A Phase 1 randomized, open-label clinical trial to evaluate the effect of a far-infrared emitting patch on local skin perfusion, microcirculation and oxygenation
Abstract
Far-infrared radiation (FIR) has been investigated for reduction of pain and improvement of dermal blood flow. The FIRTECH patch is a medical device designed to re-emit FIR radiated by the body. This phase 1 study was conducted to evaluate the local effects of the FIRTECH patch on local skin perfusion, microcirculation and oxygenation. This prospective, randomized, open-label, parallel designed study admitted 20 healthy participants to a medical research facility for treatment for 31 h on three anatomical locations. During treatment, imaging assessments consisting of laser speckle contrast imaging, near-infrared spectroscopy, side-stream dark-field microscopy, multispectral imaging and thermography were conducted regularly on patch-treated skin and contralateral non-treated skin. The primary endpoint was baseline perfusion increase during treatment on the upper back. Secondary endpoints included change in baseline perfusion, oxygen consumption and temperature of treated versus untreated areas. The primary endpoint was not statistically significantly different between treated and non-treated areas. The secondary endpoints baseline perfusion on the forearm (least square means [LSMs] difference 2.63 PU, 95% CI: 0.97, 4.28), oxygen consumption (LSMs difference: 0.42 arbitrary units [AUs], 95% CI: 0.04, 0.81) and skin temperature (LSMs difference 0.35°C, 95% CI: 0.16, 0.6) were statistically significantly higher in treated areas. Adverse events observed during the study were mild and transient. The vascular response to the FIRTECH patch was short-lived suggesting a non-thermal vasodilatory effect of the patch. The FIRTECH patch was well tolerated, with mild and transient adverse events observed during the study. These results support the therapeutic potential of FIR in future investigations.
Abbreviations
-
- ANCOVA
-
- Analysis of covariance
-
- FDR
-
- False Discovery Rate
-
- FIR
-
- Far-infrared radiation
-
- ICH GCP
-
- International Conference on Harmonization Good Clinical Practice
-
- LSCI
-
- Laser Speckle Contrast Imaging
-
- LSM
-
- Least square mean
-
- LTH
-
- Local thermal hyperaemia
-
- MedDRA
-
- Medical Dictionary for Regulatory Activities
-
- MSI
-
- Multispectral Imaging
-
- NIRS
-
- Near-Infrared Spectroscopy
-
- NO
-
- Nitric oxide
-
- NOS
-
- Nitric oxide synthase
-
- PD
-
- Pharmacodynamic
-
- PORH
-
- Post-occlusive reactive hyperaemia
-
- SDFM
-
- Side-stream Dark-Field Microscopy
-
- SEM
-
- Standard error of mean
-
- TEAE
-
- Treatment emergent adverse events
1 INTRODUCTION
Far-infrared radiation (FIR) is radiation in the electromagnetic spectrum ranging between 3000 nm and 100 μm. This type of radiation has been the subject of investigation for its therapeutic benefits when delivered through various powered or non-powered modalities such as FIR-emitting bioceramics and belts, lamps and saunas.1 Clinical and preclinical evidence point to FIR therapy exerting beneficial effects on cardiovascular and endothelial function by improving vascular endothelial function, increasing endothelial nitric oxide synthase (NOS) activity and by increasing exercise tolerance,2, 3 and it has been also found to have therapeutic benefits in acute and chronic pain conditions such as musculoskeletal pain.4-7 The mechanism of action of FIR is not yet fully known but vascular effects of FIR may be mediated through an increase in nitric oxide (NO) bioavailability and reduction in oxidative stress,8-11 and improvement of mitochondrial function by FIR has also been shown ex vivo.12, 13 Some effects of FIR may be due to heat associated with the absorption of the radiation; however, it induces the nuclear translocation of promyelocytic leukaemia zinc finger protein in the cells, which affects microcirculation independently from thermal effects. Few studies have highlighted a significant and quickened wound healing process upon exposure to non-thermal FIR therapy that do not heat the skin but still seem to have therapeutic benefits.1
The FIRTECH patch* is a non-medicated thin medical device containing titanium dioxide dispersed in an adhesive layer. The FIRTECH patch is designed to absorb emitted body heat and re-emit this energy in the form of FIR. This mode of delivery of FIR is hypothesized to not heat the skin while exerting beneficial effects on local skin microcirculation, oxygenation, and mitochondrial function. Since studies with other FIR-emitting therapeutic modalities have shown promise in treatment of pain,5, 6, 14, 15 the intended mode of action of the FIRTECH patch is to alleviate acute pain across various parts of the body such as joints, tendons, bones and muscles (articular and musculoskeletal pain) by improving local microcirculation. Preclinical testing of the FIRTECH patch conducted in rabbits and guinea pigs showed the device to be safe and non-irritating. The present first-on-human clinical study aimed to elucidate the possible mechanisms of action of FIR re-emitted by the FIRTECH patch by evaluating the local effects of this patch on local perfusion, skin temperature and tissue oxygen consumption.
2 METHODS
This study was conducted at the medical research facility in accordance with the principles of the Declaration of Helsinki, the International Conference on Harmonization Good Clinical Practice (ICH GCP) and ethical principles as referenced in EU Directive 2001/20/EC. The protocol was submitted and approved in accordance with European Union Medical Device Regulation, article 82. All volunteers provided written informed consent prior to any study-related activity. The trial was prospectively registered in toetsingonline.nl (NL77899.100.21, ABR number 77899).
2.1 Subjects
Adult (age ≥18 and <55 years) male and female participants were included if no clinically significant abnormal findings were found in the medical history, physical examination, 12-lead electrocardiogram (ECG), alcohol breathalyser and clinical laboratory tests (i.e. serum chemistry, haematology, coagulation, urine drug screen and urinalysis). Pregnant and nursing women were excluded from participation, as well as subjects using any type of medication (exception paracetamol/acetaminophen up to 4 g/day) and subjects with body modifications or impediments for imaging on the locations where treatment with the study patch was planned.
2.2 Study design
This was a prospective, randomized, open-label, parallel designed study in which contralateral non-treated areas served as control for treated areas in the same subject. One cohort of 20 subjects received treatment with three FIRTECH patches for 31 h, one located on the inner surface of the lower forearm on glabrous skin, one vertically placed on the lower back and one vertically placed on the upper back. Subjects were body-site randomized to receive the FIRTECH patch (target treatment group) placed on either the left or right side of the aforementioned areas, and the same area on the opposite side was used as control. Study assessments were performed through a 1 × 1 cm ‘window’ cut in the patch which was folded up for assessing underlying skin and attached to the skin in between measurements.
2.3 Study assessments
2.3.1 Safety
Safety assessments included monitoring of adverse events and concomitant medication use and measurement of vital signs. Adverse events were coded using Medical Dictionary for Regulatory Activities (MedDRA) version 24.0 (March 2021). Vital signs were measured before treatment administration and at 1 h, 2 h, 4 h, 6 h, 8 h, 24 h, 27 h and 30 h after treatment administration.
2.3.2 Pharmacodynamic assessments
Imaging assessments of local skin microcirculation were conducted at regular intervals on treated and contralateral non-treated sites, before treatment administration and at 1 h, 2 h, 4 h 6 h, 8 h, 24 h, 27 h and 30 h after start of treatment. Imaging assessments were conducted on several locations with patches placed, as shown in Figure 1. Measurements were conducted in temperature-controlled rooms (20–24°C) at the medical research facility. Study assessments conducted to evaluate microvascular function were laser speckle contrast imaging (LSCI), side-stream dark-field microscopy (SDFM), near-infrared spectroscopy (NIRS) multispectral imaging (MSI) and thermography.
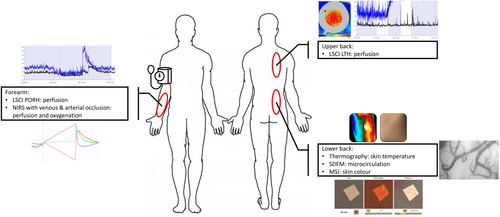
LSCI is a non-invasive imaging method that uses changes in the speckle pattern reflection when illuminating an imaged object with laser light. Changes in the speckle pattern signify movement on or inside the imaged object. When imaging human tissue, the movement of blood cells causes changes in the speckle pattern which can be used to derive an estimation of blood flow in the imaged tissue.16 LSCI imaging was performed as a baseline measurement of blood flow in treated and non-treated areas as well as in combination with two challenges (temporary occlusion and local hyperthermia) to assess microvascular responses in the imaged area. Post-occlusive reactive hyperaemia (PORH) was assessed in treated and non-treated areas on the arm. During this procedure, blood flow is temporarily occluded with a blood pressure cuff placed around the upper arm and then released. The subsequent increase in flow can be used as a measure of vascular reactivity to shear stress caused by the sudden influx of blood into the arm.17-19 Additionally, LSCI was combined with the local thermal hyperaemia (LTH) challenge, in which skin is heated to approximately 43°C while continuously measuring blood flow. The skin blood flow response to heating can be used to assess neuronal and NO-dependent vascular reactivity.20-22
SDFM is a technique used to visualize blood vessels in vivo using light with a wavelength absorbed by red blood cells. Assessment of blood vessels in the skin was done after removing the top layer of the epidermis through tape stripping to allow penetration of light up to 0.5 mm into the skin. SDFM imaging can provide information on blood vessel density and perfusion.23
NIRS was used to measure fractions of oxygenated and deoxygenated haemoglobin in treated and non-treated areas on the arm up to 3–4 cm deep. In combination with a venous and arterial occlusion challenge, NIRS allowed the quantification of tissue oxygen consumption, blood flow and vascular response to influx of blood in the arm.24, 25
MSI was used to measure skin colour and erythema. Colour was defined in the CIELAB colour space L*, a*, b*, in which L* represent lightness, a* represents the green–red colour axis and b* represents the blue–yellow axis. Lastly, thermography was used to assess changes in skin temperature on treated and non-treated sites.
2.4 Statistical analysis
All statistical analyses were conducted according to a statistical analysis plan written before unblinding of the database.
2.5 Safety data
All subjects who received ≥1 FIRTECH patch treatment were included in the safety analyses. Treatment emergent adverse events (TEAEs) were summarized, and percentages calculated by treatment, System Organ Class, preferred term, severity and study drug relatedness. Vital signs were summarized similarly, and number and percentage of out-of-range values calculated by treatment and time point. Treatment exposure was calculated in minutes using patch application as start time and patch removal as end time and summarized with mean, standard deviation (SD), minimum, maximum and median treatment duration.
2.6 Pharmacodynamic data
- The p-value associated with the second primary endpoints (LSCI: absolute blood flow measurements during post occlusive reactive hyperaemia challenges over 2 days) was interpreted in a confirmatory way only if the first primary endpoint (LSCI: absolute blood flow measurements during LTH challenges over 2 days) was statistically significant.
- The p-values associated with the main secondary endpoint (NIRS) were interpreted in a confirmatory way only if the primary objective was met and if the preceding endpoint as defined in the statistical analysis plan was met.
- The multiplicity of other secondary endpoints was handled using Benjamini–Hochberg controlling procedure in False Discovery Rate (FDR) approach, assuming FDR = 10%, in case the primary and main secondary endpoint were met.
3 RESULTS
3.1 Subject disposition
A total of 20 subjects received the study treatment and completed the study period and follow up, 10 on the right side of the body and 10 on the left. The median (min, max) of study treatment exposure was 1874.5 min on the left side (1863, 1886 min) and 1875 min on the right side (1819, 1886 min). One subject lost one treatment patch on the back during the night from Study Day 1 to Day 2. A new patch was applied upon arrival of the subject on the study site on Study Day 2. Since the subject was without patch for a maximum of 8 h, treatment exposure was at least 25/33 h or 75% for this subject.
3.2 Baseline characteristics
Baseline characteristics of treatment groups are presented in Table 1. All participants were healthy male (70%) or female (30%) volunteers of predominantly white ethnicity (80%).
| Mean (SD) | Median | Min | Max | |
|---|---|---|---|---|
| Age (years) | 25.2 (7.3) | 24 | 18 | 49 |
| Height (cm) | 180.48 (7.77) | 181.3 | 163.0 | 194.5 |
| Weight (kg) | 73.64 (7.83) | 76.28 | 54.20 | 85.45 |
| BMI (kg/m2) | 22.64 (2.57) | 22.5 | 18.4 | 28.9 |
- Abbreviations: Max, maximum, Min, minimum, SD, standard deviation.
3.3 Safety data
Data from 20 subjects were used for safety evaluation. No serious adverse events nor treatment discontinuations occurred. Observed TEAEs were mild, transient and unlikely to be related to study treatment except one possibly related adverse event with MedDRA v24.0 preferred term ‘Eczema’, which resolved spontaneously within 1 day. Vital signs evaluation showed no notable out-of-range values or changes during study treatment.
3.4 Pharmacodynamic analyses
3.4.1 LSCI
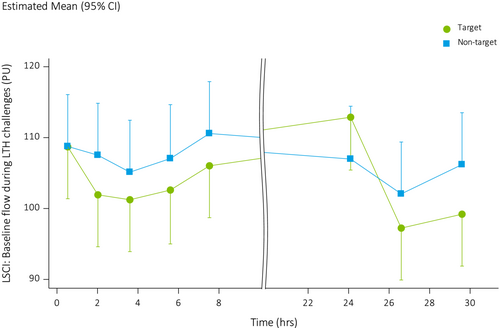
Baseline blood flow on the upper back measured with LSCI before initiating LTH was not statistically different between treated and non-treated areas (LSMs difference: −3.06 PU, 95% CI: −6.55, 0.44) (Figure 2). Due to the application of the hierarchical procedure, p-values of all other endpoints were therefore interpreted exploratively. Due to the effects of the LTH technique, baseline flow was already increased before start of LTH challenges when compared to PORH measurements. Plateau and peak flow during heating was higher in the non-treated areas compared to the treated areas (LSMs: −4.02 vs. −14.18, LSMs difference −10.16, 95% CI: −15.72, −4.59).
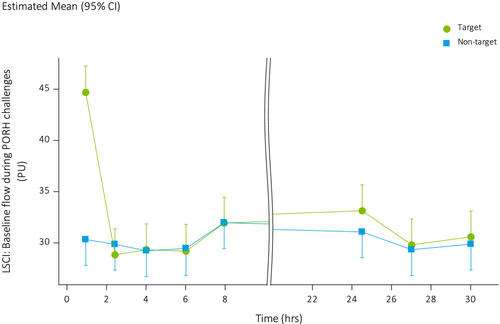
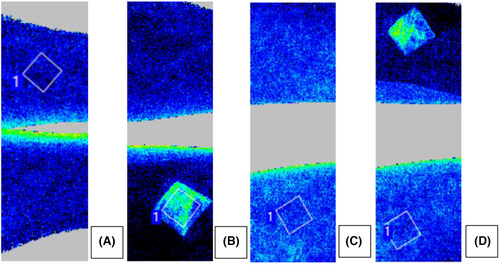
Baseline blood flow on the forearm measured before initiating PORH was overall higher in treated areas when compared to non-treated areas (LSMs difference: 2.05, 95% CI: 0.54, 3.56) (Figure 3), owing to an approximately 50% higher perfusion in the treated areas (44.62 AU, SD: 17.31) versus non-treated areas (30.33 AU, SD: 5.12) at the first measurement post treatment administration, that is, 30 min after starting treatment. Figure 4 shows an example of increased blood flow underneath the patch in two subjects. Peak blood flow after PORH was not significantly different between treated and non-treated areas (LSMs: 6.61 vs. 5.66, LSMs difference: 0.94, 95% CI: −1.42, 3.30).
3.4.2 NIRS
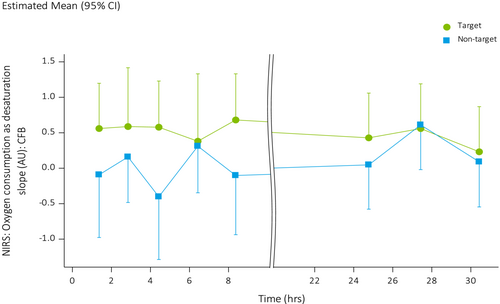
Oxygen consumption was higher in treated areas on the forearm when compared to non-treated areas (LSMs difference 0.42 AU, 95% CI: 0.04, 0.81) (Figure 5). Other evaluated NIRS endpoints, including blood flow, duration of PORH and percent increase in blood flow after arterial occlusion did not differ significantly between treated and non-treated areas.
3.4.3 SDFM
There were no clinically or statistically significant effects of the patch on SDFM readouts on the lower back.
3.4.4 MSI
There were no clinically relevant differences between treated and non-treated areas as measured with MSI on the lower back. Specifically, no statistically significant differences in redness (LSMs difference 0.01, 95% CI: −0.27, 0.28) or haemoglobin content (LSMs difference −0.32, 95% CI: −1.13, 0.49) of the skin were found.
3.4.5 Thermography
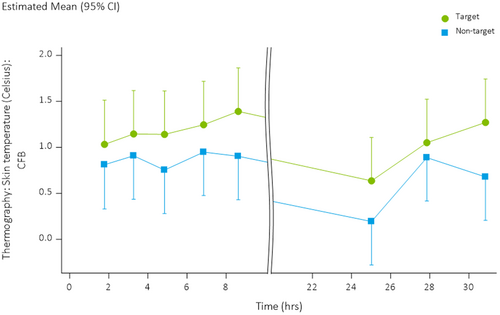
Skin temperature was higher in treated areas on the lower back compared to non-treated areas (LSMs: +1.11°C vs. +0.76°C, LSMs difference 0.35, 95% CI: 0.15, 0.56) (Figure 6).
4 DISCUSSION
In the present first-on-human study, FIRTECH patch application was associated with a local increase in dermal blood flow, increased oxygen consumption and increased skin temperature, although the primary endpoint was not met. Dermal blood flow improved approximately 30 min post administration of treatment and returned to baseline after 2 h, while oxygen consumption and skin temperature in treated areas remained elevated during the entire patch administration. In addition, the FIRTECH patch showed an excellent safety and tolerability profile. The FIRTECH patch thus has the potential to non-invasively modulate local microvascular function.
Previous literature has already reported the positive effects of FIR on the microvasculature, believed to be primarily mediated by the induction of local NOS expression and stimulation of this enzyme9, 26, 27 increasing local NO bioavailability and thereby local vasodilation. However, NO also exerts other beneficial effects, including anti-inflammatory and anti-analgesic. It is noteworthy that the vascular response that was observed in this study was generally short-lived as opposed to the longer-lasting increase in skin temperature, suggesting a non-thermal effect of the FIRTECH patch immediately post application. Hypothetically, the short-lived nature of the effect might be caused by the induction of buffering mechanisms in the skin. In this case, local NO production is still increased, and non-vascular beneficial effects might extend beyond the vascular effects. This is supported by the observation that the NO-dependent plateau flow during LTH showed a decrease over time in treated areas, possibly owing to the increased local NO production creating a ceiling for the increase of blood flow by heating.
Increased resting oxygen consumption as measured with NIRS28, 29 on the forearm was observed in this study throughout the patch application duration. To date, evidence of the effects of FIR on oxygen consumption have been mixed, with some studies showing reduced oxygen uptake during exercise and others showing increases or no changes in oxygen consumption or concentration.30-33 In vitro and in vivo evidence however points towards FIR promoting mitochondrial function, thereby possibly leading to increased aerobic metabolism and oxygen consumption.12, 13, 34, 35 The findings in this study indicate that the FIRTECH patch can increase oxygen consumption, hypothetically through increasing mitochondrial activity. Notably, results from LSCI analysis showed a hypothetical lasting increase in NO availability, which is associated with decreased mitochondrial respiration.36 The FIR effect increasing mitochondrial respiration was apparently greater than the possible negative effect of NO.
In our study, we also observed an effect of the FIRTECH patch on the local skin temperature. Potentially, this is caused by the FIR being reflected onto the skin, or the prevention of heat loss from the underlying skin by the fabric of the patch itself. This temperature increase possibly contributes to the measured increase in oxygen consumption, as well as an increase in flow.37 Whether increasing the local skin temperature has beneficial effects on pain perception is not known, as this may be dependent on pain type.
For logistical reasons as well as to reduce subject burden, the imaging assessments in this study were performed on different locations, that is, forearm, upper and lower back. Although measurements were compared to the same location on the other side of the body, it is possible that characteristics of skin on the various measurement sites influenced the ability of the imaging techniques to detect effects of the FIRTECH patch. However, epidermal thickness is comparable on the volar forearm and back,38 as are stratum corneum thickness and skin roughness,39 indicating comparability of skin characteristics. This increases the likelihood that the observed differences are due to either intra-individual variation, or that distinct physiological aspects of microcirculation were assessed by the employed imaging techniques and differed between the measurement sites.
Both improved blood flow40 as well as improved mitochondrial function41 could be promising mechanisms in the treatment of various types of pain such as acute musculoskeletal pain since both mitochondrial dysfunction and reduced blood flow have been implicated in the development of various pain syndromes.42-44 Previous studies with IR therapy have shown promise for its application in relieving a variety of pain conditions,6, 15, 45-47 and the present study further supports the physiological effects of treatment with FIR which could potentially be used in the treatment of pain.
4.1 Study Limitations
In this study, treatment with the FIRTECH patch was not compared to a placebo or mock patch, thereby introducing the possibility of a placebo effect as well as lack of blinding. However, the assessments used provide objective information on blood circulation and oxygenation on a microscopic level and are therefore not likely to have been affected by placebo effects. As all materials re-emit infrared (IR) to various degrees, the effects of the FIRTECH patch may not be limited to the ceramic particles, but also a result of the other components of the patch. As it is not possible to manufacture a patch that is entirely IR inert, comparison to non-treated sites can be considered the most objective evaluation of IR effects.
5 CONCLUSION
The present study shows that the FIRTECH patch is a safe treatment with beneficial effects on both skin microcirculation and oxygen consumption which warrant further investigation of its efficacy.
6 AUTHOR CONTRIBUTIONS
Sebastiaan JW van Kraaij, Hayet Kechemir, Iva Igracki-Turudic, Yalçin Yavuz, Robert Rissmann and Pim Gal conceived of and designed the study. Sebastiaan JW van Kraaij and Pim Gal performed the research. Sebastiaan JW van Kraaij, Bill Giannokopoulos, Moritz Heinz, Yalçin Yavuz, Robert Rissmann and Pim Gal analysed and interpreted the data. Sebastiaan JW van Kraaij, Robert Rissmann and Pim Gal wrote the manuscript. All authors critically revised the manuscript and approved of the final version.
ACKNOWLEDGEMENTS
This study was performed at Centre for Human Drug Research, Leiden, the Netherlands. Coordination for the development of this manuscript and assistance with the revisions was provided by Mohamed Amessou, PhD, from Sanofi. Editorial assistance in the preparation of this article was provided by Vijay Rayasam, PhD, from Sanofi. The results of this study will be presented as a poster presentation at the IASP 2022 World Congress on Pain.
FUNDING INFORMATION
This study was funded by Sanofi.
CONFLICT OF INTEREST STATEMENT
Sebastiaan JW van Kraaij, Yalçin Yavuz, Robert Rissmann and Pim Gal these authors have no conflicts of interest to declare. Michael R Hamblin declares the following potential conflicts of interest. Scientific Advisory Boards: Transdermal Cap Inc., Cleveland, Ohio; Hologenix Inc., Santa Monica, California; Vielight, Toronto, Canada; JOOVV Inc., Minneapolis-St. Paul Minneapolis; Consulting; USHIO Corp, Japan; Sanofi-Aventis Deutschland GmbH, Frankfurt am Main, Germany. Stockholding: Niraxx Light Therapeutics, Inc., Irvine, California; JelikaLite Corp, New York. Gisele Pickering has received consultancy fees from Grunenthal, Mylan, MundiPharma and Sanofi. Bill Giannokopoulos, Hayet Kechemir, Moritz Heinz and Iva Igracki-Turudic are Sanofi employees and may hold shares and/or stock options in the company.
CLINICAL TRIAL REGISTRATION NUMBER
NL77899.100.21.
Suppliers: Sanofi-Aventis Recherche & Developpement to Sanofi, Recherche & Developpement
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- * Suppliers: Sanofi-Aventis Recherche & Developpement to Sanofi, Recherche & Developpement.




