Brain-Derived Tau as an Outcome Marker in Guillain-Barré Syndrome: A Retrospective Cohort Study
Funding: This study was supported by grants from the Edit Jacobson Foundation, the Rune and Ulla Amlövs Foundation for Neurological Research (#2022-358 and #2023-405), the Gothenburg Society of Medicine (#GLS972383), the Göteborg Foundation for Neurological Research (#2023-00015), and NEURO Sweden.
ABSTRACT
Background and Objectives
Biomarkers for predicting disease severity and outcome in Guillain-Barré syndrome (GBS) are scarce. We aimed to determine if brain-derived tau in serum (sBD-tau) and cerebrospinal fluid (CSF BD-tau) are associated with long-term outcome and disease severity in GBS.
Methods
In this retrospective study of 100 GBS patients, we measured sBD-tau and CSF BD-tau at diagnosis. Outcome was defined as GBS disability scale (GBSDS) > 2 and overall neuropathy limitation scale (ONLS) at 12 months, disease severity as respiratory support and ONLS at nadir. BD-tau levels were compared between groups and correlated with ONLS scores. Regression analyses and receiver operator characteristic curve analyses were performed for GBSDS > 2 at 12 months.
Results
BD-tau levels were higher for GBSDS > 2 at 12 months in serum and CSF. Odds ratio for sBD-tau was 1.9 (95% CI 1.08–3.2, p = 0.03) and for CSF BD-tau was 5.9 (95% CI 1.4–25, p = 0.02). Area under curve for sBD-tau was 0.75 (95% CI 0.57–0.9, p < 0.001) and for CSF BD-tau was 0.78 (95% CI 0.65–0.9, p = 0.001). ONLS at 12 months correlated with sBD-tau (ρ = 0.34 [95% CI 0.12–0.53], p = 0.002) and CSF BD-tau (ρ = 0.33 [95% CI 0.08–0.54], p = 0.01). Statistically significant difference in BD-tau levels was not seen for respiratory support or ONLS at nadir.
Conclusion
BD-tau at GBS onset is associated with long-term outcome but not disease severity. Because BD-tau is essentially a CNS biomarker, our results suggest that CNS involvement influences recovery.
1 Introduction
Guillain-Barré syndrome (GBS) is considered a postinfectious autoimmune disease that typically affects the peripheral nervous system (PNS). However, the central nervous system (CNS) can be involved to a varying degree. CNS involvement is particularly evident in the Miller-Fisher (MF) and Bickerstaff encephalitis variants [1, 2]. The inflammatory process in GBS can affect the intrathecally located nerve roots and extend as proximally as the anterior horn cell of the spinal cord [3]. Additionally, evidence from cases of GBS associated with hyperreflexia suggests affection of the spinal interneurons or the corticospinal tract [4]. GBS may therefore be considered a spectrum disorder with varying degrees of PNS and CNS involvement.
Disease severity and outcome in GBS are highly variable, and there is an unmet need for reliable predictive biomarkers. In the past two decades, associations between neurofilament light (NfL), a biomarker of neuroaxonal injury, and outcome in GBS have been reported [5-8]. Whether NfL levels measured in the blood of GBS patients originate primarily from the CNS or the PNS remain to be determined [8, 9]. Impairment of the blood-CSF barrier (BCSFB) has been linked to progression and clinical severity and may influence the distribution of biomarkers between the CSF/CNS compartment and systemically [10].
Previous studies have reported an association between GBS outcome and total tau (t-tau) in CSF but not in blood [11-13]. The isoforms of tau proteins from the CNS differ from those originating from the PNS. With the development of an ultrasensitive assay that uses anti-tau antibodies that exclusively bind to CNS-specific tau proteins, it is possible to measure brain-derived tau (BD-tau) in the blood and investigate a possible CNS involvement in GBS [14].
The aim of the study was to determine if levels of BD-tau in serum and CSF are associated with disease severity and long-term outcome in GBS.
2 Methods
2.1 Study Design, Cohort, and Outcome Measures
This is a retrospective observational cohort study conducted in the Västra Götaland region in Sweden. We searched the local and national patient registers for patients who received the ICD-10 diagnostic code for GBS (G61.0) between 2011 and 2021 in the study area. The results were matched with a clinical database at the regional microbiology laboratory, Sahlgrenska University Hospital, to identify those with stored serum and CSF samples in the laboratory's biobank. We reviewed medical records to confirm the diagnosis, and only those who fulfilled the Brighton diagnostic criteria for GBS were included [15]. Patients with coexisting neurological diseases were excluded as it might affect BD-tau concentrations and functional outcomes.
Nerve conduction studies (NCS) were performed at diagnosis and interpreted by board-certified neurophysiologists. Results were classified as compatible with demyelinating subtype (AIDP), axonal subtype (AMAN/AMSAN), MF variant, or normal. Those where NCS could not distinguish between demyelinating and axonal subtypes were classified as equivocal. Clinical subtype was defined as classic, paraparetic, Miller-Fisher syndrome (MFS), or pharyngeal-cervical-brachial variant (PCB) using the Wakerly criteria [16].
Disability at diagnosis, nadir, and after 12 months was assessed based on information from medical records and graded on the GBS disability scale [17] and overall neuropathy limitation scale (ONLS) [18]. Disease severity was measured as the need for respiratory support and ONLS at nadir. Outcome was defined as inability to walk unaided (GBS disability scale [GBSDS] > 2) and ONLS score 12 months after diagnosis.
2.2 Sample Collection and Biomarker Analyses
Serum and CSF samples were collected for serological analyses during the diagnostic workup and thereafter stored for various lengths of time at −20°C until study analyses. BD-tau was measured on the Simoa HD-X platform. CSF and serum BD-tau were measured according to the previously published method, using TauJ5.H3 (Bioventix) as the capture antibody and Tau12 (BioLegend, #SIG-39416) as the partner antibody [14, 19]. A dilution factor of four was used for the serum samples, whereas a dilution factor of 30 was used for measurements in CSF.
The analyses of serum and CSF NfL were performed using the Simoa NEUROLOGY NF-light Advantage Kit (Quanterix, Billerica, MA, USA). Albumin levels were measured by immunonephelometry on a Beckman Immage Immunochemistry system (Beckman Instruments, Beckman Coulter, Brea, CA, USA) and the CSF/serum albumin quotient (Qalb) calculated.
2.3 Statistical Analysis
Statistical analyses were conducted in IBM SPSS Statistics (version 29.0.2.0) and GraphPad Prism 10 (version 10.2.2). Variables that were not normally distributed, including BD-tau levels, are expressed as median and interquartile range (IQR).
Nonparametric tests, Mann–Whitney U and Kruskal–Wallis, were used to compare groups of different clinical characteristics and outcomes. Correlation between levels of different biomarkers and between BD-tau levels and ONLS was assessed using the Spearman rank correlation test and expressed as ρ (Spearman rho) and 95% confidence interval (CI). As storage time affects BD-tau levels in CSF, we carried out multiple linear regression analyses between CSF BD-tau and the other biomarkers, with storage time in years as a covariate.
According to stratification by age group < 30, 30–64, and ≥ 65 years, the oldest group had a higher proportion of patients with unfavorable outcomes compared with the other two groups. We performed simple logistic regression analyses for BD-tau, age > 65, and axonal subtype. To account for the confounding effect of older age on outcome, we performed logistic regression analysis (multivariate binary and ordinal were appropriate) on logarithmically transformed BD-tau using the forward likelihood ratio procedure. We included storage time in the regression analyses for BD-tau in CSF. Axonal subtype was included in the multiple regression analysis for sBD-tau.
Receiver operator characteristics (ROC) curves were carried out on logarithmically transformed BD-tau, logarithmically transformed sNfL levels, and Qalb to determine the area under the curve (AUC) for GBSDS > 2 at 12 months. Statistical significance was defined as p < 0.05.
2.4 Standard Protocol Approvals, Registrations, and Patient Consents
Informed consent was not required as all data are presented on a group level and anonymized. According to the local clinical routine, samples gathered for microbiological analyses are stored in a biobank for 10 years in case of further analysis. The patient is given the opportunity to withdraw their consent at the time of sampling. The study was approved by the Swedish Ethical Review Authority (Dnr 2020-02558 and 2021-04229).
3 Results
3.1 BD-Tau Levels According to Clinical Characteristics and Correlation With Other Biomarkers
A total of 100 patients were included. The cohorts' demographics and clinical characteristics are shown in Table 1.
| N = 100 | |
|---|---|
| Age, mean (range) | 52 (3–82) years |
| Sex, N (%) | |
| Male | 68 |
| Female | 32 |
| Brighton criteria level, N | |
| 1 | 45 |
| 2 | 37 |
| 3 | 18 |
| Clinical subtype, N | |
| Classic | 83 |
| Paraparetic | 8 |
| PCB | 2 |
| MFS | 7 |
| Neurophysiological subtype, N | |
| AIDP | 61 |
| AMAN/AMSAN | 9 |
| MFS | 6 |
| Normal | 12 |
| Equivocal | 4 |
| Not done | 8 |
| Treatment, N | |
| None | 3 |
| IVIG | 76 |
| Plasmapheresis | 6 |
| IVIG + Plasmapheresis | 8 |
| IVIG > 1 | 7 |
- Abbreviations: AIDP, acute inflammatory demyelinating polyneuropathy; AMAN/AMSAN, acute motoric/sensory axonal neuropathy; IVIG, intravenous immunoglobulins; MFS, Miller-Fisher syndrome; PCB, pharyngeal-cervical-brachial variant.
Sixty-six patients had paired serum and CSF samples, 28 had only serum samples, and six had only CSF samples. The median time from symptom onset to sampling was 6 days (IQR 3–14 days). BD-tau levels according to different clinical characteristics are shown in Table 2 and Figure S1.
| sBD-tau, pg/mL | CSF BD-tau, pg/mL | |
|---|---|---|
| Age, ρ (95% CI) |
0.3 (0.09 to 0.5) p = 0.004 |
0.3 (0.05 to 0.5) p = 0.015 |
| Days from debut to sampling, ρ (95% CI) |
0.2 (−0.3 to 0.4) p = 0.07 |
0.06 (− 0.19 to 0.3) p = 0.6 |
| Storage time in years, ρ (95% CI) |
ρ = 0.02 (−0.2 to 0.19) p = 0.8 |
−0.43 (−0.6 to −0.2) p < 0.001 |
| GBSDS at diagnosis, Med (IQR) | ||
| 1 | 0.22 (0.19 to 0.27) | 5.7 (4.7 to 11) |
| 2 | 0.22 (0.17 to 0.29) | 7.4 (5.0 to 12) |
| 3 | 0.28 (0.21 to 0.39) | 11 (6.7 to 17) |
| 4 | 0.39 (0.25 to 3.0) | 13 (7.0 to 24) |
| p = 0.009a | p = 0.03b | |
| Clinical subtype, Med (IQR) | ||
| Classic | 0.26 (0.19 to 0.32) | 8.3 (5.1 to 14) |
| Paraparetic | 0.22 (0.19 to 0.34) | 11 (6.8 to 16) |
| PCB | 0.22 (0.16 to 0.28) | 11 (5.7 to 16) |
| MFS | 0.26 (0.22 to 0.42) | 6.8 (4.9 to 17) |
| p = 0.9 | p = 0.8 | |
| Neurophysiological subtype, Med (IQR) | ||
| AIDP | 0.25 (0.19 to 0.29) | 8 (5 to 12) |
| AMAN/AMSAN | 0.37 (0.21 to 0.60) | 22 (8.9 to 31) |
| MFS | 0.26 (0.18 to 0.35) | 7.3 (6.8 to 17) |
| Normal | 0.20 (0.17 to 0.26) | 6.8 (5.7 to 8.7) |
| Equivocal | 0.51 (0.26 to 20) | 4.5 (4.2 to 24) |
| p = 0.13 | p = 0.13 | |
| Preceding infection, Med (IQR) | ||
| Respiratory | 0.25 (0.19 to 0.29) | 7.3 (5.2 to 13) |
| Gastrointestinal | 0.21 (0.18 to 0.28) | 9.9 (4.7 to 18) |
| p = 0.5 | p = 0.4 | |
- Abbreviations: AIDP, acute inflammatory demyelinating polyneuropathy; AMAN/AMSAN, acute motor/sensory axonal neuropathy; GBSDS, Guillain-Barré syndrome disability scale; MFS, Miller-Fisher syndrome; PCB, pharyngeal-cervical-brachial variant.
- a Age-adjusted p value 0.005.
- b Age-adjusted p value 0.007.
We showed a clear correlation between storage time and CSF BD-tau levels, whereas no such correlation was found for sBD-tau.
3.2 BD-Tau Levels and Outcome
BD-tau levels at diagnosis were significantly higher for GBSDS > 2 compared to GBSDS ≤ 2 at 12 months in both serum (median 0.49 pg/mL [IQR 0.25–1.5 pg/mL] vs. 0.22 [0.18–0.28], p = 0.004) and CSF (median 14 pg/mL [IQR 7.6–32 pg/mL] vs. 7.3 pg/mL [IQR 5–12.5 pg/mL], p = 0.02) (Figure 1a,b).
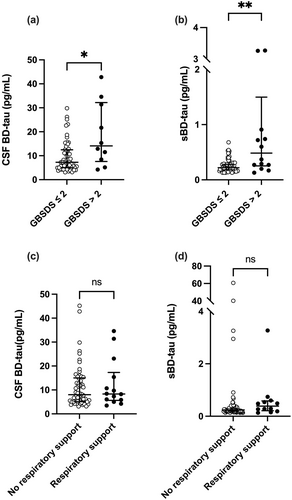
ONLS at 12 months correlated with both sBD-tau (ρ = 0.34 [95% CI 0.12–0.53], p = 0.002) and CSF BD-tau (ρ = 0.33 [95% CI 0.08–0.54], p = 0.01) levels.
No statistically significant difference was seen in BD-tau levels for those who needed respiratory support in serum (median 0.38 pg/mL [IQR 0.21–0.59 pg/mL] vs. 0.24 pg/mL [IQR 0.19–0.30 pg/mL], p = 0.07) or CSF (median 8.3 pg/mL [IQR 5.7–17 pg/mL] vs. 8 pg/mL [IQR 5.1–15 pg/mL], p = 0.8) (Figure 1c,d).
ONLS at nadir correlated with sBD-tau levels at diagnosis (ρ = 0.32 [95% CI 0.12–0.5], p = 0.002) but not CSF BD-tau levels (ρ = 0.09 [95% CI −0.12 to 0.3], p = 0.4).
Results from multiple regression analyses for outcome at 12 months and ONLS at nadir are shown in Table 3 and Table S1.
| Outcome | sBD-taua | CSF BD-taub | ||
|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | |
| GBSDS > 2 at 12 months | 1.9 (1.1–4.0)c | 0.03 | 5.9 (1.4–25) | 0.02 |
| ONLS at 12 months | 1.95 (1.15–3.3) | 0.01 | 2.6 (1.2–2.8) | 0.046 |
| ONLS at nadir | 1.5 (0.99–2.1) | 0.06 | — | — |
- Note: Calculations were carried out on logarithmically transformed BD-tau levels.
- Abbreviations: CSF BD-tau, brain-derived tau in cerebrospinal fluid; GBSDS, Guillain-Barré syndrome disability score; ONLS, overall neuropathy limitation scale; sBD-tau, brain-derived tau in serum.
- a Adjusted for age > 65 years.
- b Adjusted for age > 65 years and storage time in years.
- c Adjusted for axonal subtype.
ROC analysis for GBSDS > 2 at 12 months for BD-tau in serum and CSF at diagnosis, the AUC for CSF BD-tau adjusted for storage time was 0.78 (95% CI 0.58–0.97, p = 0.006), and AUC for sBD-tau 0.75 (95% CI 0.57–0.9, p < 0.001). For comparison, ROC analysis was carried out for sNfL, AUC 0.78 (95% CI 0.65–0.9, p = 0.001) and Qalb, AUC 0.64 (95% CI 0.44–0.84, p = 0.1). ROC curves for BD-tau and sNfL are shown in Figure 2.
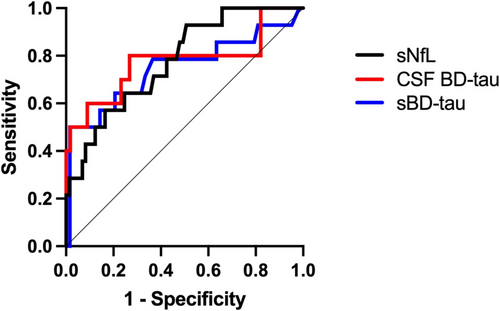
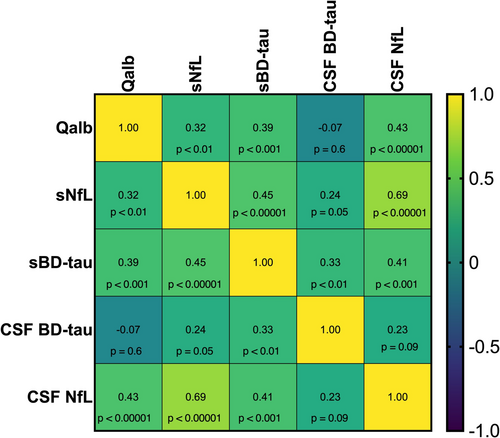
3.3 Correlation With Other Biomarkers
We performed correlation analysis between BD-tau and NfL in serum and CSF and Qalb (Figure 3). BD-tau in serum (sBD-tau) correlated with all the above-mentioned biomarkers, but CSF BD-tau did not. CSF and serum BD-tau correlated, ρ = 0.33 (95% CI 0.09–0.54, p < 0.01). Linear regression analyses accounting for the confounding effects of storage time on CSF BD-tau levels showed stronger associations with the other biomarkers, statistically significant for sBD-tau and sNFL but not CSF-NfL or Qalb (Table S2).
4 Discussion
Our results show an association between serum and CSF levels of BD-tau and long-term outcome in GBS. Higher BD-tau levels at diagnosis were associated with worse functional status after 12 months, independent of age, and show similar ability to discriminate between favorable and unfavorable outcomes as sNfL and superior to Qalb.
Higher BD-tau levels were associated with disease severity at diagnosis but not at nadir and not with the need for respiratory support. The lack of association between BD-tau and the need for respiratory support could be that respiratory failure in GBS is related to peripheral phrenic nerve affection rather than CNS engagement. This is to some extent supported by previous studies that show associations between phrenic nerve amplitude and latency and the development of respiratory failure in GBS [20, 21].
We found no significant difference in BD-tau levels between neurophysiological or clinical subtypes. A previous study reported higher CSF t-tau levels in the axonal subtype and positive anti-GM1 antibodies [11, 12]. Interestingly, exaggerated deep tendon reflexes, a CNS symptom, have been associated with the AMAN subtype and anti-GM1 antibodies [4]. In our study, the axonal subtype had higher BD-tau levels than the other subtypes. The difference did not reach statistical significance, which could be due to the low number of patients with the axonal subtype included in the study. The MF variant is more frequently associated with CNS symptoms than the other subtypes. However, we did not see higher BD-tau levels in this group, which might indicate that BD-tau in GBS primarily originates from the proximal nerve roots.
Peripherally released tau isoforms, called high molecular weight tau (HMW tau), can be distinguished from centrally derived isoforms by an additional large peptide transcribed from an exon between exons 4 and 5 of the MAPT gene. HMW tau is also expressed in the CNS, principally in the parts that extend to the PNS. Tau proteins in the blood consist primarily of HMW tau, whereas in CSF, they are mainly of the lighter tau isoforms [22]. The method used to measure BD-tau is based on a monoclonal antibody that specifically binds to the junction between exons 4 and 5, only detecting the lighter tau proteins derived from the CNS. BD-tau levels have been shown to correlate in serum and CSF, whereas t-tau levels in the same population did not. Furthermore, CSF BD-tau and CSF t-tau correlated strongly [14].
BD-tau has been associated with outcomes in acute stroke and traumatic brain injury as well as a diagnostic biomarker for Alzheimer's disease [23-26]. To our knowledge, levels in GBS have not previously been published. There are, however, few studies on the association of t-tau with outcome in GBS. Our study supports the findings of two earlier studies where higher t-tau levels in CSF at diagnosis were associated with poorer outcomes [11, 12]. One study on both plasma and CSF t-tau levels did not show any association with functional outcomes [13]. The association of outcome with serum and CSF BD-tau and t-tau in CSF but not in blood suggests that the degree of CNS involvement is an important factor for disability and recovery in GBS.
We find that sBD-tau, NfL in serum and CSF, and Qalb correlate, and all are biomarkers for outcome in GBS [5-8]. It is currently unknown how BD-tau and NfL transfer from the CNS to the peripheral circulation. The correlation of Qalb with the serum biomarkers could indicate that they pass through the permeable BCSFB. As t-tau in plasma and CSF does not correlate with Qalb, our findings support BD-tau as a more selective CNS marker [13]. We find no correlation between CSF BD-tau and the other biomarkers and a weaker correlation with sBD-tau than previously reported [19]. This is explained by the effects of storage on CSF BD-tau but not sBD-tau.
Our results show that BD-tau is a robust biomarker in serum and is preserved over long periods at −20°C temperatures, but similar stability does not appear to exist for BD-tau in CSF. This finding supports a higher reliability for sBD-tau if the preanalytical storage is performed at 20°C temperatures for longer periods and emphasizes the practical utility of sBD-tau as a potential clinical biomarker in GBS and other conditions [19].
Our study has some limitations that might have impacted the results. We could not adjust sBD-tau levels for renal function as we did not have the information available for our cohort. BD-tau blood levels correlate with renal function but not CSF BD-tau [23]. Although we excluded patients with known neurological comorbidities, some subjects in our cohort might have subclinical or undiagnosed degenerative neurological disease that could contribute to BD-tau levels as well as functional outcomes.
The demographics of our cohort are comparable to epidemiological reports from North America and Europe [27]. Our results are therefore generalizable to GBS patients in North America and Europe that have access to the same quality of health care as in Sweden.
In conclusion, our findings show that BD-tau is associated with outcome in GBS. As BD-tau is predominantly CNS derived, our results indicate that the degree of CNS involvement in GBS is related to functional outcome and recovery.
Author Contributions
Brynhildur Hafsteinsdóttir: conceptualization, data curation, formal analysis, writing – review and editing, writing – original draft. Fernando Gonzalez-Ortiz: conceptualization, data curation, writing – review and editing. Nina Gleisner: data curation. Ellen Alpsten: data curation. Jan Lycke: conceptualization, writing – review and editing. Igal Rosenstein: formal analysis, writing – review and editing. Lenka Novakova: writing – review and editing. Kaj Blennow: conceptualization, writing – review and editing. Tomas Bergström: data curation, writing – review and editing. Henrik Zetterberg: conceptualization, writing – review and editing. Markus Axelsson: conceptualization, data curation, supervision, writing – review and editing.
Conflicts of Interest
K.B. has served as a consultant and at advisory boards for Abbvie, AC Immune, ALZPath, AriBio, BioArctic, Biogen, Eisai, Lilly, Moleac Pte. Ltd., Neurimmune, Novartis, Ono Pharma, Prothena, Roche Diagnostics, Sanofi, and Siemens Healthineers; has served at data monitoring committees for Julius Clinical and Novartis; has given lectures, produced educational materials, and participated in educational programs for AC Immune, Biogen, Celdara Medical, Eisai, and Roche Diagnostics; and is a cofounder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program, outside the work presented in this paper. H.Z. has served at scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZpath, Amylyx, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, LabCorp, Merry Life, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Quanterix, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave; has given lectures sponsored by Alzecure, BioArctic, Biogen, Cellectricon, Fujirebio, Lilly, Novo Nordisk, Roche, and WebMD; and is a cofounder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work). J.L. has received travel support and/or lecture honoraria and has served on scientific advisory boards for Amgen, Almirall, Biogen, Bristol Myers Squibb, Celgene, Genesis Pharma, Janssen, Merck, Novartis, Roche, Sanofi, and Sandoz; and has received unconditional research grants from Biogen and Novartis, and financial research support from Sanofi. L.N. has received lecture honoraria from Biogen, Novartis, Teva, Sanofi, and Merck, and has served on advisory boards for Merck, Janssen, and Sanofi. M.A. has received compensation for lectures and/or advisory boards from Biogen, Genzyme, and Novartis. I.R. has received compensation for lectures from Biogen, Novartis, and Sanofi, and has served on advisory boards for Sanofi. Other authors have nothing to disclose.
Open Research
Data Availability Statement
Anonymized data not published within this article will be made available upon reasonable request from any qualified investigator.




