Characteristics and temporal evolution of asymptomatic diffusion-weighted imaging lesions in patients with cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL)
Abstract
Background and Purpose
The role of asymptomatic diffusion-weighted imaging-positive (aDWI+) lesions in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) patients remains unclear, and their radiographic features may differ from those of symptomatic diffusion-weighted imaging-positive (sDWI+) lesions. We aimed to investigate the clinicoradiographic characteristics of aDWI+ lesions in CADASIL patients.
Methods
We conducted a retrospective analysis using data from the Taiwan CADASIL Registry. aDWI+ lesions were defined as incidentally detected DWI+ lesions without corresponding acute neurological deficits. We compared the baseline clinical characteristics of patients with and without aDWI+ lesions and analyzed their radiological features and evolution in relation to sDWI+ lesions.
Results
Among 154 enrolled patients (mean age 62 ± 10 years), 17 (11%) had aDWI+ lesions. Baseline clinical characteristics were similar in the two groups, but those with aDWI+ lesions had more lacunes (median 8 vs. 2), multiple cerebral microbleeds (CMBs; 85% vs. 40%), and anterior temporal white matter hyperintensity (WMH; 47% vs. 14%). Multivariable analysis showed that aDWI+ lesions were associated with anterior temporal WMH (odds ratio 5.7, 95% confidence interval 1.5–21.0) after adjusting for multiple lacunes, multiple CMBs, and total WMH score. Compared to sDWI+ lesions, aDWI+ lesions were more often small infarcts (<1 cm; 89% vs. 23%) and less likely to involve the corticospinal tract (11% vs. 96%). Among the 11 aDWI+ lesions with follow-up magnetic resonance imaging, seven became microinfarcts, three became lacunes, and one disappeared.
Conclusions
aDWI+ lesions in CADASIL are not uncommon and are associated with higher burdens of small vessel disease and anterior temporal WMH. Further research is needed to assess their long-term impact on CADASIL.
INTRODUCTION
Cerebral small vessel disease (CSVD) is an important cause of neurodegeneration, leading to stroke, cognitive impairment and gait disturbance. Standard neuroimaging features of small vessel disease (SVD) detected on brain magnetic resonance imaging (MRI) include white matter hyperintensity (WMH), recent small subcortical infarcts (RSSIs), lacunes, cerebral microbleeds (CMBs), enlarged perivascular spaces, and brain atrophy [1]. Cross-sectional studies have shown that subclinical, or asymptomatic diffusion-weighted imaging-positive (aDWI+) lesions can be observed in patients with SVD, such as those with cerebral amyloid angiopathy and hypertension-associated SVD [2, 3]. Several studies have indicated that diffusion-weighted imaging-positive (DWI+) lesions in individuals with SVD are associated with a higher burden of SVD MRI markers at baseline [4, 5]. Growing evidence suggests that aDWI+ lesions may contribute to the progression of SVD markers, such as WMH or cortical microinfarcts [6-8]. Thus, these lesions are increasingly recognized as potential surrogate markers of disease activity in SVD [2].
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is the most prevalent hereditary CSVD [9, 10]. It is caused by mutations in the NOTCH3 gene on chromosome 19p13.12, which encodes a transmembrane receptor expressed on vascular smooth muscle cells and pericytes [9, 10]. In addition to the typical MRI features of SVD, anterior temporal WMH is highly specific to CADASIL [11]. A cross-sectional study from the United Kingdom found aDWI+ lesions in two of 19 CADASIL patients [2]. However, the exact role of aDWI+ lesions in the progression of CADASIL remains uncertain.
We aimed to investigate the prevalence and clinical and imaging features of aDWI+ lesions in patients with CADASIL. It is unclear whether the appearance of aDWI+ lesions in CADASIL patients is associated with the severity of underlying arteriopathy or how it impacts short-term clinical outcomes compared to symptomatic diffusion-weighted imaging-positive (sDWI+) lesions; therefore, we compared the radiographic characteristics, temporal evolution, and stroke incidence of aDWI+ lesions with those of sDWI+ lesions.
METHODS
Study population
This was a retrospective analysis of the data collected from the prospective Taiwan CADASIL Registry cohort at the National Taiwan University Hospital, as previously described in detail [12]. Study participants with genetically confirmed cysteine-altering variants in NOTCH3, recruited consecutively from February 2019 to September 2023, were included for clinical and neuroimaging analysis. Baseline clinical characteristics documented at enrollment included age, sex, education level, modified Rankin Scale score, family history of stroke, presence of the NOTCH3 p.R544C variant, medical history, and clinical manifestation. High family burden of stroke was defined as having at least two individuals among parents or siblings who have experienced a stroke. Cognitive impairment was defined as subjective cognitive complaints with a Mini-Mental State Examination score <24 or a Clinical Dementia Rating scale score ≥0.5 [13]. Patients without a brain MRI scan available after enrollment were excluded. All enrolled patients were regularly followed up at outpatient visits with MRI scans at intervals of 1–2 years. Any stroke incidence, death, or loss of follow-up was recorded. Follow-up was censored at the time of patient death, loss to follow-up, or on May 1, 2024.
Brain MRI acquisition and analysis
A 3-Tesla brain MRI scan (TIM Trio; Siemens) was obtained for each participant after enrollment, regardless of any prior MRI conducted for other clinical purposes. The acquisition sequences included three-dimensional (3D) T1-weighted magnetization-prepared rapid gradient echo imaging (voxel size 1.0 × 1.0 × 1.0 mm3), axial T2-weighted imaging (voxel size 0.6 × 0.6 × 4 mm3), 3D fluid-attenuated inversion recovery (FLAIR) imaging (voxel size 1.0 × 1.0 × 1.0 mm3), susceptibility-weighted imaging (SWI; voxel size 0.8 × 0.8 × 1.6 mm3), diffusion-weighted imaging (DWI; voxel size 1.8 × 1.8 × 5.0 mm3), diffusion tensor imaging, and apparent diffusion coefficient (ADC) mapping.
This study included a baseline MRI scan conducted at the outpatient visit following enrollment, with follow-up MRI scans performed at intervals of 1–2 years. If individuals presented to the emergency department with acute neurological symptoms, the study MRI was performed at least 3 months after the acute stage of the index stroke.
Identification of DWI+ lesions
We defined DWI+ lesions as hyperintensity lesions on DWI scans that were hypo- or iso-intense on the ADC map. To exclude T2 shine-through artifacts that might mimic DWI+ lesions, we carefully correlated DWI findings with ADC maps to confirm true restricted diffusion. Confirmation of DWI+ lesions was achieved through a consensus meeting with a senior neuroradiologist (Y.F.C.). An experienced neurologist (Y.C.S.) reviewed the medical records and categorized all DWI+ lesions as asymptomatic or symptomatic. aDWI+ lesions were defined as incidentally detected DWI+ lesions without corresponding acute neurological deficits, while sDWI+ lesions were those corresponding to acute stroke symptoms suggestive of recent infarction. Final confirmation of aDWI+ or sDWI+ lesions was determined by the senior investigator (S.C.T.). To compare the radiographic features, we defined lesions with an axial diameter of <1 cm as small infarcts and those with an axial diameter of ≥2 cm as large DWI+ lesions. The diffusion tensor imaging-based fiber tract map, a color-coded fractional anisotropy map, was used to identify corticospinal tract involvement, which was considered present if any portion of the tract was deviated or showed decreased anisotropy.
Other MRI markers of SVD
Radiological markers of SVD were defined according to the STandards for ReportIng Vascular changes on nEuroimaging (STRIVE) criteria [14]. The severity of WMH in the periventricular and deep white matter was assessed on FLAIR sequences and graded using the Fazekas scale [15]. The total WMH score was defined as the sum of the periventricular and deep white matter WMH scores. Enlarged perivascular spaces were visualized on T2-weighted imaging, with their severity in the basal ganglia and centrum semiovale evaluated using a 4-point rating scale [16]. The presence and number of lacunes were assessed using T1, T2, and FLAIR imaging. The location and number of CMBs were evaluated on SWI scans [17]. We defined multiple lacunes as having > 5 lacunes and multiple CMBs as having > 10 CMBs. All MRI scans were reviewed independently by two investigators blinded to clinical and outcome data (Y.W.C., C.H.C.); any discrepancies were resolved by a senior neuroradiologist (Y.F.C.).
Assessment of DWI+ lesion evolution
The temporal evolution of DWI+ lesions on the nearest follow-up MRI scans was evaluated by two investigators (Y.C.S. and C.H.C.) and confirmed by a senior neuroradiologist (Y.F.C.). The evolution of DWI+ lesions can be classified into one of the following features: merging into WMH, becoming a lacune (with an axial diameter between 3 and 15 mm) or a microinfarct (defined as a lacune-like lesion with an axial diameter <3 mm) [18], becoming a CMB, or disappearing. To distinguish a microinfarct from enlarged perivascular spaces, a lesion was classified as a microinfarct only if its location corresponded to a previously documented DWI+ lesion. To assess changes in DWI+ lesions, images from various modalities, including DWI, T2-weighted imaging, FLAIR imaging, and SWI, were co-registered with the T1-weighted images at the time of lesion occurrence.
Statistical analyses
Continuous variables are presented as median (interquartile range [IQR]) or mean (standard deviation [SD]), while categorical variables are presented as counts (percentages). Baseline clinical and MRI characteristics were compared between patients with and without any aDWI+ lesions during the study period using nonparametric tests due to the small sample size and non-normal distribution. Variables that were statistically significant in the univariable analysis were included in a multivariable logistic regression model to estimate the odds ratio (OR) and 95% confidence interval (CI) for aDWI+ lesions. The multivariable model included multiple lacunes, multiple CMBs, anterior temporal WMH, and total WMH score. Kaplan–Meier analysis with the log-rank test was used to compare stroke-free survival rates between patients with aDWI+ and sDWI+ lesions during the follow-up period. Survival curves were plotted using PRISM software. The two-tailed significance level was set at 0.05. All statistical analyses were performed with SPSS, version 19.0 for Windows (IBM Corp., Armonk, NY, USA).
Standard protocol approvals, registrations, and patient consents
The study was conducted with the approval of the Institutional Review Board (no. 201807044RIND) of the National Taiwan University Hospital, and in accordance with their guidelines. Written informed consent was obtained from all participants or their relatives.
RESULTS
Prevalence of aDWI+ lesions
From February 2019 to September 2023, 157 patients were enrolled in the Taiwan CADASIL Registry. Three patients were excluded due to a lack of available MRI scans (Figure 1). The remaining 154 patients (mean age 62.1 ± 10.3 years, 52% male) were included in this study. At baseline, 13 of the 154 patients (8.4%) exhibited aDWI+ lesions. During a median (IQR) follow-up period of 3.2 (1.7–4.5) years, with a median (IQR) of 2 (1–3) MRI scans, four additional patients developed aDWI+ lesions. One patient had aDWI+ lesions at both baseline and a follow-up MRI scan. Overall, 17 of the 154 patients (11%) had aDWI+ lesions either at baseline or during follow-up.
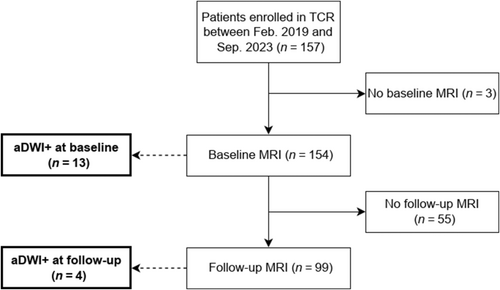
Differences between patients with and without aDWI+ lesions
Baseline clinical characteristics were comparable between patients with and without any aDWI+ lesions (Table 1). There was no significant difference in the number of MRI scans between the two groups (1 [2, 3] for aDWI+ vs. 2 [1–3] for non-aDWI+; p = 0.264). The baseline MRI markers for both groups are summarized in Table 2. Patients with aDWI+ lesions had more lacunes (median 8 [6–15] vs. 2 [0–9]; p = 0.003), a higher prevalence of multiple lacunes (n ≥ 5, 77% vs. 39%; p = 0.004), multiple CMBs (n ≥ 10, 85% vs. 40%; p = 0.003), and mixed CMBs (85% vs. 52%; p = 0.04). They also had higher periventricular WMH scores (3 [3] vs. 3 [2, 3]; p = 0.04), subcortical WMH scores (3 [3] vs. 3 [2, 3]; p = 0.03), and total WMH scores (6 [6] vs. 5 [4–6]; p = 0.01), and a higher prevalence of anterior temporal WMH (47% vs. 14%; p = 0.003) compared to those without aDWI+ lesions. In multivariate logistic regression analysis (Table S1), aDWI+ lesions were associated with anterior temporal WMH (OR 5.65, 95% CI 1.52–21.00; p = 0.01) after simultaneously adjusting for multiple lacunes, multiple CMBs, and total WMH score.
| aDWI+ (n = 17) | Non-aDWI+ (n = 137) | p value | |
|---|---|---|---|
| Demographics | |||
| Age at enrollment, years | 63 (56–65) | 63 (56–70) | 0.409 |
| Male, n (%) | 10 (59) | 70 (51) | 0.613 |
| BMI, kg/m2 | 25 (23–25) | 24 (22–27)a | 0.855 |
| Education level, yearsb | 14 (12–16) | 12 (10–16)c | 0.274 |
| Modified Rankin scale score | 1 (0–2) | 0 (0–1) | 0.499 |
| High family burden of stroked, n (%) | 8 (53) | 59 (47) | 0.786 |
| NOTCH3 p.R544C variant, n (%) | 14 (82) | 128 (93) | 0.131 |
| Medical history, n (%) | |||
| Hypertension | 12 (71) | 77 (56) | 0.306 |
| Diabetes mellitus | 3 (18) | 28 (20) | 1.000 |
| Hyperlipidemia | 10 (59) | 75 (55) | 0.801 |
| Coronary artery disease | 1 (6) | 4 (3) | 0.447 |
| Atrial fibrillation | 0 (0) | 8 (6) | 0.599 |
| History of stroke | 10 (59) | 72 (53) | 0.798 |
| Only IS | 9 (53) | 53 (39) | 0.300 |
| Only ICH | 2 (12) | 28 (20) | 0.528 |
| Both IS and ICH | 1 (6) | 9 (7) | 1.000 |
| Smoking, ever | 5 (29) | 35 (26) | 0.771 |
| Alcohol, ever | 1 (6) | 12 (9) | 1.000 |
| Clinical manifestation, n (%) | |||
| Psychiatric symptoms | 5 (29) | 42 (31) | 1.000 |
| Headache | 2 (12) | 28 (20) | 0.528 |
| Cognitive impairmente | 7 (41) | 51 (37) | 0.794 |
| Gait disturbance | 7 (41) | 60 (45) | 1.000 |
- Note: Data are median (interquartile range) unless otherwise stated.
- Abbreviations: aDWI+, asymptomatic diffusion-weighted imaging-positive; BMI, body mass index; CADASIL, cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy; ICH, intracerebral hemorrhage; IS, ischemic stroke; MRI, magnetic resonance imaging; y, years.
- a Only 129 patients had complete data.
- b Refers to years of schooling.
- c Only 128 patients had complete data.
- d Refers to having at least two individuals among parents or siblings who have experienced a stroke.
- e Refers to subjective cognitive complaints with a Mini-Mental State Examination score <24 or a Clinical Dementia Rating scale score ≥0.5.
| aDWI+ (n = 17) | Non-aDWI+ (n = 137) | p value | |
|---|---|---|---|
| Number of lacunes | 8 (6–15) | 2 (0–9) | 0.003* |
| Multiple lacunes (n ≥ 5), n (%) | 13 (77) | 54 (39) | 0.004* |
| Number of CMBs | 14 (11–30)a | 5 (1–27)b | 0.054 |
| Multiple CMBs (n ≥ 10), n (%) | 11 (85)a | 54 (40)b | 0.003* |
| Location of CMBs, n (%) | |||
| None | 1 (6)a | 42 (31)b | 0.042* |
| Pure lobar | 1 (8)a | 5 (4)b | 0.430 |
| Pure deep | 1 (8)a | 26 (19)b | 0.464 |
| Mixed | 11 (85)a | 70 (52)b | 0.038* |
| Macrohemorrhage | 2 (15)a | 30 (22)b | 0.735 |
| Periventricular WMH score | 3 (3–3) | 3 (2–3) | 0.042* |
| Subcortical WMH score | 3 (3–3) | 3 (2–3) | 0.026* |
| Total WMH score | 6 (6–6) | 5 (4–6) | 0.014* |
| Anterior temporal WMH, n (%) | 8 (47) | 19 (14) | 0.003* |
| External capsule WMH, n (%) | 12 (71) | 78 (57) | 0.311 |
| Basal ganglia PVS score | 2 (2–3) | 2 (2–3) | 0.939 |
| Centrum semiovale PVS score | 1 (1–2) | 1 (1–2) | 0.661 |
| CSVD score | 4 (3–4) | 4 (3–4) | 0.159 |
- Note: Data are median (interquartile range) unless otherwise stated.
- Abbreviations: aDWI+, asymptomatic diffusion-weighted imaging-positive; CADASIL, cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy; CMB, cerebral microbleeds; CSVD, cerebral small vessel disease; MRI, magnetic resonance imaging; PVS, perivascular spaces; WMH, white matter hyperintensity.
- a Only 13 patients had available susceptibility-weighted imaging (SWI).
- b Only 135 patients had available SWI.
- * Significant p values.
Sensitivity analyses (Tables S2 and S3) excluding patients who did not have aDWI+ lesions but developed sDWI+ lesions during follow-up were conducted. There were no significant differences in baseline clinical characteristics between patients with aDWI+ lesions and those without any DWI+ lesions; however, patients with aDWI+ lesions had more lacunes (8 [6–15] vs. 2 [0–8]; p = 0.002), more CMBs (14 [11–30] vs. 4 [0–27]; p = 0.05), a higher prevalence of multiple lacunes (77% vs. 38%; p = 0.003), multiple CMBs (85% vs. 39%; p = 0.002), and mixed CMBs (85% vs. 50%; p = 0.02). They also had higher periventricular WMH scores (3 [3] vs. 3 [2, 3]; p = 0.04), subcortical WMH scores (3 [3] vs. 3 [2, 3]; p = 0.01), total WMH scores (6 [6] vs. 5 [4–6]; p = 0.01), and a higher prevalence of anterior temporal WMH (47% vs. 14%; p = 0.003).
Comparisons of MRI features between aDWI+ and sDWI+ lesions
To compare the characteristics between aDWI+ and sDWI+ lesions, we analyzed DWI scans from enrolled patients who presented to the emergency room with acute stroke symptoms at any time during the study period. In total, we identified 22 sDWI+ lesions in 19 patients. The majority of DWI+ lesions (either aDWI+ or sDWI+) were located in the subcortical region, with no significant differences in topographical distribution between the two groups (Table S4). After excluding two patients in the aDWI+ group who had both aDWI+ lesions and sDWI+ lesions on different MRI scans, and six patients in the sDWI+ group who had sDWI+ lesions before enrollment, we found that those with sDWI+ lesions were more likely to have had a prior stroke compared to those with aDWI+ lesions (53% vs. 100%; p = 0.01 [Table S5]). Table 3 shows that aDWI+ lesions were more likely to be small infarcts (<1 cm; 89% vs. 23%; p < 0.001) and less likely to involve the corticospinal tract (11% vs. 96%; p < 0.001).
| aDWI+ (n = 18)n (%) | sDWI+ (n = 22)n (%) | p value | |
|---|---|---|---|
| Small infarct (<1 cm) | 16 (89) | 5 (23) | <0.001* |
| Any large DWI+ lesion (≥2 cm) | 0 (0) | 3 (14) | 0.238 |
| Multiple DWI+ lesions | 5 (28) | 10 (46) | 0.332 |
| Corticospinal tract involvement | 2 (11) | 21 (96) | <0.001* |
- Abbreviations: aDWI+, asymptomatic diffusion-weighted imaging-positive; DWI+, diffusion-weighted imaging-positive; sDWI+, symptomatic diffusion-weighted imaging-positive.
- * Significant p values.
Additionally, we compared baseline clinical characteristics and MRI features between patients with and without any DWI+ lesions (Tables S6 and S7). The DWI+ group had a significantly higher rate of history of stroke (75% vs. 45%; p = 0.006), particularly ischemic stroke (68% vs. 31%; p < 0.001). Compared to the non-DWI+ group, the DWI+ group had more lacunes (8 [4–13] vs. 2 [0–8]; p < 0.001), more CMBs (15 [10–32] vs. 4 [0–27]; p = 0.03), and a higher prevalence of multiple lacunes (68% vs. 38%; p = 0.005), multiple CMBs (75% vs. 39%; p = 0.001), and mixed CMBs (83% vs. 50%; p = 0.003). They also had higher periventricular WMH scores (3 [3] vs. 3 [2, 3]; p = 0.02), subcortical WMH scores (3 [3] vs. 3 [2, 3]; p = 0.003), total WMH scores (6 [6] vs. 5 [4–6]; p = 0.006), and CSVD scores (4 [4] vs. 4 [2–4]; p = 0.01), and a higher prevalence of anterior temporal WMH (32% vs. 14%; p = 0.049).
Temporal evolution and clinical outcomes of aDWI+ and sDWI+ lesions
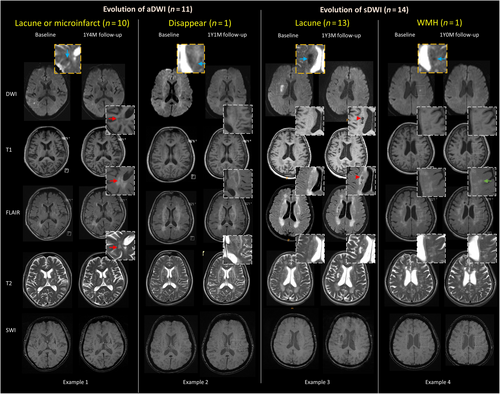
In patients with aDWI+ lesions, follow-up scans were available for 11 individuals (Figure S1), with a median (IQR) MRI interval of 1.5 (1.3–2.1) years. Among these, three aDWI+ lesions evolved into lacunes, seven became microinfarcts, and one disappeared on the follow-up scan. No aDWI+ lesions evolved into CMBs. In contrast, 14 patients with sDWI+ lesions underwent follow-up MRIs, with a median (IQR) interval of 0.9 (0.7–1.2) years; 13 of these lesions evolved into lacunes, and one merged into WMH (Figure 2).
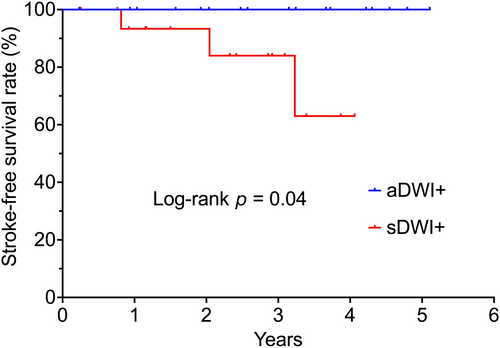
Among the 19 patients with sDWI+ lesions, three experienced clinically recurrent strokes during a median (IQR) follow-up of 3.2 (1.9–4.2) years, resulting in an annual incident rate of 7.6 per 100 person-years. In contrast, no incident strokes were observed in patients with aDWI+ lesions during a median (IQR) follow-up of 3.7 (2.5–4.6) years. Additionally, no incident strokes were noted in patients without DWI+ lesions. Kaplan–Meier survival analysis indicated that the presence of sDWI+ lesions was associated with a higher risk of stroke compared to aDWI+ lesions (log-rank p = 0.04; Figure 3).
DISCUSSION
This study demonstrates that aDWI+ lesions occur in over 8% of patients with CADASIL, with most lesions evolving into microinfarcts over time. We found that CADASIL patients with aDWI+ lesions exhibited a higher burden of SVD markers on MRI, particularly in the anterior WMH. Notably, no baseline clinical differences were observed between patients with and without aDWI+ lesions, but the presence of aDWI+ lesions was associated with a lower rate of incident symptomatic stroke during follow-up compared to the presence of sDWI+ lesions.
Previous studies have reported that the prevalence of DWI+ lesions in patients with SVD ranges from 3% to 30% [5, 18, 19], depending on the severity of the SVD in the cohort. O'Sullivan et al. [20] found that two out of 19 (10.5%) patients with CADASIL in the United Kingdom developed new aDWI+ lesions. Another study by Osman et al. [21] identified 77 small subcortical ischemic lesions (30 symptomatic, 43 asymptomatic) in 55 of 323 genetically confirmed CADASIL patients in France. Asymptomatic lesions were less likely to be located in the corticospinal tracts and more likely near preexisting WMH [21]. The authors suggested that extensive preexisting WMH, which disrupts surrounding brain function, played a more critical role in stroke symptoms than the characteristics of the lesions themselves [21]. Our study aligns with their findings, including the prevalence of aDWI+ lesions at approximately 10%, and shows that aDWI+ lesions were less likely to affect the corticospinal tract. A key strength of our study was the follow-up imaging of aDWI+ lesions and the tracking of clinical outcomes in these patients. This revealed distinct temporal evolution of aDWI+ lesions and a higher stroke risk in patients with sDWI+ lesions compared to those with aDWI+ lesions.
Additionally, our study demonstrated that, during the acute stage, aDWI+ lesions are significantly more likely to be small infarcts (<1 cm) compared to sDWI+ lesions. DWI is highly sensitive for detecting early cerebral ischemia and tissue alterations [6, 22]. Traditionally, cerebral microinfarcts were considered invisible to the naked eye but detectable under microscopy [22]. However, advancements in high-resolution and higher Tesla MRI have challenged this notion, with some cerebral microinfarcts as small as 1–2 mm now discernible on high-resolution DWI during the acute phase of infarction [23]. Tao et al. [24] found that acute subcortical cerebral microinfarcts, defined as less than 5 mm in diameter, were associated with higher burdens of SVD compared to subcortical infarcts ranging from 5 to 20 mm in diameter in patients with DWI-confirmed RSSIs. This suggests that acute, smaller subcortical cerebral infarcts might have distinct clinical implications compared to larger RSSI lesions, possibly indicating a closer association with intrinsic small vessel pathology.
Recent literature has shown that DWI+ lesions may evolve into various SVD markers, including WMH, lacunes, and CMBs, on follow-up MRI [18], suggesting they are early radiographic features in the course of SVD [6]. Ter Telgte et al. [18] demonstrated that DWI+ lesions may precede the manifestation of SVD markers on MRI in individuals with SVD, with many lesions initially located in the cortical region and disappearing on follow-up 3-Tesla MRI scans. In contrast, our study found that aDWI+ lesions, which were primarily located in the subcortical regions, evolved into microinfarcts (<3 mm) in patients with CADASIL. The differences in these evolutions may be explained by variations in target populations, as individuals with CADASIL tend to have more subcortical infarctions rather than cortical lesions. While prior studies correlating imaging with pathology have focused mainly on cortical cerebral microinfarct [22], defined by an upper size limit of 4 mm in cortical region based on the current STRIVE criteria [1], robust criteria for subcortical microinfarcts are currently lacking, and their clinical significance remains unknown. Future research is needed to determine the significance of tiny subcortical cavities on MRI, particularly in subjects with non-amyloid SVD. Our study may provide a clinical basis for considering the presence of aDWI+ lesions in CADASIL patients as an additional imaging marker of SVD burden.
Previous research has reported that, in individuals with SVD, DWI+ lesions were associated with a higher baseline SVD burden on MRI [4, 19]. Accumulated DWI+ lesions may worsen leukoaraiosis and cortical atrophy by impairing parenchymal microstructure and functional connectivity, leading to a more severe form of SVD [7, 23, 25]. Our study further demonstrates that aDWI+ lesions were independently associated with anterior temporal WMH in patients with CADASIL, even after adjusting for relevant SVD markers. Importantly, involvement of anterior temporal poles is also a diagnostic hallmark of CADASIL compared to sporadic SVD [11]. The different patterns of WMH may indicate distinct clinical phenotypes and genotypes in CADASIL. CADASIL patients in Taiwan exhibited a lower frequency of anterior temporal WMH compared to the White population but presented with an older age at onset, more severe cognitive impairment, and a higher association with NOTCH3 R544C variants [26]. Further studies are warranted to elucidate the genetic phenotype associations and long-term clinical outcomes among CADASIL patients with aDWI+ lesions.
Additionally, we found that having aDWI+ lesions does not appear to increase the short-term risk of stroke, whereas the presence of sDWI+ lesions may increase the risk of recurrent stroke. Chen et al. [27] also reported that, in CADASIL patients with stroke, the risk of recurrent stroke can be as high as 7%–10% per year. Our research extends the understanding of clinical outcomes in CADASIL patients with a stroke history and highlights that aDWI+ lesions do not increase short-term risk of overt brain infarction. However, their significance in long-term outcomes, including recurrent stroke and the development of vascular cognitive impairment, should be further investigated, particularly in comparison to patients who have never developed any DWI+ lesions.
This study has some limitations. First, it was a retrospective cross-sectional study with a relatively small sample size, which may have introduced selection bias and limit our ability to establish a solid causal inference. Second, possible embolic sources or other mechanisms of acute infarction that could manifest as diffusion restriction lesions could not be completely excluded. Third, the duration and availability of follow-up MRI varied among patients, which may have led to underestimation of the prevalence of aDWI+ lesions in our cohort. Additionally, approximately one third of the patients did not undergo follow-up MRI. To address missing follow-up data, we compared patients who were lost to follow-up with those who were not and found no significant differences, including in SVD severity, in clinical features between the two groups.
In conclusion, aDWI+ lesions in patients with CADASIL are not uncommon, tend to be small, and are associated with higher baseline MRI burden of SVD, particularly in anterior temporal WMH. However, these lesions may not increase the risk of incident stroke compared to sDWI+ lesions. Further research is needed to clarify the clinicopathological significance and long-term implications of these findings for patients with CADASIL.
AUTHOR CONTRIBUTIONS
Ying-Chi Shen: Conceptualization; methodology; writing – original draft; writing – review and editing; formal analysis; data curation. Ya-Fang Chen: Validation; writing – review and editing. Yu-Wen Cheng: Validation; writing – review and editing. Chih-Hao Chen: Methodology; validation; writing – review and editing. Jiann-Shing Jeng: Supervision; writing – review and editing. Sung-Chun Tang: Methodology; funding acquisition; project administration; supervision; writing – review and editing.
FUNDING INFORMATION
This study was supported by the grant from the Academia Sinica, Taiwan (grant AS-GC-111-L04).
CONFLICT OF INTEREST STATEMENT
The authors declare that there is no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




