Implications for cumulative and prolonged clinical improvement induced by cross-linked hyaluronic acid: An in vivo biochemical/microscopic study in humans
Abstract
In photoaged human skin, type I collagen fragmentation impairs dermal extracellular matrix (ECM) integrity, resulting in collapsed/contracted fibroblasts with reduced type I procollagen synthesis. Injections of cross-linked hyaluronic acid (CL-HA) reverse these deleterious changes. To investigate the time course and effects of biochemical changes induced by injected CL-HA, particularly whether fibroblast activation leads to accumulation/deposition of dermal collagen, we injected CL-HA into photoaged skin of human participants over 60 years-old and performed biochemical/microscopic analyses of skin samples. Beginning 1 week post-injection and lasting 6–9 months, fibroblasts exhibited activation, including increased immunostaining and gene expression of markers of type I collagen synthesis, such as heat shock protein 47 and components of the transforming growth factor-β pathway. At 1 week post-injection, multiphoton microscopy revealed elongation/stretching of fibroblasts, indicating enhanced dermal mechanical support. At 4 weeks, second-harmonic generation microscopy revealed thick collagen bundles densely packed around pools of injected CL-HA. At 12 months, accumulation of thick collagen bundles was observed and injected CL-HA remained present in substantial amounts. Thus, by occupying space in the dermal ECM, injected CL-HA rapidly and durably enhances mechanical support, stimulating fibroblast elongation and activation, which results in thick, densely packed type I collagen bundles accumulating as early as 4 weeks post-injection and continuing for at least a year. These observations indicate that early and prolonged clinical improvement following CL-HA injection results from space-filling and collagen deposition. As type I collagen has an estimated half-life of 15 years, our data provide the foundations for optimizing the timing/frequency of repeat CL-HA injections.
Abbreviations
-
- CL-HA
-
- cross-linked hyaluronic acid
-
- CTGF/CCN2
-
- connective tissue growth factor/cellular communication network factor 2
-
- ECM
-
- extracellular matrix
-
- HA
-
- hyaluronic acid
-
- HSP47
-
- heat shock protein 47
-
- MMPs
-
- matrix metalloproteinases
-
- SHG
-
- second-harmonic generation
-
- TGF-β
-
- transforming growth factor-β
-
- TβR
-
- TGF-β receptor
-
- PCR
-
- polymerase chain reaction
1 INTRODUCTION
Chronic exposure of human skin to ultraviolet irradiation causes photoaging, characterized by wrinkles, fragility and loss of support and elasticity. In large part, these changes result from deleterious molecular alterations in the dermis.1-3
The dermis is mainly composed of extracellular matrix (ECM), 80%–90% of which is type I collagen, which provides strength/support to human skin.3 Type I collagen is produced by dermal fibroblasts, and synthesized as a precursor (type I procollagen) that assembles into long fibrils, which organize into bundles that create an interwoven mesh-like scaffolding in the ECM. Importantly, interactions between dermal fibroblasts and the collagenous ECM determine cellular function.3-11
Indeed, in sun-protected skin, numerous long, intact bundles of type I collagen act as a stable scaffold onto which dermal fibroblasts bind through cell-surface receptors.3, 5, 12 As the collagenous ECM is relatively stiff and exerts mechanical forces in response to this binding, fibroblasts in this environment adopt an elongated, spread morphology.3, 13, 14 These mechanically ‘stretched’ cells demonstrate a synthetic phenotype characterized by relatively increased synthesis of type I procollagen and decreased production of enzymes, known as matrix metalloproteinases (MMPs), that break down the collagenous ECM.
As the skin undergoes photoaging, type I collagen bundles become fragmented, due to increased expression and enzymatic activity of MMPs.2, 3, 13, 15 These alterations disrupt the collagenous ECM, creating a disorganized, weakened scaffolding onto which fibroblasts cannot bind. As a result, mechanical forces exerted between fibroblasts and the surrounding dermal ECM are reduced, leading fibroblasts to collapse and contract.3, 13, 14, 16 Mechanically relaxed fibroblasts demonstrate a photoaged phenotype, characterized by relatively decreased production of type I procollagen and increased production of MMPs.3, 13, 14, 16-19 These changes create a self-perpetuating cycle, in which further dermal ECM disruption and fibroblast collapse occur.
In prior studies,20, 21 we observed that injection of the dermal filler cross-linked hyaluronic acid (CL-HA) into photoaged skin could reverse these changes, stimulating fibroblasts to adopt a stretched morphology and synthesize type I procollagen. Hyaluronic acid (HA) is a short-lived long-chain sugar molecule found naturally in the skin.2, 22 Chemical cross-linking of HA enhances its longevity, forming the basis of injectable fillers used for enhancing skin volume and reducing lines and wrinkles.23, 24 When injected into typical areas, such as the nasolabial folds, these fillers lead to clinical improvement lasting at least 6–9 months.2, 25-27
We found that activation of fibroblasts by injected CL-HA did not occur as a result of inflammation, or direct interaction between the injected filler and cells. Rather, fibroblast stimulation resulted from enhanced structural support and mechanical forces induced by the filler in the surrounding dermal ECM. These findings began to elucidate the mechanisms of CL-HA fillers.
In this study, we injected CL-HA and vehicle (saline) into photodamaged forearm skin of individuals over 60 years and took skin samples at various timepoints. We investigated when fibroblast elongation and activation begin following CL-HA injection, and the duration and mechanisms of these changes. We evaluated whether fibroblast activation leads to deposition of type I collagen, which prior studies had not specifically investigated (i.e. whether newly synthesized type I procollagen is converted to intact type I collagen bundles). As dermal type I collagen is long-lasting, with a half-life of approximately 15 years,28 our findings have implications for optimizing the quantity/timing of repeat CL-HA injections. We additionally investigated how long CL-HA lasts following dermal injection. Overall, our findings provide insights into the reversibility of fibroblast function in photoaged skin.
2 METHODS
2.1 Participants
The study was approved by the Institutional Review Board at the University of Michigan Medical School. After providing written informed consent, healthy lightly pigmented volunteers (60–88 years-old, 13 females, 21 males) were administered 4 injections of CL-HA (Restylane, Galderma, Fort Worth, TX) into the dorsal skin of one randomly assigned forearm and 4 injections of vehicle (0.9% NaCl in sterile water) into the other. Injections (each 0.5 mL) were intradermal, as typically performed when injecting into the nasolabial folds, and spaced 2–4 cm apart. Using a clear plastic template to record their locations, injection sites were located reliably and punch biopsies (4 mm) were obtained under local anaesthesia (lidocaine) at 1, 2, 3 and 4 weeks post-injection for an early group of subjects (15/16 completed the study), and 3, 6, 9 and 12 months for a late group (16/18 completed the study). Specimens were stored in optimum cutting temperature (OCT) medium at −80°C for later analysis. Only participants who completed the study were included in the analysis. There were no major adverse effects.
2.2 Immunostaining
As previously described,21, 29-31 OCT-embedded sections were stained for heat shock protein 47 (HSP47, Stressgen Biotechnologies, Victoria, Canada) and HA (Calbiochem, Burlington, MA). Staining for the latter was performed using a biotinylated HA binding protein, which according to the manufacturer, is derived from bovine nasal cartilage, binds specifically and strongly to HA, and is composed of two binding polypeptides derived from N-terminal regions of the HA binding proteoglycan and linkage proteins.22 Quantification of staining and dermal area occupied by injected CL-HA was analysed using Image-Pro Plus v4.1 (Media Cybernetics, Silver Spring, MD).
2.3 Multiphoton microscopy
As described previously,31 multiphoton microscopy was used to perform second-harmonic generation (SHG) and immunofluorescence imaging during separate scans. Briefly, skin sections (up to 100 μm) were incubated with HSP47 monoclonal antibody (Enzo, Farmingdale, NY) or HA binding protein (Calbiochem, Burlington, MA), followed by anti-mouse or streptavidin secondary antibodies conjugated with Alexa Fluor 488 (2 μg/mL for 45 min; Jackson ImmunoResearch, West Grove, PA). Fluorescent emissions were detected by a HyD detector between 440–470 nm (autofluorescence), 490–535 nm (Alexa Fluor 488) and 565–605 nm (SYTOX Orange for staining of cell nuclei, Molecular Probes, Eugene, OR). SHG emissions were detected through an externally mounted 410/10 or 483/32 bandwidth filter. Forward emissions were detected by an externally mounted forward-directed photomultiplier tube. Images were analysed using ImageJ v.1.49t (http://imagej.nih.gov/ij/), 3-D Gaussian blurred, autofluorescence subtracted, and projected using maximum projection or + 1 SD projections. Three dimensional renders were generated with FluoRender (v.2.25, University of Utah, Salt Lake City, UT).
2.4 Gene expression
As described previously,31-34 quantitative polymerase chain reaction (PCR) was performed.
2.5 Statistical analysis
Data are displayed as means + SEM. When appropriate logarithmic transformation of data was performed to achieve normality. Comparisons were assessed at each timepoint using paired, two-sided t-tests. An overall α-level of 0.05 was used to determine statistical significance.
3 RESULTS
3.1 Increased fibroblast synthetic activity as early as 1 week post-filler injection
To investigate when fibroblasts become synthetically active following CL-HA injection, we measured protein expression (immunostaining) of a specific marker of dermal collagen synthesis, heat shock protein 47 (HSP47),35 which is expressed in fibroblasts actively producing type I procollagen. In vehicle-injected skin, dermal staining was minimal at 1, 2, 3 and 4 weeks post-injection (all n = 15, Figure 1A). In CL-HA-injected skin, we noticed droplets/pools of injected filler localized to discrete pockets within the mid-to-lower dermis (Figure 1A). Around these pockets, staining occurred within fibroblasts, beginning at 1 week post-injection and appearing more intense and abundant at subsequent weeks. These fibroblasts were mostly embedded within the dermal ECM surrounding pools of injected CL-HA, but not directly contacting the filler material. Based on image analysis, staining in filler-injected skin was significantly increased 11.3 ± 4.8-fold at 1 week, and remained increased at 2, 3 and 4 weeks (28.4 ± 10.3−, 19.9 ± 5.1−, and 28.7 ± 10.6-fold, respectively), compared with vehicle-injected skin (all p < 0.05, n = 15, Figure 1B).
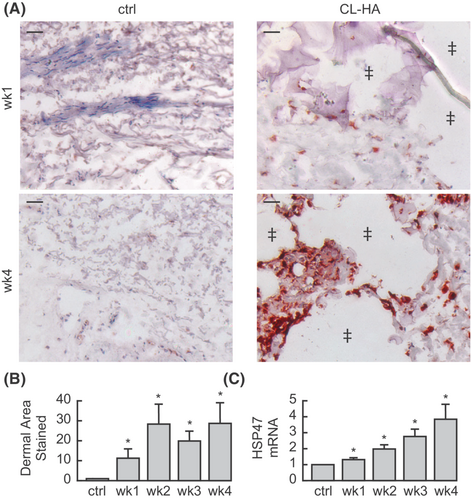
Next, we measured HSP47 gene expression, which exhibited similar changes. In filler-injected skin, expression increased significantly at 1 week (1.3 ± 0.1-fold, p < 0.05, n = 14), compared with vehicle-injected skin (Figure 1C). Gene expression of HSP47 continued increasing at 2, 3 and 4 weeks (2.0 ± 0.3−, 2.8 ± 0.5− and 3.8 ± 1.0-fold, respectively; all p < 0.05, n = 14). These data indicate that fibroblast activation begins rapidly after filler injection (as early as 1 week).
3.2 Elongation of fibroblasts, activation of the TGF-β pathway and increased type I procollagen synthesis as early as 1 week post-filler injection
To investigate mechanisms of rapid fibroblast stimulation following CL-HA injection, we performed immunofluorescence staining of HSP47 and examined the morphology of positively stained fibroblasts at high magnification. In vehicle-injected skin at 1, 2, 3 and 4 weeks, dermal staining was minimal and localized to small fibroblasts displaying a contracted/collapsed appearance, indicative of reduced mechanical support in the ECM (Figure 2A). In CL-HA-injected skin, particularly around pockets containing the injected filler, staining occurred as early as 1 week, and was robust at 4 weeks post-injection (Figure 2A). Staining was localized to large fibroblasts displaying an elongated/spread morphology, indicative of enhanced dermal mechanical support.
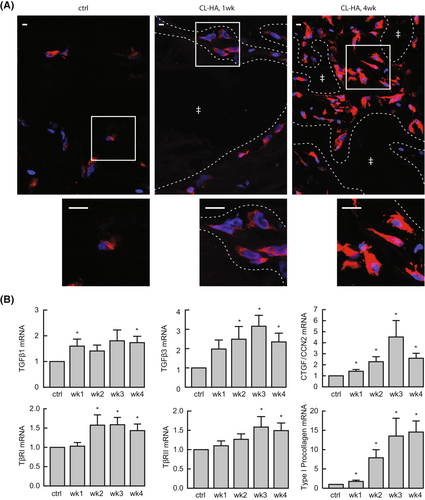
As transforming growth factor (TGF)-β is a cytokine required for type I procollagen synthesis,33, 34, 36, 37 we next measured gene expression of components of the TGF-β pathway (Figure 2B). TGF-β binds to cell-surface receptors on fibroblasts,38 and results in production of connective tissue growth factor/cellular communication network factor 2 (CTGF/CCN2), a growth factor and marker of TGF-β pathway activation that participates in upregulation of type I procollagen production.32
We found that two isoforms of TGF-β were increased at 1 and 2 weeks post-CL-HA injection, including TGF-β1 (1.6 ± 0.3-fold) and TGF-β3 (2.5 ± 0.7-fold), respectively (both p ≤ 0.05, n = 11). Expression of CTGF/CCN2 increased significantly at 1 week (1.4 ± 0.2-fold), and remained elevated at subsequent weeks (all p < 0.05, n = 14). Expression of the type I and type II receptors for TGF-β was elevated as early as 2 and 3 weeks, respectively (1.6 ± 0.3− and 1.6 ± 0.3-fold; both p ≤ 0.05, n = 14).
Additionally, we examined gene expression of type I procollagen, which showed significant elevation (1.8 ± 0.3-fold) at 1 week, and continued increasing at 2, 3 and 4 weeks (7.9 ± 2.1−, 13.5 ± 4.6− and 14.6 ± 2.9-fold, respectively; all p < 0.05, n = 14, Figure 2B). These data indicate that injection of CL-HA rapidly enhances mechanical support in the dermal ECM and activates the TGF-β pathway, stimulating type I procollagen synthesis.
3.3 Deposition of thick collagen bundles as early as 4 weeks post-CL-HA injection
To investigate whether early fibroblast elongation and synthetic activation following CL-HA-injection lead to deposition of collagen bundles in the dermal ECM, we measured gene expression of enzymes required for proper assembly of type I collagen, including procollagen N-proteinase and C-proteinase.39 Following CL-HA-injection, expression of both enzymes increased significantly at 1 week, and remained elevated at subsequent weeks, compared with vehicle-injected skin (all p < 0.05, n = 14, Figure 3A).
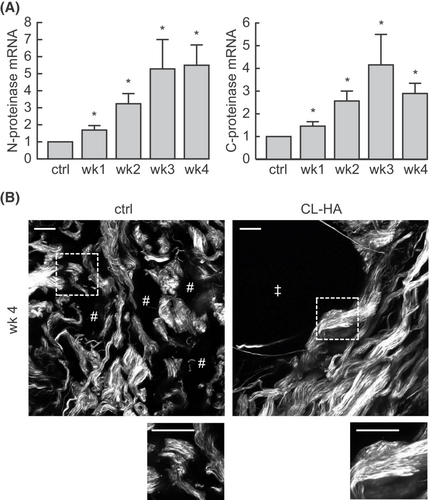
Next, we performed SHG imaging, which provides high-resolution, three-dimensional images of collagen based on its inherent optical properties.40 As type I collagen is the most abundant type of collagen in the dermal ECM, SHG imaging predominantly displays the structure and organization of this type of collagen.
In vehicle-injected skin, collagen bundles appeared shortened/fragmented, thin, loosely packed and separated from each other (Figure 3B). As early as 4 weeks in filler-injected skin, collagen bundles appeared thick and densely packed, particularly around dermal pockets containing injected filler (Figure 3B). Compared with vehicle-injected skin, these bundles appeared bright, suggesting that they were comprised of compactly arranged type I collagen fibrils. These data indicate that the enzymatic machinery needed for proper assembly of intact type I collagen is increased as early as 1 week post-filler injection, and thick, densely packed collagen bundles accumulate in the ECM by 4 weeks.
3.4 Prolonged fibroblast elongation and synthetic activity post-filler injection
To investigate the duration of fibroblast elongation and activation following CL-HA injection, we performed HSP47 immunostaining at late timepoints. In vehicle-injected skin, we observed minimal dermal staining at 3, 6, 9 and 12 months post-injection (Figure S1A). In CL-HA-injected skin, we continued to observe dermal pockets containing the injected filler material (Figure S1A). Embedded within the ECM surrounding these pockets, numerous fibroblasts remained intensely stained and markedly elongated, especially at 3 and 6 months post-injection. Image analysis revealed that staining (protein expression) remained significantly elevated at these timepoints (13.6 ± 5.9− and 13.2 ± 4.7-fold, respectively; both p < 0.05, n = 8), attenuating by 9 months (Figure S1B).
To complement these findings, we measured HSP47 gene expression, which exhibited similar changes and remained significantly increased as long as 6 months post-CL-HA injection (2.2 ± 0.5-fold, p < 0.05, n = 9), attenuating by 9 months (Figure S1C). At all late timepoints, gene expression of collagen-fragmenting MMPs was barely detectable (near the limit of detection, data not shown). These data indicate that fibroblast elongation and activation remain prolonged following CL-HA injection.
3.5 Prolonged activation of the TGF-β pathway and type I procollagen synthesis following CL-HA injection
To further investigate mechanisms promoting prolonged fibroblast activation at late timepoints following CL-HA injection, we measured gene expression of TGF-β pathway components. Expression of TGF-β1 and CTGF/CCN2 remained significantly elevated as long as 3 months post-filler injection (1.5 ± 0.2- and 1.6 ± 0.3-fold, respectively), while expression of TGF-β3 remained elevated as long as 6 months (1.9 ± 0.4-fold) (all p ≤ 0.05, n = 9, Figure S1C). Gene expression of type I procollagen was increased at 3 and 6 months (10.0 ± 2.3− and 8.1 ± 2.3-fold, respectively), and remained significantly elevated at 9 months (2.3 ± 0.5-fold) (all p < 0.05, n = 9, Figure S1C). Gene expression of procollagen N-proteinase exhibited similar changes (Figure S1C). These data indicate that prolonged fibroblast activation following CL-HA injection is associated with stimulation of the TGF-β pathway, type I procollagen, and enzymes required for assembly of type I collagen.
3.6 Accumulation of collagen bundles for at least 1 year following CL-HA injection
To investigate whether collagen bundles accumulate over time following CL-HA injection, we performed high-powered microscopy at 12 months post-injection. We used SHG imaging to examine the organization of dermal collagen bundles, coupled with immunofluorescence staining to localize injected CL-HA. In vehicle-injected skin, collagen bundles appeared fragmented, thin and loosely packed (Figure 4A). Immunofluorescence staining revealed the presence of endogenous (natural) HA, which appeared grainy and dot-like throughout the dermal ECM (Figure 4B,C).
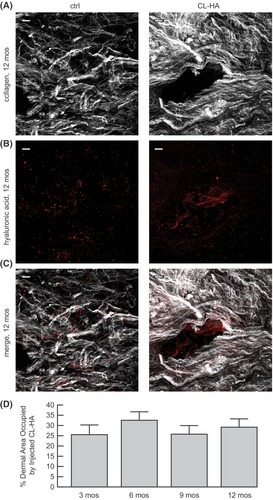
In filler-injected skin at 12 months, SHG imaging revealed numerous bright collagen bundles, which appeared long, thick and densely packed in the ECM, particularly around discrete dermal pockets (Figure 4A). Within these pockets, immunofluorescence staining demonstrated substantial amounts of injected filler appearing as droplets (Figure 4B,C). These findings are clearly shown using 3-D video rendering of images (Video S1).
We quantified the amount of space (area) in the dermis occupied by the filler. Compared with 3 months post-injection, the filler occupied just as much space in the dermis at 6, 9 or 12 months (Figure 4D). These observations indicate that collagen bundles accumulate stably for at least 1 year following filler injection, during which time substantial amounts of the filler remain physically present.
4 DISCUSSION
To translate findings to clinical observations and practice, we evaluated the temporal and biochemical changes occurring in the dermis following CL-HA injection. Our findings are summarized in Figure S2. We observed that fibroblast activation occurred as early as 1 week post-CL-HA injection. As this timepoint was our earliest, it is possible that fibroblast activation occurs even earlier following CL-HA injection.
To investigate mechanisms of this rapid stimulation, we examined fibroblast morphology. In contrast to vehicle-injected skin, fibroblasts in filler-injected skin appeared elongated/stretched, resembling cells in photoprotected skin with adequate dermal mechanical support.3, 13, 14 This stretching occurred as early as 1 week post-CL-HA injection, and suggested that space-filling by injected CL-HA rapidly activates fibroblasts by enhancing dermal mechanical support.
In addition to fibroblast elongation, key components of the TGF-β pathway were rapidly stimulated within several weeks post-filler injection. Importantly, synthesis of type I procollagen by fibroblasts is dependent on this pathway.36, 41-43 In photoaged skin, components of the pathway are decreased, reducing type I procollagen synthesis.30, 32 Conversely, the pathway can be stimulated by mechanical stretching of fibroblasts,44-46 and furthermore, TGF-β acts as a ‘mechanoregulatory’ growth factor that modulates fibroblast morphology in response to mechanical forces.5, 45, 47 Considering these observations, our data suggest that the TGF-β pathway is rapidly activated by fibroblast elongation, and stimulates type I procollagen production. Activation of the pathway likely further modulates fibroblast response/morphology (e.g. elongation) to enhanced dermal mechanical support.
We next investigated whether these changes lead to deposition of intact collagen bundles, which past studies20, 21 had not specifically evaluated. We observed that enzymes necessary for assembling intact type I collagen, including procollagen N- and C-proteinase, increased rapidly post-CL-HA injection.39 Additionally, we observed long, thick, densely packed bundles of intact type I collagen accumulating around pockets of the injected filler at 4 weeks post-injection. Further investigation may reveal whether collagen bundles start accumulating even earlier.
These observations suggest that immediately following CL-HA injection, clinical improvement (volumization) of skin is likely derived from physical presence of the filler, which enhances mechanical support (and fibroblast elongation) by occupying space in the dermal ECM. Within 1 month, deposition of long, thick, densely packed, intact collagen bundles likely provides additional mechanical support, enhancing early clinical improvement and fibroblast elongation.
Importantly, considering that collagen bundles continue accumulating noticeably for at least 1 year, our observations indicate that newly synthesized type I procollagen is converted to stable/durable dermal type I collagen following CL-HA injection. This accumulation apparently results from continued fibroblast elongation and TGF-β pathway activation for up to 6 months post-filler injection. Additionally, at 12 months substantial amounts of the injected filler remained present, suggesting that the clinical benefits of injected CL-HA are long-lasting due to both collagen deposition and space occupation by the filler. Future studies may clarify whether other properties of HA contribute to the clinical benefits of injected CL-HA.48
Our findings have additional clinical implications. Mature, intact dermal type I collagen is extremely stable, with an estimated half-life of approximately 15 years.28 As such, we predict that collagen bundles accumulating after a single CL-HA injection will persist years beyond our last timepoint of 1 year. We expect repeat CL-HA injections to enhance this collagen accumulation, such that following each injection, the need for subsequent treatment may be reduced. This concept is supported by anecdotal observations that, over time, less quantity or frequency of injections can achieve the same desired level of clinical improvement in an individual (personal observation, author JO).
Finally, our data support the concept that integrity of the dermal ECM is a major determinant of fibroblast collapse and functional decline in photoaging.1, 20 Interestingly, these cellular and functional changes are not irreversible, as fibroblasts in photoaged skin retain their synthetic capacity and can be stimulated by enhancing dermal mechanical support.
Indeed, interventions that enhance mechanical support in photoaged skin and thereby induce cellular elongation, for instance by space-filling in the dermal ECM, can activate fibroblasts rapidly and long-lastingly, leading to collagen deposition. In particular, injections of CL-HA rapidly (by 1 week) and durably (for 6–9 months) stimulate fibroblast stretching and synthetic activation, resulting in accumulation of thick, densely packed type I collagen bundles as early as 4 weeks post-injection and continuing for at least 12 months. The combination of space-filling by injected CL-HA and accumulation of dermal collagen, which potentially persists for years, likely contributes to both early and prolonged clinical improvement. These observations provide the foundations for optimizing the timing/frequency of repeat CL-HA injections.
AUTHOR CONTRIBUTIONS
Conceptualization: Frank Wang, Jeffrey S. Orringer, Sewon Kang, John J. Voorhees, Gary J. Fisher. Data Curation: Frank Wang. Formal Analysis: Frank Wang. Funding Acquisition: Frank Wang, John J. Voorhees. Investigation: Frank Wang, Thy Thy Do, Noah Smith, Gary J. Fisher. Methodology: Frank Wang, Gary J. Fisher. Resources: John J. Voorhees, Gary J. Fisher. Supervision: Frank Wang, Sewon Kang, John J. Voorhees, Gary J. Fisher. Writing—Original Draft Preparation: Frank Wang. Writing—Review and Editing: Frank Wang, Thy Thy Do, Noah Smith, Jeffrey S. Orringer, Sewon Kang, John J. Voorhees, Gary J. Fisher. All authors have read and approved the final manuscript.
ACKNOWLEDGEMENTS
We are indebted to Ken Calderone for his expertise and technical skills with multiphoton microscopy, ImageJ software and FluoRender software; Kaveri Karhade, Megan Lawlor, and Stephanie Cooke for assistance with quantitative PCR; Craig Hammerberg for assistance with immunostaining and image analysis; Heather Chubb for assistance with statistical analysis; Laura VanGoor for assistance with graphical material; and Diane Fiolek for administrative support.
FUNDING INFORMATION
Galderma donated cross-linked hyaluronic acid-containing syringes for research purposes, but had no involvement in the design or conduct of the study or in the collection, management, analysis, and interpretation of the data. Galderma was not involved in the preparation or review of the manuscript. This study was supported by the University of Michigan Department of Dermatology Cosmetic Research Fund; and a Career Development Award from the Dermatology Foundation (F.W.).
CONFLICT OF INTEREST STATEMENT
None.
Open Research
DATA AVAILABILITY STATEMENT
The data in this article will be shared on reasonable request to the corresponding author.




