Reactive Pleocytosis After Repeated Lumbar Puncture—Implications for Clinical Practice
Funding: The authors received no specific funding for this work.
Gabriel Bsteh and Martin Schmidauer have contributed equally.
ABSTRACT
Introduction
Lumbar puncture (LP) is a routine clinical procedure and, in some cases, is repeatedly performed for diagnostic or therapeutic reasons. The impact of repeated LP on cerebrospinal fluid (CSF) findings is not clear.
Objective
To investigate whether repeated LP is associated with reactive pleocytosis and disruption of blood–CSF barrier function and to determine the role of interval between repeated LP.
Methods
Patients with non-inflammatory neurological disease (NIND) and at least two consecutive LP were included. Longitudinal changes in CSF white blood cell count (WBC), CSF total protein (TP), and CSF/serum albumin quotient (Qalb) were assessed depending on the time interval between the LP.
Results
A total of 73 patients with a median age of 35 years (25th–75th percentile: 25–45) and a female predominance of 75% had second LP after 6 (3–19) days. Twenty (27%) patients developed pleocytosis with an increase of WBC count to 8/μl (6–15) with a maximum of 30/μl. Patients with pleocytosis had the follow-up LP significantly earlier than patients without pleocytosis, 3.5 (3–7) versus 7 (3–28) days. The majority of patients (90%) with CSF pleocytosis had the second LP within 10 days. Further repeated LP in a subgroup of patients revealed similar findings. CSF TP and Qalb slightly increased in patients with pleocytosis.
Conclusion
Mild “reactive” CSF pleocytosis occurred in approximately one-third of patients after repeated LP, mostly when performed within 10 days.
1 Introduction
Lumbar puncture (LP) is a clinical procedure used either to collect cerebrospinal fluid (CSF) and/or to measure CSF opening pressure for routine diagnostic work-up [1], or for therapeutic reasons, for example, to reduce intracranial pressure in idiopathic intracranial hypertension (IIH), or instillation of medication [2]. In some cases, repeated LP is necessary. Irritation of meningeal and peri-meningeal structures caused by LP might lead to reactive pleocytosis interfering with CSF results in case of subsequent LP. There is limited evidence on this topic with contradicting results, with the earliest publications dating back to the 1940s [3]. While some studies observed that repeated LP affects white blood cell count (WBC), others reported no significant changes [3-5]. Whether the time interval between repeated LP impacts CSF findings has not been investigated so far.
The aim of the present study was to investigate (i) whether repeated LP is associated with reactive pleocytosis and/or disruption of blood–CSF barrier function, and (ii) to determine the role of the time interval between repeated LP.
2 Methods
We retrospectively reviewed the CSF databases of the Medical University of Innsbruck (MUI) and the Medical University of Vienna (MUV) to identify patients who had at least two consecutive lumbar punctures within 6 months. Patients with non-inflammatory neurological diseases (NIND) or symptomatic controls according to Teunissen et al. [6] were eligible for inclusion. Patients were required to have a normal WBC count (< 5/μL) at baseline (first LP). Patient charts were reviewed to obtain clinical diagnoses; patients with leptomeningeal metastases were excluded from the study (Figure 1).

Cerebrospinal fluid samples were collected by LP; serum samples were collected concomitantly within 30 min by venous puncture. At MUV, traumatic needles ranging from 20 to 22 gauge (G) were used for LP (Spinocan, Braun, Melsungen, Germany), while at MUI either a traumatic needle 20 G (Spinal Needle, Becton Dickinson, Madrid, Spain) or an atraumatic needle 21 G (Sprotte, PAJUNK GmbH Medizintechnik, Geisingen, Germany) was used.
White blood cell and red blood cell (RBC) counts were determined using the Fuchs-Rosenthal chamber (MUI) [7] or automated by Symex, confirmed by visual counting using the Fuchs-Rosenthal chamber if counts were ≥ 5 (MUV). CSF total protein was determined by spectrophotometry [8] and albumin concentrations in CSF and serum by nephelometry (MUI and MUV) [9].
2.1 Ethics Statement
The study was approved by the ethics committees of the Medical Universities of Innsbruck (approval number 1269/2022) and Vienna (approval number: 1290/2022).
2.2 Statistical Analysis
Data were displayed as median, 25th and 75th percentile. For group comparisons, the Mann–Whitney U-test and Fisher test were applied, as appropriate. For comparison of paired samples, the Wilcoxon test and binomial test were used. According to the one-sided hypotheses, that is, an increase in WBC count, CSF total protein, and Qalb at the second (or third) LP, one-sided hypothesis testing was used. Thus, one-sided p values were considered statistically significant. All analyses were done using R [10].
3 Results
A total of 73 patients with a median age of 35 years (25th–75th percentile: 25–45) and a female predominance (75%) were included in the study. All patients were diagnosed with NIND, with the majority, 58 (79%), having IIH. Four patients (5%) had epilepsy, and four others (5%) had hydrocephalus. The remaining seven patients had other types of NIND. At baseline (first LP), the median CSF WBC count was 2/μl (1-3), CSF total protein was 28 (19–41) and the CSF/serum albumin quotient (Qalb) was 3.7 (2.4–6.4). An overview of clinical characteristics and main CSF findings is given in Table 1.
| Age (years) | 35 (25–45) |
| Sex (female) | 55 (75) |
| RBC (μ/l) | 0 (0–0) |
| WBC (μ/l) | 2 (1–3) |
| CSF total protein (mg/dl) | 28 (19–41) |
| Qalb (×10−3) | 3.7 (2.4–6.4) |
| IIH | 58 (79) |
| Hydrocephalus | 4 (5) |
| Epilepsy | 4 (5) |
| Other NIND | 7 (10) |
- Note: Data are given as median (25th–75th percentile) and n (%), as appropriate.
- Abbreviations: IIH, idiopathic intracranial hypertension; NIND, non-inflammatory neurological disease; Qalb, CSF/serum albumin ratio; RBC, red blood cell count; WBC, white blood cell count.
3.1 Increase of WBC in Consecutive CSF Samples Depends on Time
All patients underwent a second LP after a median interval of 6 days (3–19). Twenty (27%) of 73 patients showed an increase in WBC count at the second LP reaching a median WBC count of 8/μl (6–15) with a maximum of 30/μl (Figure 2A). Patients with reactive pleocytosis had the second LP significantly earlier than patients without pleocytosis, 3.5 (3–7) versus 7 (3–28) days (Figure 3A). All but two patients (90%) with CSF pleocytosis had the second LP within 10 days.

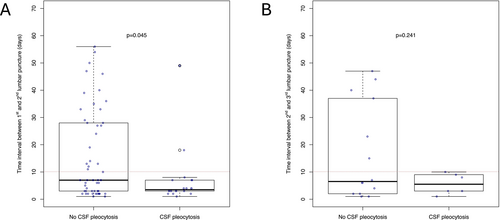
A total of 32 (43.8%) patients had a third LP after another median of 6 days (3–19). Of those 20 patients who had WBC < 5/μl at the second LP, 6 (30%) developed pleocytosis at the consecutive third LP with a median WBC count of 8/μl and a maximum of 29 /μl (Figure 2B). All patients with pleocytosis had LP within 10 days, while 6 (43%) of 14 patients without CSF pleocytosis had LP later (Figure 3B).
3.2 Change in CSF Protein Levels After Repeated Lumbar Puncture
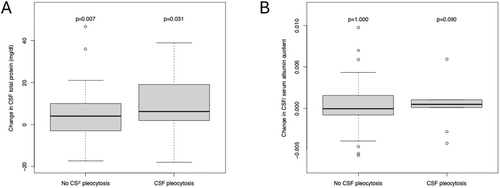
At second LP, CSF total protein concentrations were available for 68 patients and Qalb for 50 patients. CSF total protein and Qalb increased at the second LP reaching 33 mg/dL and 4.3, respectively (Table S1). These increases were seen predominantly in patients with reactive pleocytosis. The increase in CSF total protein was 50% higher in patients with reactive pleocytosis (Δ 6 mg/dL) than in those without reactive pleocytosis (Δ 4 mg/dL) (Figure 4A). While the median Qalb did not change in patients without reactive pleocytosis, the Qalb increased by 0.5 × 10−3 in patients with reactive pleocytosis (Figure 4B). The analysis of the CSF protein profile at the third LP was omitted due to the small number of available measures.
4 Discussion
Here, we aimed to investigate whether (i) repeated LP is associated with reactive pleocytosis and/or disruption of blood–CSF barrier function and (ii) to determine the role of the time interval between repeated LP.
This study demonstrates that repeated LP might be associated with mild pleocytosis, which was observed in about a third of patients and occurred predominantly when the interval between LPs was 10 days or shorter. In parallel, a slight increase in CSF total protein and Qalb was observed, particularly in those patients developing pleocytosis. Thus, “reactive” pleocytosis seems to be a frequent phenomenon, which should be considered in interpretation of CSF results of patients receiving repeated LP.
To date, evidence on reactive pleocytosis due to repeated LP is scarce and contradictory. A previous study including 41 healthy controls from phase 1 clinical trials of potential psychotropic compounds who had two consecutive LPs performed at an interval of 14 days (using 18 G Tuohy needle) reported pleocytosis in the second CSF sample in 56% of cases [5]. In contrast, another study including 176 patients who had repeated LPs after a mean of 79 days (using G21 or G22 atramautic needles) found no overall effect on WBC count. In the subgroup of 104 patients who had the second LP within 10 days, only one patient showed a mild abnormal CSF WBC count in the second LP [4]. A further study stemming from 1944 performed in 16 healthy children showed no effect of a repeated LP after 48 h on WBC [3].
There might be several explanations for the contradicting findings of previous studies. Different types of needles (traumatic vs. atraumatic, different sizes ranging from G18 to G22) were used. It might be speculated that larger needle size and/or traumatic needle type leads to pleocytosis more frequently [4, 5]. In the present study, we performed LP with traumatic needles in the vast majority of patients. This might explain why we observed reactive pleocytosis in approximately one-third of patients. We would like to highlight that we had the advantage of a broad scope of time intervals between the LPs. Of the 73 patients, those who developed reactive pleocytosis had a second LP after a median of 3.5 days, which was significantly earlier compared to patients without reactive pleocytosis. Another study which did not observe pleocytosis on the second LP might have missed it due to the short time interval between LPs [3]. Altogether, it can be hypothesized that reactive CSF pleocytosis predominantly occurs when using a traumatic needle (possibly also depending on its size) which can be detected “only” within a certain time window, that is, within approximately 10–14 days.
With regard to the CSF protein profile, we observed a slight increase in CSF total protein as well as in Qalb, especially in those patients who developed reactive pleocytosis. One might speculate that this blood–CSF barrier dysfunction is the result of the inflammatory reaction due to the initial LP. It is well known that intrathecal inflammation is associated with an increase in Qalb and CSF total protein, respectively [7]. Other hypotheses of changes in the CSF flow due to the withdrawal of larger amounts of CSF sample seem unlikely in our opinion [4].
It needs to be emphasized that none of the patients who developed pleocytosis presented any symptoms of CNS inflammation, strongly suggesting that this was indeed “reactive” and locally restricted pleocytosis, but not warranting concerns regarding performance of repeated LPs. However, the likelihood of mild pleocytosis in patients receiving LP within 10–14 days of a prior LP needs to be acknowledged by clinicians when interpreting CSF results to avoid unnecessary concern for patients or further costly diagnostic procedures.
There are some limitations of the present study. First, this is a retrospective analysis with all inherent limitations of this study design, for example, the availability of longitudinal samples was determined within clinical routine. Accordingly, the majority of patients had a diagnosis of IIH. However, this is a clear non-inflammatory neurological disease, and a baseline WBC count < 5/μl was an inclusion criterion [6]. So, we are confident that the observed CSF pleocytosis is indeed due to repeated LP and not due to the underlying disorders. Sensitivity analyses did not show any differences due to diagnosis (IIH vs. other NIND) (Table S2). Second, we were not able to consider the precise needle type used for LP, even though LP was done by traumatic needle (G20–22) in the vast majority of patients. Also, we were not able to consider the withdrawn CSF sample volume. Another limitation of the study is that WBC counts at the two centers were determined using different methods. While WBC counts were counted visually at MUI, automated cell counting was employed at the MUV. However, at the MUV, low WBC counts were all confirmed by visual counting [11]. Sensitivity analyses did not show any differences due to center (Table S2).
Altogether, we provide evidence that reactive pleocytosis can occur in repeated LP, especially within the first 10 days, together with minimal changes in the CSF protein profile. This should be considered when interpreting CSF results.
Author Contributions
Martin Schmidauer: data curation, writing – original draft, investigation, formal analysis. Fabian Föttinger: writing – review and editing, data curation. Klaus Berek: writing – review and editing. Michael Auer: writing – review and editing. Robert Barket: writing – review and editing. Franziska Di Pauli: writing – review and editing. Nik Krajnc: writing – review and editing, data curation. Laura Stichaller: writing – review and editing. Sina Zaic: writing – review and editing, data curation. Anne Zinganell: writing – review and editing. Florian Deisenhammer: writing – review and editing. Janette Walde: writing – review and editing, formal analysis. Gabriel Bsteh: writing – review and editing. Harald Hegen: conceptualization, supervision, resources, project administration, formal analysis, writing – review and editing, investigation, methodology, visualization.
Conflicts of Interest
Martin Schmidauer has participated in meetings sponsored by or received travel grants from Novartis, Sanofi-Genzyme, and Amgen. Fabian Föttinger has participated in meetings sponsored by and received speaker honoraria and travel funding from Novartis. Klaus Berek has participated in meetings sponsored by and received travel funding or speaker honoraria from Roche, Teva, Merck, Biogen, Sanofi, and Novartis. He is an associate editor of Frontiers in Immunology/Neurology, Section Multiple Sclerosis and Neuroimmunology. Michael Auer has received speaker honoraria and/or travel grants from Biogen, Merck, Novartis, Sanofi Genzyme, Horizon Therapeutics/Amgen, and Zentiva. Robert Barket has participated in meetings sponsored by or received travel grants from Novartis, Janssen-Cilag, and Sanofi-Genzyme. He has received honoraria from Janssen-Cilag and Biogen. Franziska Di Pauli has participated in meetings sponsored by, received honoraria (lectures, advisory boards, consultations), or travel funding from Bayer, Biogen, Celgene, BMS, Merck, Novartis, Sanofi-Genzyme, Teva, and Roche. Nik Krajnc has participated in meetings sponsored by, received speaker honoraria or travel funding from Alexion, BMS/Celgene, Janssen, Merck, Novartis, Roche, and Sanofi. He held a grant for a Multiple Sclerosis Clinical Training Fellowship Programme from the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS). Laura Stichaller has nothing to disclose. Sina Zaic has nothing to disclose. Anne Zinganell has participated in meetings sponsored by, received speaking honoraria, or travel funding from Biogen, Merck, Novartis, Sanofi-Genzyme, Janssen, and Teva. Florian Deisenhammer has participated in meetings sponsored by or received honoraria for acting as an advisor/speaker for Alexion, Almirall, Biogen, Celgene, Merck, Novartis, Roche, and Sanofi-Genzyme. His institution received scientific grants from Biogen and Sanofi-Genzyme. Janette Walde has nothing to disclose. Gabriel Bsteh has participated in meetings sponsored by, received speaker honoraria or travel funding from Biogen, Celgene/BMS, Janssen, Lilly, Medwhizz, Merck, Novartis, Roche, Sanofi-Genzyme, and Teva, and received honoraria for consulting Adivo Associates, Biogen, Celgene/BMS, Janssen, Merck, Novartis, Roche, Sanofi-Genzyme, and Teva. He has received unrestricted research grants from Celgene/BMS and Novartis. He serves as the Executive Committee Member of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS). Harald Hegen has participated in meetings sponsored by, received speaker honoraria or travel funding from Bayer, Biogen, Bristol Myers Squibb, Horizon, Janssen, Merck, Novartis, Sanofi-Genzyme, Siemens, Teva, and received honoraria for acting as a consultant for Biogen, Bristol Myers Squibb, Novartis, Roche, Sanofi-Genzyme, and Teva.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.




