Voxel-Based Lesion Symptom Mapping to Predict Poststroke Epilepsy After Mechanical Thrombectomy
Funding: The authors received no specific funding for this work.
ABSTRACT
Introduction
Poststroke epilepsy (PSE) is an important long-term complication after stroke. Data regarding predictors of PSE in patients with large-vessel occlusion stroke receiving mechanical thrombectomy (MT) are scarce. Voxel-based lesion symptom mapping on brain MRI might be a valuable tool in the risk prediction of PSE. This study aims to assess PSE risk after acute stroke treated with MT via voxel- and volumetric-based analyses.
Methods
In this bi-center study from two tertiary-care stroke centers, we included consecutive acute ischemic stroke patients who had received MT between 2011 and 2017, and had postinterventional brain MRI as well as long-term follow-up data available. Infarct volume and location were assessed on MRI. Following semiautomated lesion outlining and generation of binarized lesion masks, lesion symptom mapping was applied to identify relevant topographical lesion patterns in PSE.
Results
Of 348 analyzed patients, 97 cases had to be excluded due to insufficient image quality and inaccurate registration results. Finally, lesion maps from 251 patients (median age: 66, 45.4% women) were considered for lesion symptom mapping, including maps from 26 patients with PSE (10.4%). Mean infarct volume was higher in PSE patients (119.2 cm3 vs. 43.9 cm3, p < 0.0001). Lesion symptom mapping identified the orbitofrontal region, the operculum, and the temporal pole as brain regions associated with PSE.
Conclusion
Apart from infarct volume, lesion symptom mapping on postinterventional brain MRI identified specific brain regions associated with PSE after large vessel occlusion stroke. This information might be helpful for PSE risk stratification and follow-up care in this specific population.
1 Introduction
Epileptic seizures resemble a serious complication in ischemic stroke patients and are associated with increased mortality risk, worse functional recovery, and cognitive decline. They have detrimental effects on quality of life [1, 2] and can be divided into early and late seizures (≤ 7 versus > 7 days after stroke) [3]. Early seizures are considered a response to acute neuronal injury and are typically deemed provoked, whereas late seizures signify neuronal hyperexcitability and restructuring of impaired neural networks, meeting the criteria for poststroke epilepsy (PSE). While the risk of PSE in general ischemic stroke patients is estimated to be around 10% [2], PSE risk after mechanical thrombectomy (MT) ranges between 6% and 9% [4-6].
Various clinical predictors for PSE have been identified, such as stroke severity, large-artery atherosclerotic etiology, presence of early seizures [7], hyperglycemia [8], young age < 18 years [9], male sex as well as different vascular risk factors and diseases, such as myocardial infarction, peripheral vascular disease, hypertension, total serum cholesterol, and left ventricular hypertrophy [10]. However, only a limited number of studies have explored detailed information from brain imaging, suggesting that cortical involvement, larger infarct size, hemorrhagic transformation, and microbleeds are more prevalent in patients developing PSE [6, 11-13]. Furthermore, information regarding specific brain infarct location as potential epileptogenic regions after ischemic stroke is scarce, mostly based on computed tomography, and prior studies used different definitions of PSE [14, 15]. Available evidence suggests that infarction involving the supramarginal or superior temporal gyrus contributed to an increased risk of late-onset seizures. Additionally, one recent study from Taiwan utilizing advanced MRI techniques, including voxel-based analyses, identified the right superior frontal cortex and right frontal operculum as regions associated with PSE [16].
Based on lesion topology, this study aims to assess PSE risk after acute ischemic stroke treated with MT by semiautomated voxel-based lesion-symptom mapping.
2 Methods
The present study was carried out collaboratively between the Department of Neurology, Neuromed Campus, Kepler University Hospital, Linz, Austria, and the Department of Neurology, Medical University of Graz, Austria. We utilized data from our previous research [6], which provided a comprehensive dataset on PSE after MT. Employing a retrospective design with prospective follow-up, we included patients hospitalized for acute ischemic stroke between 2011 and 2017 who underwent MT for intracranial large vessel occlusion (i.e., internal carotid artery, middle cerebral artery, basilar artery and rarely anterior and posterior cerebral artery) and received postinterventional brain MRI. Patients were excluded if they were under 18 years old at the onset of stroke, had a history of prior epileptic seizures, preexisting brain lesions, lacked a postinterventional brain MRI, or if they passed away within 24 h of stroke onset (Figure 1). In the Linz cohort, all patients underwent structured telephone interviews utilizing a validated questionnaire for seizure detection [17]. For participants unable to respond, inquiries were directed to close relatives, nursing staff, or their general practitioner. Positive responses prompted face-to-face neurological consultations, involving comprehensive clinical assessments and electroencephalograms (EEGs), aimed at confirming the epileptic nature of reported episodes and minimizing the likelihood of seizure mimics. Follow-up data for the Graz cohort was obtained from MEDOCS, a fully-electronic medical documentation network interconnecting all public hospitals in Styria. This province has five specialized acute-care neurological departments providing acute neurological care, including management of acute epileptic seizures. Through MEDOCS, all outpatient hospital visits or admissions, including those resulting from new-onset seizures, are systematically documented [18].
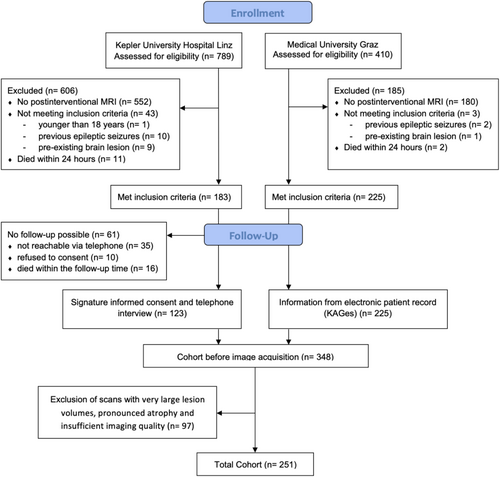
Table S1 provides a summary of the collected clinical and neuroimaging data. The ethics committees of the Johannes Kepler University Linz and the Medical University of Graz approved the study (approval numbers: Linz: 1183/2020, Graz: 32–634 ex 19/20).
2.1 Image Acquisition Protocol and Processing of MR Images
Image processing for producing lesion probability maps and lesion symptom mapping was based on fluid attenuated inversion recovery (FLAIR) scans with fast spin echo readout acquired on 1.5 T or 3 T scanners with various slice thicknesses ranging from 3 to 6 mm.
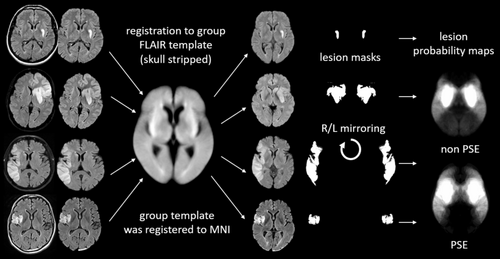
Following semiautomatic lesion outlining and generation of binarized lesion masks, all FLAIR scans and corresponding lesion masks were registered to a FLAIR group template using FLIRT (FMRIB's Linear Image Registration Tool, Oxford) as part of FSL [19]. To exclude misregistration, the signal intensities in the lesions were ignored by using the “-inweight” option. The group template was iteratively built by forming a mean FLAIR image from all patients after registration to a high-resolution FLAIR template (Winkler AM, Kochunov P, Glahn DC. FLAIR Templates. Available at http://brainder.org). This allowed us to correct for age-related atrophy and to refer all analyses to the Montreal Neurological Institute (MNI) space. Image registration was done after skull stripping with brain extraction (BET) [20] to increase the accuracy and robustness of the registration. To improve statistical power, the binary lesion masks were mirrored between the right and left hemisphere, as no significant differences in stroke lateralization were observed between patients with and without PSE (see Table 1). This resulted in almost symmetrical lesion maps with the number of lesions doubled. The processing steps of MRI images are depicted in Figure 2.
| Total cohort (n = 251; 100%) | PSE (n = 26; 10.4%) | No PSE (n = 225; 89.6%) | p | |
|---|---|---|---|---|
| Age, years, median (range) | 66 (18–89) | 61 (30–78) | 66 (18–89) | 0.126 |
| Sex, n (%) | ||||
| Male | 137 (54.6) | 16 (61.5) | 121 (53.8) | 0.452 |
| Female | 114 (45.4) | 10 (38.5) | 104 (46.2) | |
| NIHSS at admission, median (range) | 14 (1–42) | 14.5 (1–42) | 14 (4–42) | 0.171 |
| NIHSS postthrombectomy, median (range) | 5 (0–42) | 5 (0–18) | 5 (0–42) | 0.659 |
| Side of infarction, n (%) | ||||
| Left | 93 (37.1) | 13 (50) | 80 (35.6) | 0.214 |
| Right | 99 (39.4) | 10 (38.5) | 89 (39.6) | |
| Bilateral | 59 (23.5) | 3 (11.5) | 56 (24.8) | |
| Vascular risk factors, n (%) | ||||
| Arterial hypertension | 176 (70.1) | 18 (69.2) | 158 (70.2) | 0.539 |
| Dyslipidemia | 141 (56.2) | 13 (50.0) | 128 (56.9) | 0.321 |
| Atrial fibrillation | 100 (39.8) | 8 (30.8) | 92 (40.9) | 0.217 |
| Diabetes mellitus | 37 (14.7) | 2 (7.7) | 35 (15.6) | 0.225 |
| Chronic alcohol abuse | 11 (4.4) | 1 (3.8) | 10 (4.4) | 0.682 |
| Smoking | 45 (17.9) | 6 (23.1) | 39 (17.3) | 0.313 |
| Affected cerebrovascular territory, n (%) | ||||
| Middle cerebral artery | 209 (83.3) | 24 (92.3) | 185 (82.2) | 0.192 |
| M1 occlusion, n (%) | 131 (52.2) | 16 (61.5) | 115 (51.1) | 0.314 |
| Posterior cerebral artery | 53 (21.1) | 4 (15.4) | 49 (21.8) | 0.450 |
| Vertebrobasilar arteries | 49 (19.5) | 5 (19.2) | 44 (19.6) | 0.968 |
| Anterior cerebral artery | 22 (8.8) | 4 (15.4) | 18 (8.0) | 0.207 |
| Internal carotid artery | 15 (6.0) | 2 (7.7) | 13 (5.8) | 0.679 |
| Cortical involvement, n (%) | 229 (91.2) | 25 (96.2) | 204 (90.7) | 0.349 |
| TICI score, n (%) | ||||
| 0-2a | 20 (8.0) | 2 (7.7) | 18 (8.0) | 0.657 |
| 2b-3 | 231 (92.0) | 24 (92.3) | 207 (92.0) | |
| IV thrombolysis, n (%) | 172 (68.5) | 17 (65.4) | 155 (68.9) | 0.716 |
| Etiology, n (%) | ||||
| Large-artery atherosclerosis | 53 (21.1) | 4 (15.4) | 49 (21.8) | 0.450 |
| Cardioembolic | 113 (45.0) | 11 (42.3) | 102 (45.3) | 0.769 |
| Other/unknown | 85 (33.9) | 11 (42.3) | 74 (32.9) | 0.337 |
| Early seizures, n (%) | 4 (1.6) | 3 (11.5) | 1 (0.4) | 0.004 |
| SeLECT score post-thrombectomy, median (range) | 4.0 (0–8) | 4.0 (1–8) | 4.0 (0–8) | 0.840 |
| Symptom onset to recanalization time, median hh:mm (range) | 04:20 (01:18–21:42) | 03:50 (02:15–07:46) | 04:29 (01:18–21:42) | 0.790 |
| Follow up time (months), median (range) | 79 (42–125) | 80.5 (42–125) | 79 (42–125) | 0.585 |
| Time to first seizure (PSE), median days (range) | — | 561 (9–2577) | — | |
| Infarct volume, mean cm3 (range) [SD] | 51.7 (0.2–449.1) [46.3] | 119.2 (4.5–274.6) [87.1] | 43.9 (0.2–449.1) [56.4] | < 0.0001 |
- Note: Bold values are indicating statistically significant (p < 0.05).
- Abbreviations: NIHSS, National Institutes of Health Stroke Scale; SeLECT, severity of stroke, large-artery atherosclerotic etiology, early seizures, cortical involvement, and territory of middle cerebral artery involvement; TICI, thrombolysis in cerebral infarction.
Lesion symptom mapping was applied using the NiiStat software (https://github.com.neurolabusc/NiiStat), Version 3, with MATLAB according to the published recommendations [21]. The analyses were conducted across the whole brain in voxels affected in at least 4% (n ≥ 10) of the cases. Statistical significance was set at p < 0.05 corrected for familywise error (FWE) using Freedman-Lane permutation with 2000 permutations.
Brain regions significantly associated with PSE were identified by performing region-wise symptom lesion mapping based on parcellations from the AALCAT atlas (https://www.nitrc.org/projects/niistat/) and Harvard-Oxford Cortical and Subcortical Atlas [22]. The AALCAT atlas integrates 116 gray matter regions from the AAL atlas [23] and 34 white matter tracts from the CAT atlas [24].
Additionally, we calculated Z-values for each brain region to quantify the association between lesion locations and PSE occurrence, defining a ‘strong association’ as a minimum overlap of 10 voxels within the region of interest. (Table 2).
| Lobe | Region | Peak Z-value |
|---|---|---|
| Frontal | Gyrus rectus/olfactory gyrus | −5.69 |
| Orbitofrontal gyrus | −4.40 | |
| Inferior frontal gyrus/frontal operculum | −5.24 | |
| Orbital superior frontal gyrus | −5.20 | |
| Orbital middle frontal gyrus | −4.87 | |
| Orbital medial frontal gyrus | −4.50 | |
| Precentral gyrus | −4.48 | |
| Temporal | Superior temporal gyrus/temporal operculum | −5.42 |
| Middle temporal gyrus | −5.30 | |
| Transverse temporal gyrus (Heschl's gyrus) | −4.97 | |
| Temporal pole | −4.85 | |
| Piriform cortex | −4.33 | |
| Parietal | Rolandic operculum | −5.14 |
| Supramarginal gyrus | −5.11 | |
| Postcentral gyrus | −4.77 | |
| Superior parietal gyrus | −4.01 | |
| Inferior parietal gyrus | −3.99 |
We also conducted a secondary analysis for the subgroup of patients with middle cerebral artery M1 segment occlusion to assess the implication of lesions on PSE risk in a more homogeneous cohort regarding infarct extension and location (Appendix S1).
3 Results
We included 251 patients with available lesion symptom maps (median age: 66 years, 45.4% women, 10.4% PSE). 97 cases, in particular those with very large lesion volumes, had to be excluded after careful visual assessment of the registration results because of midline shifts. Furthermore, patients with pronounced atrophy and insufficient imaging quality (due to motion artifacts) had to be excluded (Figure 1).
The median time between the index stroke and the first late epileptic seizure (PSE diagnosis) was 561 days (range, 9–2577). Early seizures were more likely in the PSE group (PSE: 11.5% vs. non-PSE: 0.4%; p = 0.004). The mean infarct volume in non-PSE patients was considerably larger compared to PSE patients (119.2cm3 vs. 43.9cm3, p < 0.0001). Detailed information regarding demographic and clinical characteristics dichotomized by the occurrence of PSE is provided in Table 1.
Infarcts in the PSE group were more likely to involve fronto-temporal cortical regions as well as the temporal pole, as seen in the lesion probability maps (Figure 3). By performing lesion symptom mapping, we identified that patients with infarction in the following areas had a significantly increased risk of PSE: orbitofrontal region, operculum, temporal superior and middle gyrus, pre- and postcentral gyrus, temporal pole (esp. piriform cortex with area tempestas), supramarginal as well as parietal superior and inferior gyrus. Brain regions that are significantly implicated in the development of PSE, sorted by Z-values, are shown in Table 2.

No other clinical or demographic factors were found to be significantly associated with PSE in this cohort. Variables such as age, sex, NIHSS scores (both at admission and after MT), side of infarction, affected cerebrovascular territory, cortical involvement, vascular risk factors, TICI scores, etiology, SeLECT score [7] postthrombectomy, and time to recanalization did not show a statistically significant correlation with PSE (Table 1).
In the secondary analysis (Tables S2 and S3 and Figure S1) on patients with middle cerebral artery M1 segment occlusion (n = 131), the following regions remained significantly associated with PSE: temporal superior and middle gyrus, temporal pole, operculum, transverse temporal gyrus, inferior frontal gyrus, supramarginal gyrus, and parietal superior gyrus.
4 Discussion
To the best of our knowledge, this is the first study utilizing lesion symptom mapping to identify regions that are associated with an increased risk for PSE in patients after MT for large vessel occlusion stroke. We identified specific cortical and subcortical brain regions associated with PSE, which can be grouped into orbitofrontal regions, the temporal pole (particularly the piriform cortex and area tempestas), as well as the frontal, parietal, and temporal opercula.
Several of the brain regions identified in this study have been associated with epileptogenesis and seizure propagation. The superior and middle temporal gyri, along with the inferior, middle, and superior frontal gyri, are critical in the initiation and spread of seizure activity, particularly in focal epilepsy [25]. Seizures originating in these areas can propagate through interconnected cortical networks. The precentral and postcentral gyri, which comprise the primary motor and somatosensory cortices, are frequently involved in motor and sensory seizures. Moreover, the supramarginal gyrus and superior and inferior parietal gyri are integral to sensory integration and spatial awareness, making them susceptible to seizures that affect these functions [25, 26]. In addition, certain regions of the temporal cortex, particularly the areas covering the insula, have been highlighted in the literature as critical for epileptogenesis [27]. The hippocampus and amygdala are known to play key roles in seizure initiation and propagation, given their high synaptic plasticity and interconnection with cortical and subcortical networks. These structures, alongside the olfactory cortex, are often implicated in the spread of limbic seizures. The involvement of these regions underscores the complexity of seizure networks and the importance of considering both cortical and subcortical structures in PSE. The area tempestas, located within the piriform cortex, plays a crucial role in the context of PSE. This region is particularly vulnerable to epileptogenic processes following brain injuries, including stroke. The piriform cortex has a low threshold for seizure generation and is a common site for seizure onset due to its extensive connectivity with other brain regions, particularly those involved in olfactory and limbic functions. Imaging studies often reveal abnormalities in the piriform cortex in cases of PSE, suggesting its involvement in the reorganization of neural circuits that contribute to the development of seizures [11, 28, 29].
Two CT-based studies from Heuts-Van Raak et al. conducted in the 1990s concluded that patients with infarction involving the middle temporal, postcentral, supramarginal, and superior temporal gyrus had an increased PSE risk [14, 15]. In contrast to our study, these studies employed different imaging techniques and applied Bories' method for semiquantitative topographical analysis of cortical involvement. Our approach, however, encompassed a broader investigation of all brain regions, with a particular emphasis on complex neuroanatomical networks implicated in seizure propagation, such as the limbic system [28].
In comparison to the study by Chou et al., which identified specific cortical “hot spots” on MRI for PSE risk primarily in the central region, superior parietal lobule, and frontal operculum, our study offers a broader analysis of both cortical and subcortical regions associated with PSE. Although both studies employed voxel-based analyses, our research focused on a cohort of patients who underwent MT, providing a unique perspective on PSE in this specific population. Additionally, the follow-up period in our study was longer, and we included a larger number of patients compared to Chou et al., who studied a more general population of stroke patients with a one-year follow-up [16].
Recent evidence suggests that seizure propagation is not strictly confined to one hemisphere and can involve complex interhemispheric spread, as seizures often recruit bilateral networks, particularly in cases of temporal lobe epilepsy [30]. This underscores the importance of considering both hemispheres in seizure propagation analysis. Notably, neither in our study nor in previous works significant differences in stroke lateralization regarding PSE risk have been identified [6, 7, 16], further emphasizing the bilateral nature of seizure involvement in PSE.
In our population, the significant association between infarct volume and higher PSE occurrence highlights the relevance of lesion size in the development of PSE. While specific brain regions and networks involved in seizure generation and propagation remain critical, our analysis suggests that larger infarcts increase the likelihood of affecting these key areas. This reinforces the idea that infarct volume plays a pivotal role, potentially increasing the vulnerability of critical neuroanatomical pathways. The greater the infarcted area, the higher the chance that important networks involved in seizure activity may be compromised, thereby contributing to a higher incidence of PSE. In a secondary analysis on patients with middle cerebral artery M1 segment occlusion, we identified that the temporal superior and middle gyrus, temporal pole, rolandic operculum, transverse temporal gyrus, frontal inferior gyrus, supramarginal gyrus, and parietal superior gyrus were most strongly associated with PSE risk. This partly allows us to separate the effect of infarct location from infarct volume and potentially to better understand whether the identified regions on LSM are truly implicated in PSE risk or are simply areas affected by larger infarcts. However, we have to acknowledge that this subanalysis is limited by small sample size with reduced statistical power.
Interestingly, our study found no significant association between the affected cerebrovascular territory and the development of PSE. These findings suggest that the risk of PSE is not primarily related to the vascular territory affected but may rather depend on specific neuroanatomical structures and networks involved in seizure generation.
Additionally, our analysis found no association between cortical involvement and PSE. While previous studies have suggested that cortical strokes confer a higher risk for seizures, the high percentage of cortical involvement in both groups of our cohort (PSE: 96%, non-PSE: 91%) may have limited our ability to detect a significant difference. This high prevalence likely reflects the patient selection criteria inherent to MT-treated stroke populations, where large vessel occlusion strokes frequently lead to substantial cortical infarcts.
Furthermore, our study did not find an association between the SeLECT score [7] post-thrombectomy and PSE occurrence. The SeLECT score has been previously described as a tool for predicting late seizures after stroke, but its applicability in the specific context of post-thrombectomy patients remains uncertain. The absence of a significant correlation in our study could indicate that additional factors, beyond those incorporated in the SeLECT score, influence seizure risk in this distinct patient population.
While these findings are not directly attributable to the thrombectomy procedure itself, the inclusion of MT patients provides insights into a high-severity stroke population, often requiring advanced interventions. These patients typically represent the subset of strokes with the most extensive infarcts, which may inherently contribute to higher PSE risks and allow for detailed exploration of associated neuroanatomical factors.
Another strength of this study is the extended follow-up period, which surpasses that of similar studies. This longer duration enables a more accurate assessment of late-onset seizures, thereby providing a more complete understanding of the long-term risks associated with PSE after MT. Furthermore, we excluded patients with preexisting brain lesions and prior epilepsy, which enhances the homogeneity of the study population and reinforces the validity of the findings.
Despite these strengths, several limitations should be acknowledged. The retrospective design of the study, although applying a prospective follow-up, inherently carries the potential for selection bias and inaccuracies in historical clinical data. Additionally, the study's focus on MT-treated patients with the availability of postinterventional brain MRI may limit the generalizability of the findings to broader stroke populations, particularly those not receiving such an intervention. The final number of patients included with PSE remains moderate. Moreover, the exclusion of cases with very large lesion volumes due to difficulties in registration might have led to the omission of data from patients at potentially higher risk for PSE, thus possibly underestimating the overall PSE risk. Further, the voxel-based lesion-symptom maps suffer from reduced statistical power due to the need for correction for multiple comparisons across many voxels. In contrast, the region-wise analysis has more statistical power due to the aggregation of all voxels in a defined region and less need for correction for multiple comparisons, but lacks fine regional details. These limitations should be carefully considered when interpreting the results of this study.
In conclusion, this study represents a step forward in understanding the neuroanatomical and clinical underpinnings of PSE in patients treated with MT. By employing advanced voxel-based lesion symptom mapping, we identified specific cortical and subcortical regions, including the orbitofrontal areas, temporal pole, and opercula, which are critically implicated in seizure generation and propagation. These findings underscore the importance of infarct volume and the involvement of neuroanatomical networks in PSE risk, highlighting the need for targeted poststroke monitoring and personalized management strategies. Future research with larger cohorts and prospective designs is warranted to validate these results and further elucidate the intricate mechanisms underlying PSE.
Author Contributions
Joachim Gruber: writing – original draft, conceptualization, investigation, methodology. Stefan Ropele: methodology, software, visualization, formal analysis. Daniela Pinter: methodology. Christian Enzinger: methodology, supervision, writing – review and editing. Raimund Helbok: writing – review and editing. Hannes Deutschmann: writing – review and editing. Michael Sonnberger: visualization, writing – review and editing. Markus Kneihsl: writing – review and editing. Tim J. von Oertzen: supervision. Thomas Gattringer: supervision, writing – review and editing, validation, methodology, conceptualization.
ACKNOWLEDGEMENT
Open access funding provided by Medizinische Universitat Graz/KEMÖ.
Ethics Statement
The ethics committees of the Johannes Kepler University Linz and the Medical University of Graz approved the study (approval numbers: Linz: 1183/2020, Graz: 32–634 ex 19/20).
Consent
Informed consent to participate and publish their data was obtained from individual participants from Linz included in the study.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




