The Results of ADVANCE-CIDP IVIG Trial: Intravenous Immunoglobulin 10% Therapy With GAMMAGARD LIQUID/Kiovig for Treatment of Relapse in Chronic Inflammatory Demyelinating Polyradiculoneuropathy
Funding: This work was supported by Takeda Development Center Americas, Inc.
The affiliations for Colin Anderson-Smits, Shabbir Hasan, and Jamie Wood were at the time of the study.
ABSTRACT
Background
ADVANCE-CIDP IVIG evaluated the efficacy and safety of immune globulin infusion (human) 10% solution (IVIG 10%; GAMMAGARD LIQUID, also known as Kiovig) in chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) as a rescue treatment for patients relapsing during the ADVANCE-CIDP 1 trial.
Methods
Open-label ADVANCE-CIDP IVIG included adult patients with confirmed CIDP relapse (≥ 1-point increase in adjusted Inflammatory Neuropathy Cause and Treatment [INCAT] disability scores from pre-treatment baseline) during ADVANCE-CIDP 1, which assessed the efficacy and safety of hyaluronidase-facilitated subcutaneous immunoglobulin (fSCIG) 10%. Patients received an induction IVIG 10% dose (2 g/kg) followed by maintenance infusions at the same monthly equivalent dose of pre-randomization IVIG, 3-weekly for 6 months. The primary outcome was the responder rate (≥ 1-point decrease in adjusted INCAT scores at treatment cessation vs. pre-IVIG 10% baseline, in patients receiving placebo in ADVANCE-CIDP 1). Other outcomes included the responder rate across all patients relapsing on fSCIG 10% or placebo in ADVANCE-CIDP 1, time to functional improvement (≥ 1-point decrease in adjusted INCAT score), and change in adjusted INCAT scores and Rasch-built Overall Disability Scale (R-ODS) centile scores from pre-IVIG 10% baseline.
Results
Overall, 20 patients received IVIG 10% (n = 4 [fSCIG 10%-relapse group]; n = 16 [placebo-relapse group]). Responder rate (95% confidence interval) was 100.0% (80.6%–100.0%) in the placebo-relapse group and 95.0% (76.4%–99.1%) in the overall-relapse population. Across all patients, median time to functional improvement was 25 days. At treatment cessation, mean changes from pre-IVIG 10% baseline in adjusted INCAT and R-ODS centile scores were −1.9 and 12.9, respectively.
Conclusions
IVIG 10% effectively treated CIDP relapse and improved functional abilities.
1 Introduction
Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) is an autoimmune-mediated inflammatory condition characterized by peripheral nerve demyelination, resulting in progressive weakness and sensory loss in limbs [1-4]. CIDP is a slowly progressing and commonly relapsing disease that can impair activities of daily living [5], with additional patient burden brought about by associated fatigue, pain, and anxiety/depression [6, 7]. The current guideline from the European Academy of Neurology and Peripheral Nerve Society recommends intravenous immunoglobulin (IVIG) as a first-line induction or maintenance therapy option for patients with CIDP [8].
Immune globulin infusion (human) 10% solution (GAMMAGARD LIQUID [GGL]; Baxalta US Inc., a Takeda company, Cambridge, MA, USA) is an IVIG 10% therapy approved in the USA for the treatment of primary immunodeficiency diseases in adults and children aged 2 years or older, and as maintenance therapy for adults with multifocal motor neuropathy [9]. GGL is approved under the name Kiovig (Takeda Pharmaceuticals International AG, Vienna, Austria) in the EU for the treatment of CIDP in adults, adolescents, and children [10], and has also recently received US approval as therapy to improve neuromuscular disability and impairment in adults with CIDP [9] based on the results of the ADVANCE-CIDP IVIG trial described in this paper. GGL/Kiovig (or IVIG 10%) has been used extensively prior to its approval in the real-world setting [7, 11]. A large, real-world, observational study evaluated the safety of IVIG 10% versus other comparator IVIGs approved in the USA for patients with CIDP, and found no differences in the risks of IVIG-associated adverse events (AEs) [11]. Other studies have evaluated the safety and efficacy of IVIG 10% in CIDP [12-19], although the numbers of patients enrolled were small.
The ADVANCE-CIDP 1 trial evaluated the efficacy, safety, and tolerability of hyaluronidase-facilitated subcutaneous immunoglobulin (fSCIG) 10% (HYQVIA; Baxalta US Inc., a Takeda company, Cambridge, MA, USA) as a maintenance therapy to prevent relapse in patients with CIDP (NCT02549170). fSCIG 10% has now been approved in the USA for maintenance treatment in adults with CIDP on the basis of this study [20, 21], as well as in the EU as maintenance therapy in CIDP in adults, adolescents, and children following stabilization with IVIG [22]. In the event of relapse while receiving fSCIG 10% or placebo during ADVANCE-CIDP 1, patients were offered enrollment in ADVANCE-CIDP IVIG, which aimed to assess the efficacy, safety, and tolerability of IVIG 10% for the treatment of relapse in patients with CIDP. This report provides the results of the ADVANCE-CIDP IVIG study.
2 Methods
2.1 Study Design
ADVANCE-CIDP 1 was a phase 3, prospective, randomized, double-blind, placebo-controlled study, of which ADVANCE-CIDP IVIG was the open-label phase conducted between December 2016 and July 2020, enrolling patients from 15 sites in 10 countries (NCT02549170). Full details of the study design, eligibility criteria, and results from ADVANCE-CIDP 1 were recently published [20]. To enter the parent trial, ADVANCE-CIDP 1, participants had to be at least 18 years of age at screening, with a documented diagnosis of definite or probable CIDP [23]. Patients also had to have responded to immunoglobulin therapy in the past, have had at least 12 weeks of stable IVIG treatment equivalent to 0.4 to 2.4 g/kg per month, and had an adjusted Inflammatory Neuropathy Cause and Treatment (INCAT) disability score between 0 and 7 (inclusive). To enter ADVANCE-CIDP IVIG, eligible patients had to have a confirmed CIDP relapse (a ≥ 1-point increase in adjusted INCAT disability scores from pre-treatment baseline, as determined by two consecutive assessments obtained ≤ 7 days apart) during placebo or fSCIG 10% treatment arms in ADVANCE-CIDP 1 (Figure 1). IVIG 10% was administered at an induction dose of 2 g/kg divided over 2–5 consecutive days, followed by maintenance infusions at the same monthly equivalent dose as patients' pre-randomization IVIG, and was administered every 3 weeks for 6 months in total. The IVIG 10% dose was adjusted at the discretion of the investigator, as medically necessary and/or as tolerated by the patient, with a maximum dose of 100 g/1000 mL per day.
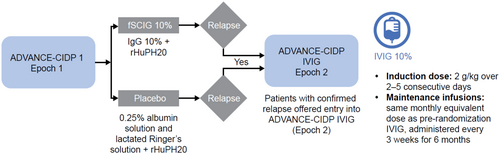
2.2 Study Outcomes
The primary efficacy outcome was the responder rate, defined as the proportion of patients who had an improvement in functional disability. Response was defined as a ≥ 1-point decrease in the adjusted INCAT disability score at treatment cessation or the last treatment visit relative to the pre-IVIG 10% baseline among patients who had a relapse while receiving placebo in ADVANCE-CIDP 1 (placebo-relapse group). The responder rate was also evaluated based on all patients experiencing relapse during ADVANCE-CIDP 1 (placebo-relapse group and fSCIG 10%-relapse group combined; n = 20). The responder rate was further evaluated based on patients experiencing relapse while receiving placebo in ADVANCE-CIDP 1 stratified by the size of the increase in the adjusted INCAT disability score (a ≤ 2- and a > 2-point increase) relative to the ADVANCE-CIDP 1 baseline.
The secondary efficacy outcome was the proportion of patients with a clinically meaningful improvement in functional ability. This was a composite outcome defined as a ≥ 1-point decrease in adjusted INCAT disability score at two consecutive time points, a ≥ 8 kPa increase in grip strength (in the more affected hand), or a ≥ 4-point increase in Rasch-built Overall Disability Scale (R-ODS) raw summed score at study completion relative to pre-IVIG 10% baseline.
Tertiary outcomes included the median time to functional improvement, defined as the time from the date of first IVIG 10% administration to a ≥ 1-point decrease in the adjusted INCAT disability score relative to pre-IVIG 10% baseline, as well as the overall mean change from pre-IVIG 10% baseline in adjusted INCAT disability score (median values also calculated), hand grip strength, and R-ODS centile score at treatment cessation. The proportion of patients whose grip strength in the most affected hand returned to pre-SCIG baseline (or better) in ADVANCE-CIDP IVIG, after worsening by ≥ 8 kPa in ADVANCE-CIDP 1, was also assessed. In addition, the proportion of patients whose R-ODS scores returned to or improved versus pre-SCIG baseline in ADVANCE-CIDP IVIG, after worsening by ≥ 4 points (raw scores) or ≥ 8 points (centile scores) in ADVANCE-CIDP 1, was evaluated. Patient-reported health-related quality of life (HRQoL) was evaluated using the Short Form-36 Health Survey (SF-36) Physical Component Summary score and EuroQoL Visual Analogue Score (EQ-VAS), while treatment satisfaction was investigated using the 9-item Treatment Satisfaction Questionnaire for Medication (TSQM-9). For each measure, the mean change in scores from pre-IVIG 10% baseline (assessments made at the time of evaluation for relapse in ADVANCE-CIDP 1 and just prior to initiation of intravenous treatment) to the end of the ADVANCE-CIDP IVIG treatment period was assessed.
Safety outcomes included the incidence of AEs, regardless of causality, including the rates of systemic and local AEs among all patients who received IVIG 10% during ADVANCE-CIDP IVIG. The number and proportion of infusions for which the infusion rate had been reduced and/or the infusion had been interrupted or stopped owing to intolerability and/or AEs were also evaluated.
2.3 Statistical Analysis
The primary outcome measure (responder rate) was described using the number (%) of patients in the placebo-relapse group who were responders to IVIG 10%. Assuming a responder rate of 65% for IVIG 10%, based on responder rates of 55% and 77% from previous studies [24, 25], the estimated sample size of at least 19 patients in the placebo-relapse group provided > 90% power to reject the null hypothesis that the responder rate is at most 24% at the two-sided 5% significance level and allowing for a 15% dropout rate. The overall responder rate was described using the number (%) of patients responding to IVIG 10% in the fSCIG 10%-relapse and placebo-relapse groups. The Wilson score method [26] was used to calculate 95% confidence intervals (CIs) for single proportions, and the two-sided Wilson score CI was not presented for any cohort with ≤ 5 patients. The analysis of responder rate stratified by change from pre-IVIG 10% baseline in adjusted INCAT disability score (a ≤ 2- and a > 2-point increase) was described using the number (%) of patients in the placebo-relapse group. Time to functional improvement was estimated using the Kaplan–Meier method. Changes in parameters from pre-IVIG 10% baseline were summarized using descriptive statistics. SF-36 Physical Component Summary, EQ-VAS, and TSQM-9 scores were summarized using descriptive statistics. AEs were coded using the Medical Dictionary for Regulatory Activities version 24.1 [27] or higher, and were summarized descriptively.
3 Results
3.1 Patient Demographics and Baseline Characteristics
During ADVANCE-CIDP 1, 28 patients experienced CIDP relapse (fSCIG 10%: n = 6; placebo: n = 22). Of these, 21 provided consent to enter ADVANCE-CIDP IVIG, and 20 patients received IVIG 10% (4 and 16 patients treated with fSCIG 10% and placebo, respectively, during ADVANCE-CIDP 1). One patient from a US site received GAMUNEX-C because this was the only IVIG therapy approved by the US Food and Drug Administration at the time of the study. Herein, the results reported are for patients receiving IVIG 10% (n = 20). All 20 patients who entered ADVANCE-CIDP IVIG completed the study with no loss to follow-up. The mean patient age was 50.9 years, 55.0% of patients were female, and 80.0% were White (Table 1).
| Characteristic | Placebo relapse (n = 16) | fSCIG 10% relapse (n = 4) | Total (N = 20) |
|---|---|---|---|
| Age, years, mean (SD) | 52.0 (13.7) | 46.3 (23.6) | 50.9 (15.5) |
| Sex, n (%) | |||
| Male | 7 (43.8) | 2 (50.0) | 9 (45.0) |
| Female | 9 (56.3) | 2 (50.0) | 11 (55.0) |
| Race, n (%) | |||
| White | 12 (75.0) | 4 (100) | 16 (80.0) |
| American Indian or Alaskan Native | 2 (12.5) | 0 | 2 (10.0) |
| Not reported | 2 (12.5) | 0 | 2 (10.0) |
| Ethnicity, n (%) | |||
| Hispanic or Latino | 6 (37.5) | 0 | 6 (30.0) |
| Not Hispanic or Latino | 6 (37.5) | 4 (100) | 10 (50.0) |
| Not reported | 4 (25.0) | 0 | 4 (20.0) |
| BMI, kg/m2, mean (SD) | 28.0 (4.0) | 28.0 (6.2) | 28.0 (4.3) |
| Time since first symptoms of CIDP in ADVANCE-CIDP 1 enrollment, years | |||
| n | 15 | 4 | 19 |
| Mean (SD) | 6.10 (4.2) | 4.70 (3.0) | 5.81 (4.0) |
| Median (range) | 5.40 (0.5–15.7) | 4.20 (2.2–8.2) | 5.40 (0.5–15.7) |
| Time since first diagnosis of CIDP in ADVANCE-CIDP 1 enrollment, years | |||
| n | 16 | 4 | 20 |
| Mean (SD) | 4.43 (3.3) | 2.78 (2.0) | 4.10 (3.1) |
| Median (range) | 3.70 (0.3–11.5) | 2.55 (0.6–5.4) | 3.35 (0.3–11.5) |
| Age at first diagnosis of CIDP, years | |||
| n | 16 | 4 | 20 |
| Mean (SD) | 47.5 (14.2) | 43.5 (22.9) | 46.7 (15.6) |
| Median (range) | 49.5 (25–66) | 41.5 (18–73) | 47.5 (18–73) |
| Time to relapse in ADVANCE-CIDP 1, days | |||
| n | 16 | 4 | 20 |
| Mean (SD) | 63.0 (41.7) | 91.3 (73.7) | 68.7 (48.6) |
| Median (range) | 49.0 (20–176) | 80.0 (20–185) | 51.0 (20–185) |
| Adjusted INCAT disability score at pre-IVIG 10% baseline | |||
| n | 16 | 4 | 20 |
| Mean (SD) | 5.2 (1.7) | 4.8 (1.9) | 5.1 (1.7) |
| Median (range) | 5.0 (3.0–8.0) | 5.5 (2.0–6.0) | 5.0 (2.0–8.0) |
| Maximum hand grip strength (most affected hand) at pre-IVIG 10% baseline | |||
| n | 16 | 4 | 20 |
| Mean (SD) | 26.6 (23.6) | 48.5 (13.0) | 31.0 (23.4) |
| Median (range) | 17.5 (0.0–74.0) | 46.0 (36.0–66.0) | 33.0 (0.0–74.0) |
| Centile R-ODS score at pre-IVIG 10% baseline | |||
| n | 15 | 4 | 19 |
| Mean (SD) | 37.9 (17.1) | 53.0 (22.3) | 41.1 (18.7) |
| Median (range) | 41.0 (6.0–63.0) | 46.5 (36.0–83.0) | 41.0 (6.0–83.0) |
- Abbreviations: BMI, body mass index; CIDP, chronic inflammatory demyelinating polyradiculoneuropathy; fSCIG 10%, hyaluronidase-facilitated subcutaneous immunoglobulin 10%; INCAT, Inflammatory Neuropathy Cause and Treatment; IVIG, intravenous immunoglobulin; R-ODS, Rasch-built Overall Disability Scale; SD, standard deviation.
3.2 Efficacy Outcomes
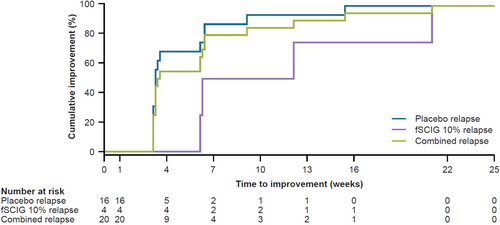
The responder rate (95% CI; primary endpoint) was 100.0% (80.6%–100.0%) in the placebo-relapse group (Table 2). One patient in the fSCIG 10%-relapse group, who initially responded to IVIG 10% with a 1-point reduction in INCAT score, subsequently developed clinical deterioration with a 1-point increase in INCAT score while still receiving IVIG 10% prior to the completion of the 6-month treatment period. Hence, the overall responder rate (95% CI) across all patients in both treatment relapse groups (n = 20) was 95.0% (76.4%–99.1%). Irrespective of the severity of relapse in the placebo-relapse group (> 2-point [n = 5/16; 31.3%] or ≤ 2-point [n = 11/16; 68.8%] increases in adjusted INCAT disability score), the responder rate was 100%. All patients achieved clinically meaningful improvement in functional ability based on the composite outcome (Table 2), with 100.0% of patients showing improvement in INCAT disability score, 60.0% of patients displaying improvement with respect to grip strength in the more affected hand, and 55.0% of patients with an increase of ≥ 4 points in R-ODS raw summed score. The median time to improvement in functional ability was 25 days from pre-IVIG 10% baseline for all patients, with a longer median time to improvement in the fSCIG 10%-relapse group than in the placebo-relapse group (65 vs. 23 days; Figure 2). Across all patients at treatment cessation (n = 20), clinically meaningful improvements from pre-IVIG 10% baseline in INCAT disability score (−1.9 points [mean]; −1.0 points [median]), hand grip strength (12.2 kPa in the more affected hand [mean]), and R-ODS centile score (12.9 points, at Week 24 [mean]) were observed (Table 2). Overall, there were 9 patients whose grip strength in the most affected hand had worsened by ≥ 8 kPa in ADVANCE-CIDP 1. The proportion of patients treated with IVIG 10% whose grip strength returned to pre-SCIG treatment baseline or better was 88.9% (8/9 patients) during ADVANCE-CIDP IVIG. In addition, there were 12 patients whose raw R-ODS scores had worsened by ≥ 4 points, and 9 patients whose centile R-ODS scores had worsened by ≥ 8 points, in ADVANCE-CIDP 1. The proportion of patients receiving IVIG 10% in ADVANCE-CIDP IVIG whose raw R-ODS scores had returned to pre-SCIG treatment baseline or better was 66.7% (8/12 patients) for those worsening by ≥ 4 points, and 77.8% (7/9 patients) for those with centile scores worsening by ≥ 8 points, respectively.
| Placebo relapse (n = 16) | fSCIG 10% relapse (n = 4) | Total (N = 20) | |
|---|---|---|---|
| Primary efficacy outcome | |||
| Primary analysis of responder rate based on placebo-relapse group, n (%) | 16 (100.0) | NA | NA |
| Wilson 95% CIa | 80.6–100.0 | NA | NA |
| Responder rate analysis based on all observed cases, n (%) | 16 (100.0) | 3 (75.0) | 19 (95.0) |
| Wilson 95% CIb | 80.6–100.0 | NA | 76.4–99.1 |
| Responder rate analysis stratified by increase in adjusted INCAT disability score, based on placebo-relapse group | |||
| Increase from baseline in adjusted INCAT disability score of ≤ 2 points, n (% of patients responding)c | 11 (100.0) | NA | NA |
| Increase from baseline in adjusted INCAT disability score of > 2 points, n (% of patients responding)c | 5 (100.0) | NA | NA |
| Secondary efficacy outcomes | |||
| Improvement of functional ability, composite outcomed | |||
| Patients who improved during ADVANCE-CIDP IVIG, n (%) | 16 (100.0) | 4 (100.0) | 20 (100.0) |
| Wilson 95% CIb | 80.6–100.0 | NA | 83.9–100.0 |
| Met INCAT component criterion, n (%) | 16 (100.0) | 4 (100.0) | 20 (100.0) |
| Met grip strength component criterion, n (%) | 10 (62.5) | 2 (50.0) | 12 (60.0) |
| Met R-ODS raw summed score component criterion, n (%) | 11 (68.8) | 0 | 11 (55.0) |
| Tertiary efficacy outcomes | |||
| Adjusted INCAT disability score | |||
| Patients with data on change from pre-IVIG 10% baseline in adjusted INCAT disability score at treatment cessation in ADVANCE-CIDP IVIG, n | 15 | 4 | 19 |
| Change in adjusted INCAT disability score from pre-IVIG 10% baseline at treatment cessation | |||
| Mean (SD) | −2.2 (1.42) | −1.0 (0.82) | −1.9 (1.39) |
| Median (min, max) | −2.0 (−5.0, −1.0) | −1.0 (−2.0, 0.0) | −1.0 (−5.0, 0.0) |
| Grip strength (in the more affected hand) | |||
| Patients with data on change from pre-IVIG 10% baseline in grip strength (in the more affected hand) at treatment cessation in ADVANCE-CIDP IVIG, n | 15 | 4 | 19 |
| Change in grip strength (in the more affected hand) from pre-IVIG 10% baseline at treatment cessation, kPa | |||
| Mean (SD) | 13.9 (13.53) | 6.0 (7.30) | 12.2 (12.73) |
| R-ODS | |||
| Patients with data on change from pre-IVIG 10% baseline in R-ODS centile score at treatment cessation (Week 24) in ADVANCE-CIDP IVIG, n | 15 | 4 | 19 |
| Change in R-ODS centile score from pre-IVIG 10% baseline at treatment cessation (Week 24) | |||
| Mean (SD) | 17.0 (17.58) | −2.3 (3.40) | 12.9 (17.53) |
- Abbreviations: CI, confidence interval; CIDP, chronic inflammatory demyelinating polyradiculoneuropathy; fSCIG 10%, hyaluronidase-facilitated subcutaneous immunoglobulin 10%; INCAT, Inflammatory Neuropathy Cause and Treatment; IVIG, intravenous immunoglobulin; NA, not applicable; R-ODS, Rasch-built Overall Disability Scale.
- a 95% Wilson CI for the estimated response rate expressed as a percentage; the lower limit was compared with a historical-control placebo response rate of 24%.
- b Two-sided 95% Wilson CIs for single proportions expressed as a percentage are not presented for any group with ≤ 5 patients.
- c The overall number of patients with a change from baseline in adjusted INCAT disability score of ≤ 2 and > 2 points was 11 and 5, respectively. 95% CIs are not reported owing to 100.0% response rates.
- d A clinically meaningful improvement in functional ability was defined as a ≥ 1-point decrease in the adjusted INCAT disability score at two consecutive time points or CIDP improvement (defined as a ≥ 8 kPa increase in grip strength in the more affected hand or a ≥ 4-point increase in raw R-ODS score at completion of the IVIG 10% treatment period [6 months] or at the last study visit of the IVIG 10% treatment period, relative to the pre-IVIG 10% treatment baseline score).
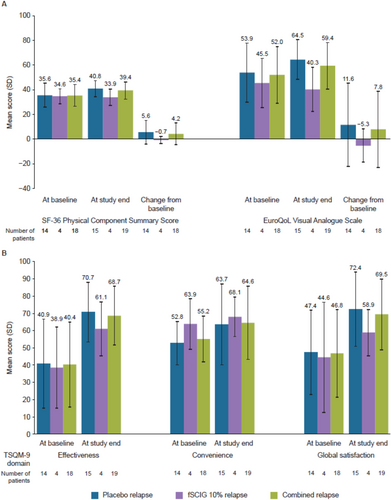
3.3 Patient-Reported Outcomes
Across all patients, the SF-36 Physical Component Summary score and the EQ-VAS score were maintained (marginal differences vs. baseline) or meaningfully improved during treatment with IVIG 10%, with the change from baseline typically greater for patients who relapsed on placebo during ADVANCE-CIDP 1 (Figure 3A). At the end of treatment, the overall mean TSQM-9 global satisfaction score was 69.5 for the placebo-relapse and fSCIG 10%-relapse combined group. For all patients with available data, the overall mean scores were 68.7 for effectiveness and 64.6 for convenience at the end of ADVANCE-CIDP IVIG (Figure 3B).
3.4 Safety Outcomes
Overall, 60 AEs were reported in 14 patients (70.0%), with event rates of 0.15 per infusion and 3.00 per patient (Table 3). Treatment-related AEs occurred in 11 patients (55.0%), with 2 patients (10.0%) experiencing a severe event (headache reported by one patient and pain in the body, arms, legs, and joints reported by one patient). The most frequently reported AEs (> 5% of patients) were headache (40.0%) and pyrexia (10.0%). No patients experienced local AEs, and no patients reported serious AEs or AEs leading to study drug withdrawal or early discontinuation. No deaths were reported during the study. Overall, 389 infusions were administered, of which less than 1% had the infusion rate reduced or the infusion interrupted or stopped owing to intolerability.
| Category, n (%) | Placebo relapse (n = 16) | fSCIG 10% relapse (n = 4) | Total N = 20) |
|---|---|---|---|
| Patients experiencing AEs | 11 (68.8) | 3 (75.0) | 14 (70.0) |
| Patients experiencing systemic AEs | 11 (68.8) | 3 (75.0) | 14 (70.0) |
| Patients experiencing local AEs | 0 | 0 | 0 |
| Patients experiencing AEs related to study treatment | 8 (50.0) | 3 (75.0) | 11 (55.0) |
| Severity of AEs | |||
| Mild | 8 (50.0) | 1 (25.0) | 9 (45.0) |
| Moderate | 2 (12.5) | 1 (25.0) | 3 (15.0) |
| Severe | 1 (6.3) | 1 (25.0) | 2 (10.0) |
| Total infusions, n | 328 | 61 | 389 |
| Infusions that were interrupted/stopped and/or had the rate reduced owing to intolerability | 1 (0.3) | 1 (1.6) | 2 (0.5) |
| Category | Events per infusion | Events per patient | Events per infusion | Events per patient | Events per infusion | Events per patient |
|---|---|---|---|---|---|---|
| All AEs | 0.11 | 2.19 | 0.41 | 6.25 | 0.15 | 3.00 |
| All systemic AEs | 0.11 | 2.19 | 0.41 | 6.25 | 0.15 | 3.00 |
| All temporally associated AEs | 0.08 | 1.56 | 0.18 | 2.75 | 0.09 | 1.80 |
| Any systemic AEs related to study treatment | 0.07 | 1.44 | 0.13 | 2.00 | 0.08 | 1.55 |
- Abbreviations: AE, adverse event; fSCIG 10%, hyaluronidase-facilitated subcutaneous immunoglobulin 10%.
4 Discussion
This study demonstrates that IVIG 10% is an efficacious induction treatment for CIDP relapse. The responder rate in the placebo-relapse group was 100%; the lower CI limit (80.6%) exceeded the historical control placebo responder rate of 24%, providing strong supportive evidence for the efficacy of IVIG 10% for the treatment of CIDP. Further analysis of treatment response revealed that all but 1 patient in the overall study population responded to IVIG 10% treatment, and the responder rates did not differ between subsets with and without a > 2-point increase in adjusted INCAT disability score during the relapse (100.0% in both groups). The secondary efficacy composite outcome provided further support for the primary analysis results by showing that functional improvement after initiating IVIG 10% was maintained during the remainder of the study in 95% of the patients. Clinically meaningful improvements were observed in various functional metrics, including the adjusted INCAT disability score, hand grip strength, and R-ODS score versus the pre-IVIG 10% baseline. After initiating IVIG 10%, the response to treatment was rapid, with a median time to improvement of 25 days.
IVIG 10% treatment was associated with improvement in HRQoL versus baseline, especially for patients in the placebo-relapse group. Treatment satisfaction also improved among patients who received IVIG 10% relative to pre-IVIG 10% baseline, with overall scores at the end of treatment supporting increased treatment satisfaction. In addition, IVIG 10% demonstrated a beneficial safety and tolerability profile during the study, with no serious AEs or AEs leading to early discontinuation or deaths reported [28].
The current study examined a subset of patients experiencing relapse in the context of receiving fSCIG 10% or placebo therapy. These groups may reflect different subsets of CIDP, with potential variation in treatment response. Notably, a longer median time to improvement in functional ability was observed in the fSCIG 10%-relapse group than in the placebo-relapse group. Patients in the fSCIG 10%-relapse group were also generally younger than those in the placebo-relapse group at enrollment and had a shorter duration since their first CIDP symptoms or diagnosis. Further studies are warranted to understand whether the population experiencing relapse while receiving fSCIG 10% represents a distinct CIDP phenotype characterized by a poor response to immunoglobulin treatment. We acknowledge that the small number of patients in the fSCIG 10%-relapse group may limit the interpretation of the results.
The limitations of this study require consideration. The sample size was small, resulting in large CIs for the point estimate for the primary endpoint. However, the lower limit of the 95% CI was 80.6%, supporting that IVIG 10% exhibits profound efficacy in the treatment of CIDP relapse. It should also be noted that the results of this study are applicable only to patients who have previously responded to immunoglobulin treatment. Furthermore, ADVANCE-CIDP IVIG was an open-label phase of the ADVANCE-CIDP 1 study, in which some outcomes were dependent on subjective patient perception; hence, the results may have potentially been affected by associated biases. To reduce the possibility of bias, the investigators who performed the INCAT assessments did not have access to information related to any AEs experienced by patients, and the investigators who performed these assessments for a particular patient remained consistent throughout the study. IVIG 10% has been approved in multiple countries for the treatment of primary immunodeficiency and immune disorders, such as multifocal motor neuropathy and CIDP, demonstrating a favorable safety profile [29]. The current study results are consistent with the known safety profile of IVIG 10% and do not indicate any new safety signals [11, 28]. IVIG 10% thus recently received US approval for the treatment of CIDP, based on the results described in this paper [9].
In conclusion, IVIG 10% effectively treated CIDP relapse, achieving a 100.0% response rate in patients who had a relapse on placebo (primary outcome) and 95.0% in the overall population, as well as improved functional ability with a favorable safety and tolerability profile.
Author Contributions
Mamatha Pasnoor: writing – review and editing, investigation, validation, visualization. Colin Anderson-Smits: writing – review and editing, conceptualization, formal analysis, methodology, resources, supervision, validation, visualization, writing – original draft. Todd Levine: writing – review and editing. Vera Bril: writing – review and editing, investigation, methodology, resources. Juan Marcos Solano: writing – review and editing, investigation. Konrad Rejdak: writing – review and editing, investigation. Josep Gamez: writing – review and editing, conceptualization, investigation, funding acquisition, writing – original draft, methodology, validation, visualization, formal analysis, project administration, data curation, supervision, resources. Elisabeth Chroni: writing – review and editing, investigation. Carlos Casasnovas: writing – review and editing, validation, visualization. Enrico Marchioni: writing – review and editing, data curation, investigation. Gabriele Siciliano: writing – review and editing, conceptualization, investigation. Dario Cocito: writing – review and editing, investigation. K. Sivakumar: writing – review and editing, data curation, investigation, supervision. Alberto Rivero: writing – review and editing, data curation, investigation. Kim Duff: writing – review and editing, conceptualization, investigation, project administration. Erin Greco: writing – review and editing, conceptualization, formal analysis, investigation, methodology, project administration, resources, software, validation, visualization. Massimo Corbo: writing – review and editing, investigation. Shabbir Hasan: writing – review and editing, resources. Amir Dori: writing – review and editing, data curation, investigation. Jens Schmidt: writing – review and editing, investigation. Jamie Wood: writing – review and editing, conceptualization, writing – original draft. Zhaoyang Li: writing – review and editing, data curation, formal analysis, investigation, methodology, resources, supervision. Hakan Ay: writing – review and editing, conceptualization, investigation, funding acquisition, writing – original draft, methodology, validation, visualization, software, formal analysis, project administration, data curation, supervision, resources.
Acknowledgments
The authors thank the patients who participated in this trial, their caregivers, the study-site personnel, and the investigators.
Ethics Statement
This study was conducted in accordance with the Code of Federal Regulations pertaining to clinical trials, the Declaration of Helsinki, the International Conference on Harmonization Guidelines for Good Clinical Practice, and all applicable national and local regulations. The regional and local Institutional Review Boards providing approval were: Copernicus Group Institutional Review Board; Quorom Review Institutional Review Board; National Research Ethics Service Committee West of Scotland; Dokuz Eylul University Clinical Trials Ethics Committee; Ethics Committee for La Fe University and Polytechnic Hospital; EK-UN Bratislava University, Hospital St. Cyril and Methodius; Ethics Committee of Serbia; Bioethics Committee for Scientific Research at the Medical University of Gdańsk; Regional Committees for Medical and Healthcare Research Ethics (Norway); Clinical Ethics Committee of the Pisa University Hospital; National Ethics Committee (Greece); Ethics Committee for the Department of Medicine of the Philipps University of Marburg; Scientific Medical Ethics Committees (Denmark); Ethics Commission for the Ostrava University Hospital; Agency for Medicinal Product and Medical Devices of Croatia; National Research Ethics Commission (Brazil); University Health Network Research Ethics Board (Ontario, Canada); University of Alberta Health Research Ethics Board; Western University Office of Research Ethics Board (Ontario, Canada); Cleveland Clinic Institutional Review Board; The Walton Centre NHS Foundation Trust; North Bristol NHS Trust; R&D King's Health Partners; Ethics Commission of the Nitra University Hospital; EK-UN Bratislava University Hospital-Academician Ladislav Dérer Hospital; Ethics Committee of Clinical Center Niš; Ethics Committee of Clinical Center Serbia; Ethics Committee of Military Medical Academy (Serbia); National Institute of Medical Sciences (Mexico); Ethics Committee for the Health and Science, City of Health and Science University Hospital Turin; Single Regional Ethics Committee (Udine, Italy); Ethics Committee for Tor Vergata Polyclinic University Hospital; Scientific Ethics Committee for Gaetano Martino University Hospital; IRCCS San Martino Polyclinic Hospital; Ethics Committee IRCCS San Raffaele Hospital; Ethics Committee of Milan (Area B); Ethics Committee for Chaim Sheba Medical Center; Scientific Committee of University General Hospital of Patra; Ethics Committee of the University Medical Center Göttingen; Ethics Committee of the Medical Faculty of the University of Leipzig; Ethics Committee of the Motola University Hospital; Ethics and Research Committee of the IPS University of Columbia; Biomedical Ethics and Research Committee (Argentina); British Hospital Institutional Review Committee (Argentina); Research Protocols Ethics Committee (Argentina); Research Ethics Committee of the Curitiba Neurology Institute/Ecoville Hospital; Research Ethics Committee of the Clinical Hospital of the Faculty of Medicine of Ribeirão Preto University of São Paulo. All patients agreed to participate in the study by providing written informed consent.
Conflicts of Interest
M.P. has served as a consultant or medical advisor for Alexion, argenx, Catalyst Pharmaceuticals, CSL Behring, Immunovant, Janssen, Momenta (Johnson & Johnson), Takeda Pharmaceuticals Ltd., Terumo BCT, and UCB Pharma. C.A.-S. was an employee of Takeda Development Center Americas, Inc. and a Takeda shareholder at the time of the study, and is currently an employee of Gilead Sciences, Inc. T.L. has served as a consultant for Alexion, FFF Enterprises, and Immunovant. V.B. has acted as a consultant for Akcea Therapeutics, Alnylam Pharmaceuticals, argenx, AstraZeneca-Alexion, CSL Behring, Grifols, Immunovant, Ionis Pharmaceuticals, Janssen, Momenta (Johnson & Johnson), Novo Nordisk, Octapharma, Pfizer, Powell Mansfield, Inc., Roche, Sanofi, Takeda Pharmaceuticals Ltd., and UCB Pharma, and has received research support from Akcea Therapeutics, argenx, AstraZeneca-Alexion, CSL Behring, Grifols, Immunovant, Ionis Pharmaceuticals, Momenta (Johnson & Johnson), Octapharma, Takeda Pharmaceuticals Ltd., and UCB Pharma. J.M.S. has received funds for an investigator-initiated research project and travel expenses from Takeda Pharmaceuticals Ltd. K.R. has received speaker honoraria and travel expenses for participation in scientific meetings, and has participated in advisory boards for Bayer, Biogen, Merck, Novartis, Roche, Sanofi-Genzyme, and Teva Pharmaceuticals. J.G. has received speaker honoraria fees from CSL Behring and Takeda Pharmaceuticals Ltd. E.C. has received speaker honoraria and advisory board membership fees from argenx-Medison, AstraZeneca-Alexion, Genesis Pharm, ITF Hellas, Takeda Pharmaceuticals Ltd., and UCB Pharma. C.C. has received funding for advisory boards from Alexion-AstraZeneca Rare Diseases, Alnylam Pharmaceuticals, argenx, CSL Behring, Pfizer, PharmaNext, and UCB Pharma, speaker honoraria from Alexion-AstraZeneca Rare Diseases, Alnylam Pharmaceuticals, CSL Behring, Ferrer, Pfizer, and Sobi, and research support from Alexion-AstraZeneca Rare Diseases, CSL Behring, and Pfizer. E.M., D.C., K.S., A.R., and M.C. have nothing to disclose. G.S. has served as a consultant or medical advisor for Alexion, Amicus, argenx, Biogen, CSL Behring, Janssen, and Sanofi. K.D., E.G., Z.L., and H.A. are employees of Takeda Development Center Americas, Inc. and are Takeda shareholders. S.H. was an employee of Takeda Development Center Americas, Inc. and a Takeda shareholder at the time of the study, and is currently a consultant for Logical Apex LLC. A.D. has served as a medical advisor for Takeda Pharmaceuticals Ltd., and has received payments for speaker honoraria and travel expenses from Pfizer and Sanofi and for research projects from Alnylam Pharmaceuticals, Biogen, and Pfizer. J.S. has received payments for advisory boards, speaker honoraria, travel expenses, and research projects from Abcuro, Alnylam Pharmaceuticals, argenx, Biotest, CSL Behring, Euroimmun, Janssen, Kezar Life Sciences, LFB, Novartis, Octapharma, and UCB Pharma. J.W. was an employee of Takeda Development Center Americas, Inc. and is a Takeda shareholder, and is currently an employee of argenx US Inc.
Open Research
Data Availability Statement
The data sets, including the redacted study protocol, redacted statistical analysis plan, and individual participant data supporting the results reported in this article, will be made available within 3 months from the initial request to researchers who provide a methodologically sound proposal. The data will be provided after its de-identification, in compliance with applicable privacy laws, data protection, and requirements for consent and anonymization.




