bullous congenital icthyosiform erythroderma, ichthyosis bullosa of siemens, epidermolytic palmoplantar keratoderma, monilethrix, etc.157 Keratinases have also been used in topical preparations for hair removal.158 Sanghvi et al. (2016) investigated the dehairing efficiency of keratinase from B. subtilis DP1and the keratinase-based cream was more effective in hair removal than the chemical-based counterpart.159 Keratinase from Bacillus licheniformis is present in commercial products for the treatment of nail disorders and removal of corns and calluses.151 Also, keratinase from Paecilomyces lilacinus was investigated in cryogel patches for antimicrobial treatment.160
Most of the documented keratinases are monomeric enzymes with molecular weight up to 58 kDa.147 The enzyme from Bacillus licheniformis presents molecular weight and pH stability of 31.4 kDa and 8.5, respectively. It is stable at pH 5–12, and the optimal temperature is 50°C–55°C.161 Keratinase from Doratomyces microsporus presents isoelectric point at 9, while the optimal pH and temperature are 8–9 and 50°C, respectively. The enzyme presents 100% relative activity when human stratum corneum is used as a substrate in comparison with 22% in human nails and 14% in porcine nails.162 The kM value for the enzymes from Paecilomyces marquandii and Doratomyces microsporus are 0.17 ± 0.02 and 1.03 ± 0.17 mM, respectively.163
3.2 Papain
Papain (EC 3.4.22.2) is a cysteine hydrolase globular protein extracted from the papaya fruit latex (Carica papaya) with molecular weight of approximately 23.4 kDa and a pI value of 4.4.164 Papain is heat stable at temperatures from 60 to 80°C, the highest being best. Optimal pH is 6–9 but the enzyme is stable from pH 6 to 11.165 The presence of four disulphide bridges contributes to the preserved stability and activity under harsh conditions of temperature and pH.166 The catalytic site is located between two protein domains, an ɑ-helix and an antiparallel β-sheet, and is composed of cysteine-25 and histidine-159.166
Papain is commonly used in some cultures and countries for wound healing since the 1950s owing to its effectiveness and low cost.167, 168 The easiest and cheaper topical use of papain consists of papaya pulp directly in the wound,169, 170 although the most common formulation corresponds to a papain dried extract.167 The enzyme powder is dissolved into distilled water, applied to the wound and protected with a dry gauze. Some care must be taken to avoid metallic material near the enzyme that could result in oxidation. Nonetheless, papain home-based preparations are considerably unstable.169 The fermented phytopreparation from papaya is also used topically to treat burns in most regions of Africa.170, 171 Research indicates that papain modified with polyethylene glycol presents increased stability in comparison with free papain.172 Papain holds several beneficial properties such as degradation of proteins including collagen,173 anti-inflammatory activity174 and is an antioxidant.174, 175 In 1993, Velasco investigated a gel formulation of 0.4% w/v of papain in patients and found good therapeutic effect, easy application, reduced cost, high enzymatic stability and good wound adherence.176 In fact, papain is extensively used for wound healing not only on viable tissue but also on necrotic tissue, in patients with diabetic ulcers, pressure ulcers and venous ulcers. In all cases, treatment is associated with an improvement in wound healing and reduction of the wound area.177-181 Liposome-formulated papain has been shown to present advantages over the free protein. It not only protects the enzyme but also promotes higher skin drug deposition compared to free papain. This formulation inhibited hypertrophic scar in rabbit ears, by inhibiting angiogenesis and fibroblast invasion, and reducing collagen synthesis.173 A poly (ɣ-glutamic acid) (ɣ-PGA) chitooligosaccharide hydrogel with papain was also investigated to treat hypertrophic scars in rabbit ears. The hydrogel inhibited excessive collagen deposition during wound healing, while preserving enzyme activity.182 Papain formulations can also be used as depilatory agents in a safe way.183, 184
According to FDA, approximately 35 unapproved papain-based topical products were on the USA market in 2015 and the Agency demonstrated concern regarding the safety of these products since several reports of adverse effects related to hypersensitivity were registered.185 Based on that, research on the safety and delivery of papain increased. For example, biocompatible wound dressings incorporating papain were investigated and found to preserve enzyme stability over 28 days186 and a papain-based wound cleanser exhibited accelerated healing process in rats (97% in comparison with untreated skin), promoting complete re-epithelization and collagen deposition under the wound.187 Besides, papain can also be used as absorption enhancer in human skin.184
3.3 Collagenase
Collagenase (EC 3.4.21.32) is a serine endopeptidase, a matrix metalloproteinase produced by fibroblasts.188, 189 As others, matrix metalloproteinases are responsible for degrading collagen to help tissue renewal.190 This enzyme is essential to natural wound healing and is present at high concentrations in early proliferative phases of inflammatory wound, decreasing during remodelling of scar tissue and after complete epithelialization.164, 191 Collagenase activity can be both desirable and not. Matrix metalloproteinase-1 (MMP-1) is the main collagenase present in the skin, and its upregulation is triggered by repeated sun UVB exposure192, 193 and UVA radiation.194, 195 This increase is responsible to accumulate degraded fibrillar collagens (types I and III in skin), characterizing the photoageing process—wrinkling of photodamaged skin,196 since collagen is related with skin elasticity, hydration and its absence can be observed with the presence of fine lines and wrinkles.197 MMP-1 is the major collagenase implicated in wound healing, enabling keratinocytes migration to re-epidermisation process.198 Further, clinical studies showed that exogenous collagenase in ointment formulations may prevent burn wound conversion, diminish hospital stay, overall need for surgery and blood transfusions.199
The enzyme is commercially available in several commercial formulations, all derived from Clostridium histolyticus. It is used to promote debridement of necrotic tissue in burns, skin ulcers200, 201 and to treat Dupuytren's contracture,202, 203 Peyronie's disease204, 205 and cellulite.206 Collagenase can be used not only in adults for wound debridement but also in infants and neonates in a safe and effective way.207 Even in eschar tissue, contaminated or not, it has a great potential to debride most chronic wounds.200, 208, 209 It can also be used to treat fibrosis210 and to reduce keloids.211 Collagenase formulated in polymeric nanoparticles of acrylamide, aminoethyl methacrylate, ethylene glycol dimethacrylate and methylenebis(acrylamide) has been investigated to treat fibrosis.210 A single dose of this formulation substantially decreased the collagen area in comparison with the same dose of free collagenase in mice.
Collagenase from Clostridium histolyticus presents optimal activity at pH 7.4 in phosphate buffer, and it is stable at pH 5.6.212 The molecular weight is approximately 76–96 kDa.213 The kM value for rat type I, bovine type II and human type III collagen, is 4.0, 4.0 and 5.0 μM, respectively.213-264
4 ENZYMES FOR REPAIR OF DNA DAMAGE
DNA repair enzymes have been investigated as an active strategy against the effects of UVR. Conventional and widely used sunscreens usually absorb, disperse or reflect UVR, reducing DNA photodamage but without enhancing DNA repair. On the other hand, active photoprotection could reverse DNA damage (Figure 3).214-216
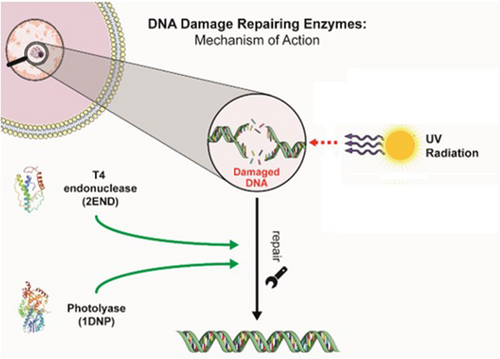
4.1 T4 endonuclease V
T4 endonuclease V (T4N5) (EC 3.1.21.7) was originally isolated from Escherichia coli infected with T4 bacteriophage.217 This enzyme can cleave the nucleotides in the internal regions of the DNA, specifically the UVR-induced CPDs, initiating their removal, which when unrepaired contribute to mutations that result in actinic keratoses and keratinocyte cancers. T4N5 repairs CPD by catalysing two reactions, the first uses glycosylase, which releases one thymine (from the CPD). In addition, another reaction involving AP lyase incises the phosphodiester backbone at the site of the missing base, causing a single stranded break.218 Its kM value for 49-mer oligonucleotide is 0.004–0.024 mM,219 the molecular weight is 20 kDa,220 stability pH ranging from 6 to 9 and optimal temperature ranging from 70 to 90°C.221 T4N5 from Pyrococcus furiosus has a pI value of 9.8.222
Early work with T4 endonuclease V showed that topically applied T4N5-loaded liposomes efficiently removed DNA photoproducts in human and mouse skin and reduced the incidence of skin photocarcinogenesis in mice.223, 224 In clinical trials, topical application of T4 endonuclease V-loaded liposomes reduced the incidence of basal cell carcinomas by 30% and that of actinic keratoses by at least 68% in xeroderma pigmentosum patients. Adverse effects were rare and allergic or irritant contact dermatitis not observed.225
4.2 Photolyase
Photolyases (EC 4.1.99.13) repair DNA photodamage by employing the energy of blue light (350–500 nm).226, 227 They are found in bacteria, plants, reptiles, amphibians and marsupials but not in placental mammalian cells. Two types of photolyases have been identified with different substrate specificity: CPD and (6–4)-photolyase.226, 227 The observation that bacterial DNA repair enzymes could be used to repair damage in human cells first happened nearly 45 years ago.218 In 2015, Aziz Sancar was recognized with the Nobel Prize in Chemistry for his work elucidating the DNA repair mechanisms by photolyase.228 The enzyme binds to the dimer with high affinity and specificity, which can occur in the dark. Following, photoreduction or dimer reversal takes place but only in the presence of light, specifically blue light. Photolyase presents a non-covalently bound chromophore/cofactor that can be a folate or a deazaflavin, and a two electron-reduced FAD as a catalytic cofactor.229
As mentioned above, photolyase is not innately present in human cells; the homologous human gene has been changed by evolution and we use the blue light for the circadian rhythm.218 Humans have other DNA repair mechanisms, such as the nucleotide excision repair (NER) pathway capable of repairing UVR-induced DNA damage. Nonetheless, this mechanism is more effective in recognizing and repairing pyrimidine–primidone type lesions and relatively inefficient in repairing CPDs.223 According to studies in photolyase-transgenic mice, the enzyme can remove CPDs from the genome of basal keratinocytes and substantially diminish the incidence of skin tumours; however, it does not affect the UVB-mediated immunosuppression.230 On the other hand, studies in human skin show that photolyase topical application induces dimer reversion and leads to immunoprotection.231, 232 The possibility of incorporating photolyase into a sunscreen and therefore repair DNA photodamage is exciting; in the words of Stege et al. ‘Exogenous application of photolyase differs from conventional photoprotection through its ability to remove damage that has already occurred. This enzyme therapy approach could thus be ideally combined as an after-sun strategy with conventional sunscreens to provide photoprotection and repair at the same time’.224
Photolyases are monomeric proteins of 50–70kDa.173 Regarding the activity and stability parameters, the enzyme from Agrobacterium fabrum presents an optimal temperature of 20–25°C and stability at pH 7–7.5.233, 234 A pI value of 8.86 is reported for the Chlamydomonas sp. enzyme.235 Measuring the kinetic parameters of photolyase is not an easy task and literature data are scarce. Nonetheless, a kM value of 32 μM is reported for E. coli photolyase.236
Different studies show the advantages of photolyase compared to traditional sunscreens. A study of a sunscreen formula containing photolyase applied prior to UVR exposure over a week resulted in 93% reduction of CPD formation while the traditional sunscreens resulted in a 62% reduction.237 The treatment of human skin with the enzyme before UVR exposure reduced telomere shortening associated with premature senescence of cells.238 Photolyase-based products are already available on the market in sunscreen formulations and after-sun lotions. Nonetheless, none of them contains the purified enzyme; they combine plankton extract (source of photolyase) encapsulated into liposomes with UV filters. As a result, the real concentration of enzyme is not known and may even vary from batch to batch. Recent studies debate the beneficial effects of the plankton extract in the treatment of actinic keratosis, a benign skin neoplasm induced mainly by UV radiation and with the potential to transform into carcinoma.223, 239 Puig et al. (2019) cite 11 clinical studies with a total of 228 volunteers and demonstrate the beneficial effect of a photolyase commercial formulation in the treatment of mild to moderate actinic keratosis.239 Nonetheless, it is important to keep in mind that all clinical trials were opened, and the number of patients reduced. Moscarella et al. (2017) conducted a parallel randomized double-blind clinical trial employing a photolyase commercial formulation and a commercially available sunscreen SPF (sun protection factor) 50 as a comparator. Thirty-six patients were investigated and after 6 months, both groups showed significant improvement in actinic keratosis lesions, but the effect was more pronounced in the group treated with photolyase.240
Yarosh et al. (2019) evaluated 125 scientific publications over the last 20 years with the aim of answering the most prominent questions about the effectiveness of DNA repair enzymes for active photoprotection. The authors concluded that the topical use of DNA repair enzymes effectively promotes the removal of DNA damage, reduces the appearance of new manifestations of actinic keratosis and promotes regression of existing lesions. Additionally, according to the authors, literature data support photolyase potential of preventing photoageing and skin cancer, but further studies could either strengthen the theory or refute it.218 One of the factors that can make it difficult to evaluate the real potential of photolyase refers to the use of the plankton extract, without effective control of the enzyme concentration. In this sense, our group has been working on the biotechnological production of photolyase to be incorporated in topical formulations.
5 MAJOR OPEN QUESTIONS, CONCLUSIONS AND PERSPECTIVES
Enzymes for dermatological and dermocosmetic applications have been investigated for many years. Their diverse range of catalytic properties offers possibilities to prevent and treat several skin conditions by different mechanisms such as antioxidant, proteolytic and DNA repair properties. Nonetheless, several challenges still prevent wider use. One of which is the economic acquisition, but genetic engineering offers new and efficient techniques to produce enzymes that can be overexpressed in host cells, with specific biological properties and desirable characteristics such as temperature and pH stability and improved activity.
Since all the enzymes mentioned in this review present molecular weight >500 Da, a relevant question is: ‘how they can reach cells and other skin layers?’ According to Bos and Meinardi (2000), ‘a compound must be under 500 Da to allow skin absorption’.241 Nonetheless, we must first consider whether penetration into deeper layers of the skin is necessary for these enzymes to exert their activity. Endogenous catalase, for example, is found at higher concentrations in the outermost skin layer—stratum corneum, where it prevents oxidative stress. In addition, previous in vitro studies in pig ear skin indicate that a bovine catalase solution can eliminate lipid peroxidation throughout the epidermis, despite its high molecular weight (~ 240 kDa).94 In some of the applications such as wound healing, this rule for skin penetration becomes not relevant since the main barrier, that is, the stratum corneum is already compromised. If penetration into deeper layers is necessary, then other characteristics may influence skin penetration such as the molecule hydrophilic/hydrophobic balance and the shape (globular or not),242 as well as the topical vehicle used to deliver the active to the target site of the cutaneous tissue. According to some authors, molecules smaller than 40 nm are capable to pass through skin layers.243 Also, depending on the formulation and the molecule hydrophilic/hydrophobic balance, it prefers to leave the formulation and move to the integument.244
Another challenge of enzymes in dermatological applications is stability since proteins are susceptible to significant degradation in exogenous environments.245 One promising approach to solve this limitation has been nanoencapsulation, which can protect enzyme integrity and activity over the time.246 Bioconjugation of enzymes with polymers such as polyethylene-glycol (PEG) is also an exciting strategy to improve cutaneous delivery. It is also important to consider the skin microbiota, that is, the microorganisms found in skin that contribute to the proper functioning of the skin through the production of beneficial bacterial metabolites,247 such skin immune defence by production of antimicrobial peptides (AMPs) production and lipoteichoic acid synthesis.244 We did not find information in the literature related to the interaction between exogenous enzyme application and skin microbiome; nonetheless, some considerations can be raised. AMPs commonly provide an acidic environment and, therefore, skin pH must be considered for enzyme stability. In addition, microorganisms commonly produce proteases that can also challenge enzymes stability. Compounds like lipoteichoic acid, on the other hand, can induce inflammation recruiting cytokines that can inhibit enzymes such as catalase248 and superoxide dismutase.249
As the scientific knowledge advances in fields such as nanotechnology and nanobiotechnology, as well as in the development of biological drugs that generates important knowledge about proteins and its interactions with the human body, novel possibilities for the use of enzymes to treat dermatological conditions may arise. Such innovations will address the delivery of proteins targeting the repair of emerging processes of skin damage, the clinical consequences of which have yet to be fully realized. For example, sunlight-generated ROS can alter gene and protein function, thereby impairing skin function. This occurs because electronically excited species are formed during ROS-induced lipid peroxidation, and protein and nucleic acid oxidation, via the formation of high-energy intermediates. The decomposition of these intermediates generates ultraweak photon emission (UPE). The emitted photons are primarily in the visible light range of solar wavelengths. Blue light has the shortest wavelengths and greatest energy in the visible light spectrum. Recent studies have shown that exposure to blue light, representative of that obtainable from sunlight, increased UPE in skin tissue, and that levels of ROS are significantly higher in skin exposed to blue light than skin without blue light exposure.250 One remaining question is whether UPE generated from blue light induced ROS formation in the epidermis can be captured by cellular chromophores in the dermis. Conceivably, this could result in low levels of photodamage that over the life-course might prove significant if accumulated and not repaired.
AUTHOR CONTRIBUTIONS
CAO and CORY provided the conceptual design of the manuscript. NSN, KMTO and JR performed the bibliographic review and wrote the original draft. ARB, PFL, ARY and CORY supervised the project, as well as discussed, corrected and improved the original draft. CORY was responsible for funding acquisition and resources. All authors commented on previous versions of the manuscript, and read and approved the final manuscript.
ACKNOWLEDGEMENTS
This research was supported by grants from the State of São Paulo Research Foundation (FAPESP-Brazil, processes number: 2020/08129-6 and 2022/01138-5), the National Council for Scientific and Technological Development (CNPq-Brazil, Fellowship # 309953/2020-0), the ANID Becas Chile/Doctorado en el extranjero/2020 - 72210489 and the Coordination of Improvement of Higher Education Personnel (CAPES-Brazil, process number: 001).
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to declare.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.




