Long-Term Efficacy and Safety of Ravulizumab in Adults With Anti-Acetylcholine Receptor Antibody-Positive Generalized Myasthenia Gravis: Final Results From the Phase 3 CHAMPION MG Open-Label Extension
Funding: This work was supported by Alexion, AstraZeneca Rare Disease.
ABSTRACT
Background
Ravulizumab, an anti-complement C5 monoclonal antibody, was efficacious with acceptable safety in the randomized controlled period (RCP) and interim open-label extension (OLE) periods of the CHAMPION MG phase 3 trial in adults with anti-acetylcholine receptor antibody-positive (AChR-Ab+) generalized myasthenia gravis (gMG). Here, we report final results from the OLE.
Methods
Patients who completed the 26-week RCP could enter the OLE and receive ravulizumab for up to 4 years. Efficacy and safety were assessed throughout the OLE.
Results
Among all ravulizumab-treated patients (n = 169; median [range] ravulizumab treatment, 759.0 [14.0, 1265.0] days), 161 entered the OLE (ravulizumab-ravulizumab: n = 78; placebo-ravulizumab: n = 83). Sustained improvements were observed in Myasthenia Gravis Activities of Daily Living (MG-ADL) total scores (ravulizumab-ravulizumab, least squares mean [95% CI] change from RCP baseline at week 164: −4.0 [−5.3, −2.8]; p < 0.0001; placebo-ravulizumab, change from OLE baseline after 138 weeks of treatment: −2.1 [−3.3, −0.9]; p = 0.0005). One hundred and forty-one out of 160 (88.1%) patients achieved a ≥ 2-point improvement in MG-ADL total score, and 59/141 (41.8%) achieved a score of 0 or 1; once achieved, 32/59 (54.2%) sustained this status for > 50% of their remaining time in the study. Similar improvements were observed in Quantitative Myasthenia Gravis and Myasthenia Gravis Quality of Life-15 revised scores, and Neurological Quality of Life Fatigue subscale scores. Clinical deterioration event rates were reduced in the OLE versus placebo in the RCP. Corticosteroid usage was reduced in the OLE. Ravulizumab was well tolerated; no meningococcal infections were reported.
Conclusion
Ravulizumab demonstrated clinically meaningful and durable efficacy and safety in adults with AChR-Ab+ gMG.
1 Introduction
Oral cholinesterase inhibitors, corticosteroids, and other immunosuppressive therapies relieve symptoms in some patients with generalized myasthenia gravis (gMG); however, they are associated with serious adverse effects [1-3]. Therefore, gMG treatment aims to achieve and maintain stable disease control [1, 4], while minimizing exposure to immunosuppressive therapies, including corticosteroids.
Most patients with gMG (85%) have autoantibodies against the postsynaptic acetylcholine receptor (AChR) [5-8]. Autoantibody binding to AChR activates the complement cascade, which converges on complement component C5. Subsequent formation of the membrane attack complex (MAC) results in the destruction of the neuromuscular junction postsynaptic membrane [5-8]. The anti-C5 monoclonal antibodies eculizumab and ravulizumab, which bind with high affinity to C5 and inhibit MAC formation, have been approved to treat adults with AChR antibody-positive (AChR-Ab+) gMG [9-12]. Engineered from eculizumab (administered every 2 weeks), ravulizumab is administered intravenously (IV) every 8 weeks based on weight to achieve immediate, complete, and sustained complement inhibition over the dosing interval [13-17].
In the 26-week, double-blind, randomized controlled period (RCP) of the phase 3 CHAMPION MG study in adults with AChR-Ab+ gMG (ClinicalTrials.gov, NCT03920293; EudraCT, 2018–003243-39), ravulizumab demonstrated rapid and clinically meaningful improvement in patient- and physician-reported clinical outcomes versus placebo [4]. Ravulizumab was well tolerated, with an adverse event (AE) rate comparable to placebo and no notable differences between treatment groups in types of AEs. Following the RCP, patients could enroll in the open-label extension (OLE) phase to receive ravulizumab for up to an additional 4 years, for which a prespecified interim analysis has been previously reported [14]. Early improvements in activities of daily living, muscle strength, fatigue, and quality of life in patients who received ravulizumab in the RCP were sustained through 60 weeks in the OLE. Patients who switched from placebo in the RCP to ravulizumab in the OLE had a rapid and sustained response from first assessment after ravulizumab initiation through 60 weeks of follow-up. Here, we report long-term clinical efficacy and safety results following ravulizumab treatment in the CHAMPION MG OLE study.
2 Methods
2.1 Study Design and Patients
The trial design and procedures for the RCP and OLE of CHAMPION MG (Figure S1) have been previously published [4, 14]. Briefly, patients in the RCP were randomized to receive ravulizumab or placebo. Following RCP completion, patients could enter the OLE and receive open-label ravulizumab for up to 4 years. Patients and study personnel remained blinded to RCP treatment assignments during the OLE. Patients completed the study if they: (1) completed all study periods, including the last OLE visit; or (2) completed the study early because the study drug had become accessible via an Alexion post-trial access program or was approved according to country-specific regulations.
The study was conducted in accordance with the International Conference on Harmonisation E6 Guideline for Good Clinical Practice, World Medical Association Declaration of Helsinki, and all applicable regulatory requirements. An independent ethics committee or institutional review board at each participating institution approved the trial protocol, and all patients provided informed consent prior to study initiation.
Eligible patients were aged ≥ 18 years with AChR-Ab+ gMG, diagnosed with MG at least 6 months before screening, had a Myasthenia Gravis Foundation of America (MGFA) clinical classification of II-IV at screening, and had a Myasthenia Gravis Activities of Daily Living (MG-ADL) total score of ≥ 6 at both screening and randomization. Vaccination against Neisseria meningitidis within 3 years before initiating study drug was required; those who started complement component 5 inhibitor therapy < 2 weeks after receiving a meningococcal vaccine received prophylactic antibiotics for 2 weeks after vaccination. Patients were ineligible if they had received IV immunoglobulin or plasma exchange in the prior 4 weeks, rituximab treatment in the preceding 6 months, or previous treatment with a complement inhibitor (e.g., eculizumab).
2.2 Treatments
During the RCP, patients received a weight-based IV loading dose of ravulizumab (2400, 2700, or 3000 mg) or matched placebo on day 1, followed by weight-based maintenance doses of ravulizumab (3000, 3300, or 3600 mg) or placebo on day 15 and once every 8 weeks thereafter. Patients who entered the OLE at week 26 (OLE start/baseline) received either ravulizumab 900 mg (if assigned to ravulizumab during the RCP; ravulizumab-ravulizumab group) or a weight-based IV loading dose of ravulizumab (2400, 2700, or 3000 mg; if assigned to placebo during the RCP; placebo-ravulizumab group). From week 28 and every 8 weeks thereafter for up to 4 years, all patients in the OLE received weight-based IV maintenance doses of ravulizumab 3000, 3300, or 3600 mg. Patients receiving stable doses of cholinesterase inhibitors and immunosuppressants (including corticosteroids) at screening were allowed to continue them. Dose changes were permitted only during the OLE, at the investigator's discretion. Throughout the trial, rescue therapy with high-dose corticosteroids, IV immunoglobulin, or plasma exchange was allowed for patients experiencing clinical deterioration. Patients receiving cholinesterase inhibitors were required to abstain ≥ 10 h prior to each scheduled assessment.
2.3 End Points and Assessments
Efficacy end points included score changes from RCP and/or OLE baselines in MG-ADL [18], Quantitative Myasthenia Gravis (QMG) [19], Myasthenia Gravis Quality of Life-15 revised (MG-QOL15r) [20], and the long form of the Neurological Quality of Life (Neuro-QOL) Fatigue subscale [21]. Details on these measurement instruments and assessment schedules are included in the Appendix S1.
Additional end points included proportions of patients with ≥ 3-point improvement and minimal clinically important difference (MCID; ≥ 2-point improvement [22]) in MG-ADL score and rates for ≥ 5-point improvement and MCID (≥ 3-point improvement [19]) in QMG score from RCP baseline (ravulizumab-ravulizumab) or OLE baseline (placebo-ravulizumab).
Also assessed were changes in corticosteroid use and the proportion of patients who discontinued corticosteroid treatment during the OLE, as well as clinical deterioration (worsening to a score of 3- or a 2-point worsening from baseline on any individual MG-ADL item, other than double vision or eyelid droop, which was associated with significant symptomatic worsening per the investigator's assessment), MG crises (weakness necessitating intubation or delaying extubation following surgery), or administration of rescue therapy to a patient whose health would be in jeopardy without it.
Safety outcomes were assessed in all patients who received ≥ 1 dose of ravulizumab in the RCP or OLE. AEs were reported from the first ravulizumab dose until 56 days after the last dose. Clinical laboratory findings, vital signs, and electrocardiogram abnormalities were collected throughout the study [14].
2.4 Statistical Analyses
Efficacy end points were assessed for patients who received ≥ 1 dose of ravulizumab in the OLE (OLE analysis set). For MG-ADL, QMG, and MG-QOL15r total scores and Neuro-QOL Fatigue subscale scores, least squares (LS) mean changes from baseline were determined using a mixed-effects model for repeated measures (MMRM), with separate models for each treatment sequence utilizing both RCP and OLE baselines. RCP baseline was defined as the last available predose assessment on day 1 of the RCP, and OLE baseline was defined as the last available assessment prior to the first dose of the study drug in the OLE. Changes from baseline were assessed using two-sided 95% CIs, with significance defined as p < 0.05. Missing data were assumed to be missing at random and were not imputed. MMRM estimates for the OLE were based on fixed categorical effects of study visit, randomization stratification factor of region, and fixed covariate of baseline score.
Post hoc analyses evaluated the percentage of time from first ≥ 2- or ≥ 3-point response, minimal symptom expression (MSE; MG-ADL score, 0 or 1) [23] response rate, and percentage of time from first MSE response. Percentages of time in response were measured from the earliest visit where the response was achieved to the first subsequent visit where the response was not observed or to the last assessment if the response was maintained throughout. The statistical significance of the relationship between achieving MSE and baseline MG-ADL total score was also assessed, using a two-sample t test.
Clinical deterioration event rates were calculated using a generalized estimating equation Poisson regression repeated measures model, with the number of events serving as the dependent variable, the logarithm of patient-years in the study as the offset variable, and the study phase indicator (prestudy, ravulizumab, or placebo) as the explanatory factor.
All analyses were performed using Statistical Analysis Software Version 9.4 (SAS, SAS Institute, Cary, NC, USA).
3 Results
3.1 Patients
Of 175 patients (ravulizumab, n = 86; placebo, n = 89) enrolled in the RCP, 162 completed the RCP and 161 entered the OLE (OLE analysis set; ravulizumab-ravulizumab, n = 78; placebo-ravulizumab, n = 83; Figure 1). Among these, 60/78 patients in the ravulizumab-ravulizumab group and 63/83 in the placebo-ravulizumab group completed the OLE. Among all ravulizumab-treated patients, median (range) study follow-up was 851 (40.0, 1281.0) days in the ravulizumab-ravulizumab group and 888.0 (269.0, 1332.0) days in the placebo-ravulizumab group. Median (range) ravulizumab treatment duration was 820.5 (14.0, 1265.0) days from RCP baseline in the ravulizumab-ravulizumab group and 685.0 (63.0, 1135.0) days from OLE baseline in the placebo-ravulizumab group (Figure S2).
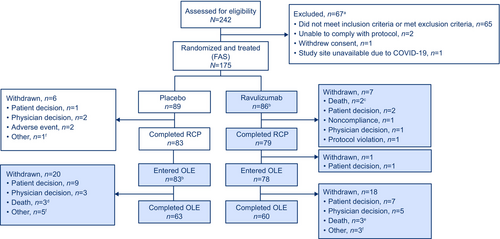
Baseline demographic and clinical characteristics were comparable across treatment groups for patients who entered the OLE (Table 1). Mean ± SD age at first infusion of study drug was 55.9 ± 15.2 years, 82/161 (50.9%) patients were female, and 118/161 (73.3%) were White. Mean ± SD baseline MG-ADL and QMG total scores were 9.0 ± 2.4 and 14.5 ± 5.2, respectively.
| Characteristics | Ravulizumab-ravulizumabb (n = 78) | Placebo-ravulizumabc (n = 83) | All patients (N = 161) |
|---|---|---|---|
| Female, n (%) | 40 (51.3) | 42 (50.6) | 82 (50.9) |
| Age, years, mean ± SD | 58.2 ± 13.6 | 53.6 ± 16.4 | 55.9 ± 15.2 |
| Race, n (%) | |||
| Asian | 13 (16.7) | 14 (16.9) | 27 (16.8) |
| Black or African American | 2 (2.6) | 4 (4.8) | 6 (3.7) |
| White | 61 (78.2) | 57 (68.7) | 118 (73.3) |
| Other/unknown/not reported | 2 (2.6) | 8 (9.6) | 10 (6.2) |
| MGFA clinical classification, n (%) | |||
| Class IIa/b | 36 (46.2) | 35 (42.2) | 71 (44.1) |
| Class IIIa/b | 37 (47.4) | 43 (51.8) | 80 (49.7) |
| Class IVa/b | 5 (6.4) | 5 (6.0) | 10 (6.2) |
| MG-ADL total score, mean ± SD | 9.2 ± 2.6 | 8.9 ± 2.2 | 9.0 ± 2.4 |
| QMG total score, mean ± SD | 14.8 ± 5.2 | 14.3 ± 5.2 | 14.5 ± 5.2 |
- Abbreviations: MG-ADL, Myasthenia Gravis Activities of Daily Living; MGFA, Myasthenia Gravis Foundation of America; OLE, open-label extension; QMG, Quantitative Myasthenia Gravis; RCP, randomized controlled period.
- a Table reprinted with permission under the Creative Commons Attribution 4.0 International (CC BY 4.0) license [14].
- b Patients treated with ravulizumab during both the RCP and OLE.
- c Patients treated with placebo during the RCP and ravulizumab during the OLE.
3.2 Long-Term Efficacy
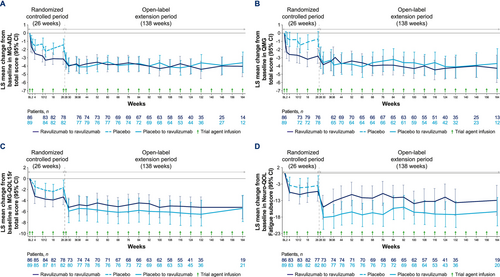
Ravulizumab was associated with sustained, long-term improvements in MG-ADL total scores (Figure 2A). In the ravulizumab-ravulizumab group, LS mean (95% CI) change from RCP baseline at week 164 was −4.0 (−5.3, −2.8; p < 0.0001) and from OLE baseline was −0.3 (−1.5, 0.8; p = 0.5772; Figure S3A). In the placebo-ravulizumab group, LS mean (95% CI) MG-ADL total score rapidly improved from OLE baseline to OLE week 2 (−1.8 [−2.6, −1.0]; p < 0.0001) and was sustained through 138 weeks of treatment (−2.1 [−3.3, −0.9]; p = 0.0005). Change from RCP baseline to week 28 (2 weeks after first/loading dose of ravulizumab) in this group was −3.3 (−4.2, −2.4; p < 0.0001) and from RCP baseline to week 164 (138 weeks of treatment) was −3.6 (−4.8, −2.3; p < 0.0001).
Extending previously reported MG-ADL responder results [14], high cumulative proportions of patients were responders at any time by week 164. Nine patients in the placebo group who achieved MSE during the RCP were excluded from the MSE analysis. Of the other 160 patients (ravulizumab-ravulizumab, 86; placebo-ravulizumab, 74), 141 (88.1%) achieved MCID (≥ 2-point improvement from baseline in MG-ADL total score) at any point during the study, and 128 (80.0%) achieved a ≥ 3-point improvement in MG-ADL total score. Among those achieving MCID, 59 (41.8%) patients achieved MSE. Median (range) percentage of time in MSE since first achieving MSE was 53.4% (1.4%, 100.0%). Thirty-two (54.2%) patients sustained MSE for > 50% of their remaining time in the study, and 20 (33.9%) sustained MSE for > 75% of their time (Table 2). Baseline (median [range]) MG-ADL scores were lower among patients who achieved MSE (8.0 [6.0, 15.0]) versus those who did not (9.0 [6.0, 24.0]; p = 0.0013).
| Ravulizumab-ravulizumab (n = 33) | Placebo-ravulizumabb (n = 26) | All patients (N = 59) | |
|---|---|---|---|
| Mean ± SD | 56.6 ± 33.6 | 54.1 ± 33.6 | 55.5 ± 33.3 |
| Median (min, max) | 55.1 (1.4, 100.0) | 49.9 (7.9, 100.0) | 53.4 (1.4, 100.0) |
| > 50%, n (%) | 19 (57.6) | 13 (50.0) | 32 (54.2) |
| > 50%–75%, n (%) | 6 (18.2) | 6 (23.1) | 12 (20.3) |
| > 75%, n (%) | 13 (39.4) | 7 (26.9) | 20 (33.9) |
- Abbreviations: MSE, minimal symptom expression; OLE, open-label extension; RCP, randomized controlled period.
- a Percent of time in MSE from first MSE was calculated as (total cumulative MSE duration/time from first MSE until end of study) × 100.
- b Placebo-ravulizumab patients who achieved MSE during the RCP were excluded.
Among patients who achieved a ≥ 2-point improvement from baseline in MG-ADL total score, 71 (77.2%) sustained a ≥ 2-point improvement for > 75% of their remaining time in the study after first response. Similarly, 68 (67.3%) of patients who achieved a ≥ 3-point improvement from baseline sustained this status for > 75% of their remaining time. Patients in the placebo arm who had the corresponding response during the RCP were excluded.
QMG total score improvements in the ravulizumab-ravulizumab group were sustained through week 164 (LS mean [95% CI] change from RCP baseline at week 164, −4.3 [−6.0, −2.7]; p < 0.0001; Figure 2B; and from OLE baseline at week 164, −0.9 [−2.3, 0.6]; p = 0.2534; Figure S3B). In the placebo-ravulizumab group, LS mean (95% CI) change from OLE baseline to OLE week 2 was −2.2 [−3.2, −1.2; p < 0.0001]. This was sustained through 138 weeks (−3.0 [−4.6, −1.4]; p = 0.0003). Change from RCP baseline to week 28 in this group was −3.0 (−4.3, −1.7; p < 0.0001) and to week 164 (138 weeks of treatment) was −3.7 (−5.5, −1.9; p < 0.0001).
Extending previously reported QMG responder results [14], high cumulative proportions of patients were responders by week 164 in the OLE, with 138/160 (86.3%) achieving a ≥ 3-point improvement (MCID), and 101 (63.1%) achieving a ≥ 5-point improvement.
Rapid improvements from RCP baseline in MG-QOL15r total (Figure S3C) and Neuro-QOL Fatigue subscale (Figure S3D) scores for the ravulizumab-ravulizumab group have been previously reported [4] and were observed from OLE baseline for the placebo-ravulizumab group. In both groups, score improvements in both measures were sustained through the OLE (Figure 2C,D).
3.3 Corticosteroid Use
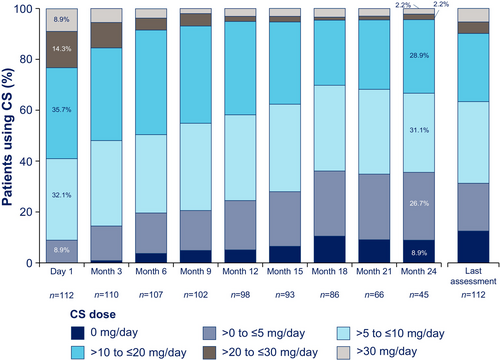
At the beginning of the OLE, 112 patients were receiving oral or enteral corticosteroids and the percentage of patients taking high-dose corticosteroids decreased from OLE baseline through the last assessment (Figure 3). One patient initiated corticosteroids during the OLE, and a small number of patients (n = 9; ravulizumab-ravulizumab, n = 4; placebo-ravulizumab, n = 5) had an increase in daily corticosteroid dose (from mean [SD; range] of 11.9 [8.9; 0.0, 30.0] mg/day at OLE start to 23.6 [13.9; 7.5, 45.0] mg/day at last reported OLE dose). Among patients taking corticosteroids at any point during the OLE (n = 113), the mean ± SD daily dose decreased from 17.5 ± 11.9 mg/day at OLE start to 11.7 ± 10.9 mg/day at last reported dose, a decrease of approximately 33% (Figure S4). The proportion of patients taking > 10 mg/day decreased by more than one-third during the OLE (from 66/113 [58.4%] at first reported dose to 42/113 [37.2%] at last reported dose). Correspondingly, as patients shifted from higher to lower dose groups, the proportions taking ≤ 5 mg/day and ≤ 10 mg/day increased from 11/113 (9.7%) and 47/113 (41.6%) at first reported dose, respectively, to 35/113 (31.0%) and 71/113 (62.8%) at last reported dose. By the last OLE assessment, 14/113 (12.4%) had completely discontinued corticosteroids.
3.4 Clinical Deterioration Events
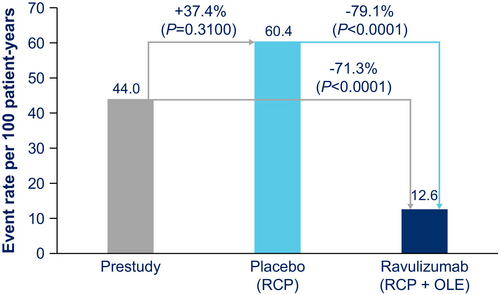
As previously reported for the RCP [4], 8/86 (9.3%) ravulizumab-treated patients experienced 10 clinical deterioration events and 15/89 (16.9%) placebo-treated patients experienced 26 clinical deterioration events. During the OLE, 10/78 (12.8%) patients in the ravulizumab-ravulizumab group experienced 18 events, and 9/83 (10.8%) in the placebo-ravulizumab group experienced 15 events. Of those during the OLE, three patients in the ravulizumab-ravulizumab group experienced four events of MG crisis (0 MG crisis events occurred in the placebo-ravulizumab group). Across both groups, high-dose corticosteroids, IV immunoglobulin, and/or plasma exchange were used for rescue therapy. The clinical deterioration event rate per 100 patient-years was 44.0 in the year before the study, 60.4 among patients receiving placebo during the RCP, and 12.6 in patients receiving ravulizumab in both the RCP and the OLE (Figure 4), corresponding to a 71.3% reduction in clinical deterioration event rate with ravulizumab versus prestudy values (p < 0.0001) and a 79.1% reduction for ravulizumab versus placebo (p < 0.0001).
3.5 Safety
During ravulizumab treatment across the RCP and OLE, 163/169 (96.4%) patients experienced ≥ 1 treatment-emergent AE, most of which were grade 1 (mild; 147/169 [87.0%]) or grade 2 (moderate; 112/169 [66.3%]) in severity (Table 3). The most common (occurring in ≥ 15% of patients) were COVID-19 (36.1%), headache (23.1%), and diarrhea (17.2%). AE rates did not increase appreciably with longer treatment duration [14]. Two COVID-19-related AEs (one of which was a serious adverse event [SAE]) led to study drug withdrawal for two patients in the ravulizumab-ravulizumab group during the OLE. Overall, 69 (40.8%) patients experienced 215 AEs related to ravulizumab.
| Events, n | Patients, n (%) | |
|---|---|---|
| Any AE | 1677b | 163 (96.4) |
| Related to trial agentc | 215 | 69 (40.8) |
| Any AE, by severityd | ||
| Grade 1 | 1061 | 147 (87.0) |
| Grade 2 | 433 | 112 (66.3) |
| Grade 3 | 162 | 67 (39.6) |
| Grade 4 | 13 | 12 (7.1) |
| Grade 5 | 8 | 8 (4.7) |
| Any SAE | 154 | 66 (39.1) |
| Related to trial agentc | 16 | 11 (6.5) |
| Deathe | 8 | 8 (4.7) |
| AEs reported in ≥ 10% of patientsf | ||
| COVID-19 | 76 | 61 (36.1) |
| Headache | 60 | 39 (23.1) |
| Diarrhea | 32 | 29 (17.2) |
| Arthralgia | 38 | 23 (13.6) |
| Nausea | 39 | 22 (13.0) |
| Back pain | 23 | 22 (13.0) |
| Urinary tract infection | 31 | 21 (12.4) |
| Nasopharyngitis | 31 | 20 (11.8) |
| Fatigue | 24 | 18 (10.7) |
| Dizziness | 24 | 17 (10.1) |
- Abbreviations: AE, adverse event; OLE, open-label extension; RCP, randomized controlled period; SAE, serious adverse event.
- a Includes data available for all patients who received ≥ 1 dose of ravulizumab in the RCP or OLE (ravulizumab treatment period).
- b During the ravulizumab treatment period: Ravulizumab-ravulizumab, 83/86 patients experienced 957 events (509.4 events per 100 patient-years); placebo-ravulizumab, 80/83 patients experienced 720 events (467.0 events per 100 patient-years).
- c As determined by the investigator.
- d Graded according to National Cancer Institute Common Terminology Criteria for Adverse Events version 4.03.
- e Two deaths occurred during the RCP (one due to COVID-19, one due to cerebral hemorrhage) and six during the OLE (three due to COVID-19, one due to drug toxicity with various agents, one due to dehydration, and one due to unknown cause); none were considered related to treatment.
- f Medical Dictionary for Regulatory Activities version 26.0 Preferred Terms.
Sixty-six of 169 (39.1%) ravulizumab-treated patients during the RCP or OLE experienced an SAE. Eleven (6.5%) patients experienced 16 SAEs related to ravulizumab. Treatment-related SAEs occurring among ravulizumab-treated patients during the RCP and up to the interim analysis have been previously reported [4, 14]. Across the RCP and OLE, ravulizumab-related SAEs included pyelonephritis, Escherichia coli bacteremia, urinary tract infection, and pancreatitis in one patient; pneumonia and mitral valve stenosis in one patient; gonococcal infection and infection (of unknown source) in one patient; cellulitis in two patients (1 episode each); and dysphagia, suppurative tendonitis, erysipelas, bacteremia related to Neisseria gonorrhoeae, MG (worsening of symptoms), and nonmeningococcal meningitis, one patient each.
Eight deaths, not related to study treatment, occurred among ravulizumab-treated patients. Of these, two occurred during the RCP (COVID-19, n = 1; cerebral hemorrhage, n = 1) and six during the OLE (COVID-19, n = 3; drug toxicity from combined effects of fentanyl, gabapentin, and alprazolam, n = 1; dehydration, n = 1; unknown cause, n = 1).
No cases of meningococcal infection occurred; as previously reported [14], a single case of meningitis with unknown etiology occurred in a patient in the placebo-ravulizumab group at approximately 63 weeks after study entry, but all cultures were negative for fungus, Mycobacterium tuberculosis, and bacteria.
No clinically important changes were observed in laboratory results, vital signs, or electrocardiogram abnormalities in either treatment group. Two patients in the placebo-ravulizumab group tested positive for antidrug antibodies at early termination/end-of-study visits, both indeterminate and nonneutralizing with low titer.
4 Discussion
These final results from the CHAMPION MG OLE extend interim analysis findings [14, 24], demonstrating clinically meaningful and durable efficacy and safety of ravulizumab for patients with AChR-Ab+ gMG. Improvements from baseline in MG-ADL, QMG, and MG-QOL15r total scores, and the NeuroQOL Fatigue subscale scores achieved by the ravulizumab-ravulizumab group during the RCP were sustained throughout the OLE. In the placebo-ravulizumab group, similar, rapid, statistically significant improvements versus OLE baseline were evident by week 2 (MG-ADL and QMG) or week 4 (MG-QOL15r and NeuroQOL Fatigue subscale). Across both groups, most patients who achieved a clinically meaningful MG-ADL response sustained that response for > 75% of their remaining time in the study. Further, 41.8% of patients who had ≥ 2-point improvement in MG-ADL total score achieved MSE, and 54.2% sustained MSE for > 50% of the study. As previously reported, 43.0% of patients with a ≥ 3-point improvement in MG-ADL total score achieved MSE [24].
During the RCP, approximately half as many patients receiving ravulizumab experienced clinical deterioration events (9.3%) versus placebo (16.9%), with approximately 60% fewer events with ravulizumab (10 events) than placebo (26 events) [4]. Ravulizumab was associated with a significantly lower exposure-adjusted clinical deterioration event rate than placebo or during the year before enrollment in CHAMPION MG, and few MG crises occurred during the OLE. Reduction of MG crises is an important potential benefit of ravulizumab treatment, considering the substantial clinical and socioeconomic burden of these events [25-27].
The magnitudes of improvement in MG-ADL and QMG total scores (−2.1 and − 3.0, respectively) in the placebo-ravulizumab group after ravulizumab initiation at OLE baseline were similar to those in the ravulizumab group during the RCP (−3.1 and − 2.8) [4]. Although mean patient score improvements after ravulizumab initiation were clinically significant in both groups, not all patients achieved MSE, suggesting that some may benefit from additional treatment(s) with different mechanisms of action.
Ravulizumab was associated with a reduction in mean corticosteroid dosage. The proportion of patients taking > 10 mg/day decreased by more than one-third during the OLE as patients shifted from higher doses to ≤ 10 mg/day by the end of the study. The proportion taking ≤ 5 mg/day increased from 11/113 (9.7%) to 35/113 (31.0%), and 12.4% of patients discontinued corticosteroid use completely.
Long-term corticosteroid use, particularly at higher doses, is associated with musculoskeletal, endocrine, gastrointestinal, cardiovascular, dermatologic, and neuropsychiatric adverse effects [28]. Treatment guidelines generally recommend an eventual maintenance dose of oral prednisolone of ≤ 10 mg/day (or equivalent) if corticosteroids are needed to achieve the treatment goal of disease control with minimal manifestations, and the use of steroid-sparing options such as nonsteroidal immunosuppressants is also encouraged [29, 30]. As demonstrated in previous studies, complement inhibition offers an additional mechanism of action that may be useful as steroid-sparing treatment [30].
Changes in concomitant corticosteroid use were also permitted in the OLE of the REGAIN eculizumab study in patients with AChR-Ab+ gMG, and similar to our findings, clinical improvements were maintained among patients who decreased or stopped immunosuppressant treatment [31]. Additionally, evidence from clinical practice has consistently shown steroid-sparing effects with eculizumab and ravulizumab. In a survey of patients with refractory AChR-Ab+ gMG (N = 36), prednisolone dose was reduced from 15.1 mg/day to 11.0 mg/day with eculizumab [32], and registry data from patients with AChR-Ab+ gMG receiving eculizumab and ravulizumab have also shown reductions in both daily corticosteroid dose and number of patients receiving high-dose corticosteroids (> 10 mg/day) following treatment [33]. Similarly, analysis of US claims data has shown a consistent reduction of prednisone usage over time, including statistically significant reductions in average daily prednisone dose after 12 months of eculizumab or ravulizumab in adults with gMG [13].
Safety data were consistent with the known safety profiles of ravulizumab and eculizumab [4, 13-15, 34, 35]. No new safety concerns were identified, and no cases of meningococcal infection were observed. During the RCP, AE frequencies were similar between ravulizumab-treated patients and placebo [4].
Strengths of this analysis include the follow-up duration of > 3 years and the diversity of patients with respect to sex, race, and disease severity. Limitations include the lack of placebo control during the OLE; however, the original RCP treatment assignment remained blinded to the investigator and patient throughout the OLE. Additionally, procedural changes in the collection of QMG scores during the COVID-19 pandemic should be considered because investigator assessment could not be collected over the phone. Finally, patients requiring rescue treatment (n = 17) were not removed from the analysis, which could have had a negative impact on efficacy outcomes.
5 Conclusions
These findings support and extend the previously reported sustained clinical efficacy and long-term safety of ravulizumab administered every 8 weeks in adults with AChR-Ab+ gMG. Furthermore, reductions in corticosteroid use observed with ravulizumab strongly support its role as a steroid-sparing therapy for patients with AChR-Ab+ gMG.
Author Contributions
Tuan H. Vu: conceptualization, investigation, methodology, writing – review and editing. Renato Mantegazza: writing – review and editing, investigation. Djillali Annane: investigation, writing – review and editing. Masahisa Katsuno: investigation, writing – review and editing. Andreas Meisel: investigation, writing – review and editing. Michael W. Nicolle: investigation, writing – review and editing. Vera Bril: investigation, writing – review and editing. Rasha Aguzzi: formal analysis, writing – review and editing. Glen Frick: formal analysis, writing – review and editing. James F. Howard Jr: conceptualization, investigation, methodology, writing – review and editing.
Acknowledgments
Medical writing and editorial support were provided by Allyson Lehrman, DPM, and Dena McWain of Apollo Medical Communications, part of Helios Global Group, and funded by Alexion, AstraZeneca Rare Disease.
Disclosure
Role of the Sponsor: The funders had a role in the design and conduct of the study; collection, management, and analysis of the data; and the review of the manuscript. The decision to submit the manuscript for publication was made by the authors.
Conflicts of Interest
T.H.V. has received research or grant support related to MG from Alexion, AstraZeneca Rare Disease, Amgen, argenx, Cartesians, COUR, Dianthus, Immunovant, Johnson & Johnson, NMD Pharma, Regeneron, and UCB; and consulted and/or served on the speakers bureau for Alexion/AstraZeneca Rare Disease, Amgen, argenx, CSL Behring, Dianthus, ImmunAbs, Johnson & Johnson, and UCB. R.M. has received funding for travel from Alexion, AstraZeneca Rare Disease, argenx, BioMarin, Catalyst, Regeneron, Sanofi, and UCB; and participated in meetings and advisory boards for Alexion, AstraZeneca Rare Disease, argenx, BioMarin, Catalyst, Regeneron, Sanofi, and UCB. D.A. has received research support (paid to institution) from Alexion, AstraZeneca Rare Disease; and serves on the CHAMPION MG study steering committee. M.K. has received honoraria from Alexion, AstraZeneca Rare Disease. A.M. served as an investigator, advisor, consultant; and/or speaker and has received grants (paid to institution) and honoraria from Alexion, argenx, Axunio, Grifols, Hormosan, Janssen, Merck, Novartis, Octapharma, and UCB; and serves as chairman of the medical advisory board of the German Myasthenia Gravis Society. M.W.N. has served as a consultant to Alexion, AstraZeneca Rare Disease, argenx, Roche, and UCB; and is involved in MG clinical trials with Alexion, AstraZeneca Rare Disease, argenx, Dianthus, Merck, Novartis, Regeneron, and UCB. V.B. has served as a consultant for Akcea, Alexion, AstraZeneca Rare Disease, Alnylam, argenx, CSL, Grifols, Immunovant, Ionis, Janssen, Momenta (now Janssen), Novo Nordisk, Octapharma, Pfizer, Powell Mansfield, Roche, Sanofi, Takeda, and UCB; and has received research support from Akcea, Alexion, AstraZeneca Rare Disease, argenx, CSL, Grifols, Immunovant, Ionis, Momenta (now Janssen), Octapharma, Takeda, and UCB. J.F.H. has received research support (paid to institution) from Ad Scientiam, Alexion AstraZeneca Rare Disease, Amgen, argenx, Cartesian Therapeutics, Centers for Disease Control and Prevention, MGFA, Muscular Dystrophy Association, NIH, NMD Pharma, PCORI, and UCB Pharma; honoraria/consulting fees from AcademicCME, Alexion, AstraZeneca Rare Disease, Biohaven Ltd., Biologix Pharma, CheckRare CME, CoreEvitas, Curie.Bio, Medscape CME, Merck EMD Serono, NMD Pharma, Novartis Pharma, PeerView CME, Physicians' Education Resource (PER) CME, PlatformQ CME, Regeneron Pharmaceuticals, Sanofi US, TG Therapeutics, UCB Pharma, and Zai Lab; and nonfinancial support from Alexion, AstraZeneca Rare Disease, argenx, Biohaven Ltd., Cartesian Therapeutics, Toleranzia AB, UCB Pharma, and Zai Lab. R.A. and G.F. are employees of Alexion, AstraZeneca Rare Disease, and hold stock or stock options in AstraZeneca.
Open Research
Data Availability Statement
Alexion, AstraZeneca Rare Disease will consider requests for disclosure of clinical study participant-level data, provided that participant privacy is assured through methods like data de-identification, pseudonymization, or anonymization (as required by applicable law), and if such disclosure was included in the relevant study informed consent form or similar documentation. Qualified academic investigators may request participant-level clinical data and supporting documents (statistical analysis plan and protocol) pertaining to Alexion-sponsored studies. Further details regarding data availability and instructions for requesting information are available in the Alexion Clinical Trials Disclosure and Transparency Policy at https://www.alexionclinicaltrialtransparency.com/data-requests/.




