PET Beta-Amyloid Tracer Uptake in Leukoencephalopathies: Comparing Metachromatic Leukodystrophy and CADASIL
Funding: This work was supported by Ministero della Salute, Ricerca corrente to Ettore Salsano.
Positron emission tomography (PET) for neurological diseases has traditionally focused on conditions affecting gray matter (GM), such as Alzheimer's disease. However, beta-amyloid tracers can also bind to the beta sheet structures of myelin basic protein (MBP), a major component of central nervous system myelin [1]. When myelin is damaged and MBP loses its beta sheet structures, these tracers can detect demyelination as areas of reduced binding [1].
In this preliminary study, three patients with adult-onset metachromatic leukodystrophy (MLD), two with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), and one control patient with mild cognitive impairment (MCI) underwent beta-amyloid PET scans using either F-18 Flutemetamol (a benzothiazole derivative) or F-18 Florbetaben (a stilbene derivative). Their clinical and genetic characteristics are summarized in the Table 1. The choice of tracer was primarily dictated by availability, as patients were scanned with the tracers accessible at the time for routine clinical use.
| Patient # | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Diagnosis | MLD | MLD | MLD | CADASIL | CADASIL | MCI |
| Gene & inheritance | ARSA, recessive | ARSA, recessive | ARSA, recessive | NOTCH3, dominant | NOTCH3, dominant | N/A |
| Mutations | c.135C> A (p.Ser45Arg); c.465+1G> A (Het) | c.256C> T (p.Arg86Trp) (Homo) | c.1174C> T (p.Arg392Trp); c.1471T> G (p.Cys491Gly) (Het) | c.1368_1373delCTGTAT (p.Ile456_457Cysdel) | p.Gly385Cys (c.1153G> T) | N/A |
| Sex | M | M | M | F | F | M |
| Age at evaluation | 49 | 67 | 50 | 68 | 50 | 48 |
| Years from onset | 2 | 5 | 5 | 20 | 1 | 1 |
| Clinical manifestations at PET scan | Behavioral and cognitive decline (MoCA: 21/30) | Behavioral and cognitive decline (MoCa: 14/30) | Behavioral and cognitive decline (MoCA: 25/30) | Migraine, but neurological findings normal, including cognition and central neurophysiology | Migraine; neurological findings normal, including cognition and central neurophysiology | Memory impairment (MoCA: 25/30) |
| Beta-amyloid radiotracer | F-18-Flutemetamol | F-18-Florbetaben | F-18-Florbetaben | F-18-Flutemetamol | F-18-Florbetaben | F-18-Florbetaben |
- Abbreviations: CADASIL, cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy; F, female; M, male; MCI, mild cognitive impairment; MLD, metachromatic leukodystrophy; MoCA, montreal cognitive assessment; N/A, not applicable.
Static PET images were acquired approximately 90 min after intravenous injection of F-18 Flutemetamol (185 MBq) or F-18 Florbetaben (300 MBq) using the same scanner, with a 20-min acquisition time, following routine clinical protocols. Whenever possible, white matter segmentation was performed on 2D MRI slices using FreeSurfer (Figure 1A), verified by two experienced neuroradiologists, and manually refined as needed. MRI and PET images were co-registered with 3D Slicer, and white matter volumes were calculated (Figure 1B). Radiotracer distribution was quantified using the standard uptake value ratio (SUVr), with the pons as the primary reference region and the cerebellum for supplementary verification. Brainstem and cerebellar involvement are exceedingly rare in adult-onset MLD, while in CADASIL, pontine involvement is less frequent than supratentorial leukoencephalopathy, and the cerebellum is typically spared [2]. SUVr was assessed in normal-appearing white matter (NAWM) and white matter hyperintensities (WMHs) for MLD patients and one CADASIL case, only in WMHs for the older CADASIL case due to their extensive presence, and in NAWM for the control subject. Gray matter (GM) tracer distribution was also evaluated. Due to the small sample size, no formal statistical subgroup analyses were conducted. The study (ClinicalTrials.gov ID NCT04880356) was approved by the Fondazione IRCCS Istituto Neurologico Carlo Besta review board, and all patients provided written informed consent.

All three MLD patients exhibited WMHs on FLAIR imaging, primarily in the frontal white matter (Figure 1C,E,G), with an average volume of 64.93 cm3 [3] (15% of total WM volume). PET imaging revealed inhomogeneous tracer distribution, with high uptake in NAWM and markedly reduced uptake in WMHs (Figure 1D,F,H), confirmed by clearly lower SUVr in WMHs (Figure 2).
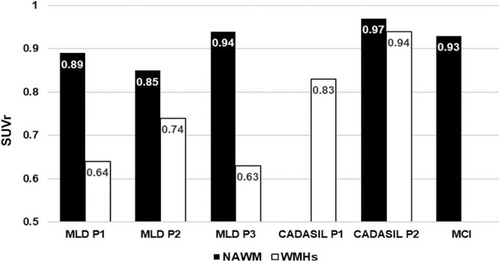
In contrast, CADASIL patients showed a tracer distribution in WMHs comparable to that in the NAWM of MLD patients, as well as the NAWM of one CADASIL patient and the control MCI subject (Figures 1I–M and 2). Similar results were obtained using the cerebellum as a reference (data not shown). Both tracers displayed low uptake in GM across all patients.
Our findings demonstrate lower beta-amyloid tracer uptake in the WMHs of MLD patients compared to NAWM, consistent with the disease's primary pathology—demyelination [3]. Beta-amyloid tracers bind to intact myelin but exhibit reduced binding in damaged areas [1]. In contrast, CADASIL patients showed preserved tracer uptake despite extensive WM changes, suggesting relatively intact myelin. Their tracer distribution in WMHs was comparable to that observed in NAWM, supporting the idea that myelin integrity is preserved. Findings from CADASIL mouse models, where no demyelination is observed in early phases, suggest that the pathology is probably characterized by intramyelinic edema and inefficient myelin debris clearance rather than demyelination [4]. The absence of clinical or neurophysiological signs of myelin loss supports this interpretation, indicating that lesion extent and nature do not reach the threshold required to produce detectable abnormalities. However, pathological data on the initial stages in humans remain scarce [3, 5].
As a preliminary investigation, this study is limited by its small sample size, a consequence of disease rarity and radiotracer constraints. Additionally, static PET acquisition was used instead of a dynamic protocol; however, this approach aligns with clinical practice and has been validated in multiple sclerosis. Lastly, the use of two different radiotracers may reduce homogeneity but also demonstrates their comparable applicability.
The need for advanced imaging tools beyond conventional MRI to differentiate white matter pathologies arose from our clinical experience in white matter diseases. In this context, beta-amyloid PET tracers offer a promising approach for the in vivo characterization of leukoencephalopathies, providing valuable insights into white matter abnormalities and complementing existing diagnostic techniques.
Author Contributions
Chiara Benzoni: writing – original draft, writing – review and editing, data curation, formal analysis, investigation. Marco Moscatelli: writing – review and editing, formal analysis, data curation, investigation. Anna Bersano: writing – review and editing. Angelo Del Sole: methodology, data curation, formal analysis, writing – original draft, writing – review and editing, investigation. Ettore Salsano: conceptualization, writing – review and editing, methodology, writing – original draft, data curation, formal analysis, investigation.
Acknowledgments
The authors thank Anna Venerando and Silvia Baratta for performing the genetic testing as part of routine clinical practice.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Anonymized data not published within this article will be made available by request from any qualified investigator.




