Video head impulse gain is impaired in myotonic dystrophy types 1 and 2
Abstract
Background and Purpose
This study was undertaken to examine vestibulo-ocular reflex (VOR) characteristics in myotonic dystrophy type 1 (DM1) and type 2 (DM2) using video head impulse testing (vHIT).
Methods
VOR gain, refixation saccade prevalence, first saccade amplitude, onset latency, peak velocity, and duration were compared in DM1, DM2, age-matched normal controls, and patients with peripheral and central vestibulopathies.
Results
Fifty percent of DM1 and 37.5% of DM2 patients demonstrated reduced VOR gain. Refixation saccade prevalence for horizontal canal (HC) and posterior canal (PC) was significantly higher in DM1 (101 ± 42%, 82 ± 47%) and DM2 (70 ± 45%, 61 ± 38%) compared to controls (40 ± 28% and 43 ± 33%, p < 0.05). The first saccade amplitudes and peak velocities were higher in HC and PC planes in DM1 and DM2 compared to controls (p < 0.05). HC slow phase eye velocity profiles in DM1 showed delayed peaks. The asymmetry ratio, which represents the percentage difference between the first and second halves of the slow phase eye velocity response, was therefore negative (−22.5 ± 17%, −2.3 ± 16%, and − 4.7 ± 8% in DM1, DM2, and controls). HC VOR gains were lower and gain asymmetry ratio was larger and negative in patients with DM1 with moderate to severe ptosis and a history of imbalance and falls compared to the remaining DM1 patients (p < 0.05). In peripheral vestibulopathies, saccade amplitude was larger, peak velocity was higher, and onset latency was shorter (p < 0.05) than in DM1. In central vestibulopathy (posterior circulation strokes), saccade peak velocity was higher, but amplitude and onset latency were not significantly different from DM1.
Conclusions
VOR impairment is common in DM1 and DM2. In DM1, refixation saccade characteristics are closer to central than peripheral vestibulopathies. Delayed peaks in the vHIT eye velocity profile observed in patients with DM1 may reflect extraocular muscle weakness. VOR impairment and VOR asymmetry in DM1 are associated with imbalance and falls.
INTRODUCTION
Myotonic dystrophy type 1 and type 2 (DM1 and DM2) are autosomal dominant, progressive, multisystem disorders with highly variable phenotypes [1]. They are caused by the unstable expansion of the CTG and CCTG nucleotides in untranslated DNA regions of their respective genes—dystrophia myotonica protein kinase (DMPK) for DM1 and cellular nucleic acid binding protein (CNBP) for DM2—resulting in missplicing of messenger RNA, which affects almost all cells and organs of the human body.
DM1 is characterized by cranial and distal limb muscle weakness, myotonia, and widespread systemic manifestations reflecting brain, ocular, cardiac, endocrine, and gastrointestinal involvement [1]. The clinical presentation of DM2 is generally milder than DM1 and muscle weakness is typically proximal and axial, with a significant phenotypic variability and multisystem involvement [1].
Impairment of smooth pursuit eye movements and saccade slowing are the main oculomotor changes described in patients with DM1 [2]. Central (dysfunction at the premotor level) or peripheral (extraocular myopathic and/or myotonic phenomena) mechanisms for saccadic slowing have been proposed [2, 3]. Several studies focusing on audiological assessment found that cochlear sensorineural hearing impairment is common in both conditions, interpreted by the investigators as early presbyacusis [4-6]. Thus far, quantitative vestibulo-ocular reflex (VOR) testing has not been undertaken in patients with DM1 and DM2.
This study examined the VOR function using the video head impulse test (vHIT). We hypothesized that the angular VOR, which begins in the semicircular canals, traverses the upper brainstem, and ends in the extraocular muscles, is highly likely to be abnormal in DM1 and DM2 principally due to extraocular muscle involvement. We expected abnormal vHITs with reduced VOR gains and absent refixation saccades. We also sought to formally compare abnormalities found in DM1 and DM2 against well-described peripheral and central vestibular disorders. Thus, vestibular neuritis (VN) and bilateral vestibular loss (BVL) patients as well as posterior circulation stroke (PCS) patients were compared against DM1 and DM2.
METHODS
This prospective case–control study was undertaken at the Institute of Neurology, Clinical Center of Serbia between November 2018 and April 2019. Sixteen DM1 patients with confirmed CGT repeats expansion in the DMPK gene and 16 patients with confirmed CCTG repeats expansion in the CNBP gene were randomly selected from the patient database (n = 350 for DM1, n = 119 for DM2) and invited to participate in the study. Genetic diagnosis was made using repeat-primed polymerase chain reaction [7, 8]. The study was approved by the ethical board of the Neurology Clinic, Clinical Center of Serbia, and all patients signed written informed consent in accordance with the Declaration of Helsinki.
Detailed sociodemographic and clinical data were collected. Any history of vertigo/dizziness, gait imbalance, recurrent falls, headache, and hearing loss was obtained using a constructed questionnaire.
Evaluation of the muscle strength in both DM1 and DM2 was done using the Medical Research Council (MRC) 0–5 scale (0—no movement, 5—normal strength) by two neurologists (V.R.-S. and S.P.) [9]. The following muscles were tested: shoulder abductors and adductors, elbow flexors and extensors, wrist and finger flexor and extensors, hip flexors, extensors, abductors and adductors, knee flexors and extensors, and plantar and dorsal ankle and toe flexors. The strength of the weakest muscle in each muscle group (upper arm, lower arm, upper legs, and lower legs) was added up [10]. The maximum total MRC score is 20, which indicates the full strength of all muscles tested. Additionally, in patients with DM1, degree of muscle weakness was categorized according to the Muscular Impairment Rating Scale (MIRS). MIRS grades muscle involvement in DM1 from 1 to 5 in accordance with the characteristic progression of weakness from distal to proximal musculature [11]. Ptosis was classified as mild (≤2 mm), moderate (2–4 mm), and severe (>4 mm), covering the pupil entirely [12]. Eye movements were assessed qualitatively for the presence of spontaneous, gaze-evoked, and positional nystagmus (Frenzel glasses were used in nine cases).
Video head impulse tests
All patients underwent three-dimensional vHIT using a GN Otometrics video system. Head impulse data were collected using previously described equipment and methods (ICS Impulse USB goggles, Otometrics) [13]. Patients sat on a straight-backed chair in a well-lit room and were instructed to visually fixate on a small dark target against a blank wall 1.5 m away. To minimize the artifact due to ptosis or long eye lashes, the eyelids were held open by the rims of the goggles. The examiner (Z.C.) stood behind the patient and delivered between 20 and 25 head impulses in the plane of each semicircular canal (SCC). High-speed video (250 Hz) of the eye was captured. Head and eye velocity data were processed offline using custom-written software (A.B., LabView v2012) as described by Pogson et al. [14]. We accepted peak velocities of 150–250°/s for horizontal canals (HCs) and 120–200°/s for the vertical canals.
The VOR gain and refixation saccade characteristics including saccade prevalence, cumulative amplitude and first saccade amplitude, onset latency, peak velocity, and duration were analysed. vHIT was performed by the same investigator. During analysis, overshooting, incomplete, or otherwise outlying head velocity traces were visually identified for each subject's canal and discarded manually.
For HCs, ACs, and PCs, the mean VOR gain and refixation saccade characteristics of DM1 and DM2 patients were compared against age-matched normal controls (NCs; n = 16, age = 43.5 ± 12.2 years, range = 26–69). vHIT VOR gain was considered reduced if mean VOR gain was <2 SD of the mean gain determined in NC (HC, <0.83; anterior canal [AC], <0.62; posterior canal [PC], <0.62).
We also computed the area under the curve for the first and the second half of the slow phase of eye velocity curve (AUC1 and AUC2). The ratio between these two components is expressed using the Jonkees Formula [15], where the asymmetry ratio (AR) = % (AUC1 − AUC2) / (AUC1 + AUC2). Thus VOR with a delayed peak would have a negative AR.
Patients with vestibular neuritis, bilateral vestibular loss, and posterior circulation stroke
We sought to compare DM saccade characteristics against those observed in well-characterized peripheral and central vestibulopathies. Patients with VN, BVL, and PCS (eight, three, and one from posterior inferior cerebellar artery, superior cerebellar artery, and anterior inferior cerebellar artery vascular territories, respectively) with matched reduction in HC VOR gain were selected for comparison against DM1. All VN patients presented with acute vestibular syndrome and had a “peripheral” HINTS plus assessment [16], with abnormal head impulse, spontaneous unidirectional horizontal nystagmus, absent skew deviation, and normal hearing. All VN patients underwent vHIT testing; the mean time to testing was 5.3 ± 7.3 days. Patients with BVL and acute PCS were selected from the database (n = 100 for BVL and n = 50 for PCS). The same investigator (Z.C.) performed and analysed vHITs.
Statistical analysis
Statistical analyses were performed with SPSS software version 23 for Windows. Descriptive statistics are reported as mean ± 1 SD unless otherwise stated. Data were tested for normality with the Shapiro–Wilk test. Two-tailed t-test was used for normally distributed data and Kruskal–Wallis test for nonnormally distributed data. Significance level was set at p = 0.05.
RESULTS
Patient characteristics
Patient sociodemographic and clinical characteristics are shown in Table S1. Fifty percent of DM1 and 43.7% DM2 patients reported gait imbalance, with recurrent falls occurring in one third of the patients (n = 5). Many patients had ocular involvement, including cataracts or prior cataract surgery (n = 14 [87.5%] in DM1, n = 6 [37.5%] in DM2). Ptosis was present in 14 (87.5%) patients with DM1 (mild, n = 9; moderate/severe, n = 5) and in four (25%) patients with DM2 (all mild ptosis). In DM1, two patients (11%) had faint gaze-evoked nystagmus, and none had spontaneous or positional nystagmus. No nystagmus was observed in patients with DM2. Subjective hearing loss was reported in six (37.5%) patients with DM1 and four (25%) patients with DM2. No patient was acutely vertiginous during the examination.
VOR gain in DM1
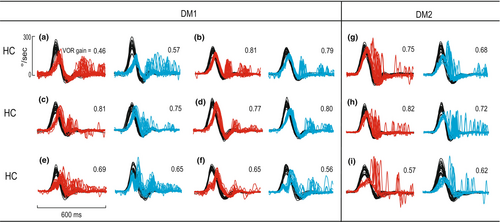
SCCs were considered as pairs horizontal/anterior/posterior (i.e., right and left HC/right and left AC/right and left PC). The mean VOR gain for HCs, ACs, and PCs was 0.89 ± 0.2, 0.90 ± 0.2, and 0.70 ± 0.2 in DM1 and 0.98 ± 0.1, 0.90 ± 0.1, and 0.83 ± 0.1 in controls. As shown in Table 1, VOR gain for HC and PC was lower in DM than controls. Gains fell below normal in 50% of DM1 patients. Table 2 shows distribution of SCC involvement in patients with DM1, and Table 3 compares abnormality rates for HC vHIT parameters in DM1 and DM2. We observed a lag in the eye velocity (acceleration phase) with respect to the head velocity in the horizontal head impulses of seven (43.7%) patients with DM1 (Figure 1a–d, Figure 2a,b).
| Characteristic | NC, n = 16 | DM1, n = 16 | DM2, n = 16 |
|---|---|---|---|
| Horizontal canal | |||
| VOR gain | 0.98 ± 0.1 | 0.89 ± 0.2a | 0.96 ± 0.2 |
| Saccade prevalence | 40 ± 28 | 101 ± 42a | 70 ± 45b |
| First saccade | |||
| Amplitude, ° | 0.7 ± 0.3 | 1.3 ± 0.7a | 1.3 ± 1.1b |
| Onset latency, ms | 369.1 ± 71.2 | 311.5 ± 60.8a | 328.7 ± 93.1 |
| Peak velocity, °/s | 65.1 ± 17.0 | 75.4 ± 19.0a | 90.6 ± 40.4b |
| Duration, ms | 25.6 ± 3.6 | 32.8 ± 17.7a | 28.2 ± 7.3 |
| Anterior canal | |||
| VOR gain | 0.90 ± 0.1 | 0.90 ± 0.2 | 0.84 ± 0.1 |
| Saccade prevalence | 20 ± 17 | 28 ± 27 | 25 ± 19 |
| First saccade | |||
| Amplitude, ° | 0.7 ± 0.3 | 0.7 ± 0.3 | 0.8 ± 0.5 |
| Onset latency, ms | 372.5 ± 68.0 | 382.4 ± 44.4 | 336.0 ± 153 |
| Peak velocity, °/s | 38.5 ± 11.7 | 40.1 ± 15.0 | 43.0 ± 22.1 |
| Duration, ms | 36.2 ± 6.2 | 37.8 ± 7.1 | 30.8 ± 15.3 |
| Posterior canal | |||
| VOR gain | 0.83 ± 0.1 | 0.70 ± 0.2a | 0.75 ± 0.2b |
| Saccade prevalence | 43 ± 33 | 82 ± 47a | 61 ± 38b |
| First saccade | |||
| Amplitude, ° | 1.0 ± 0.8 | 1.6 ± 1.0a | 1.3 ± 0.8b |
| Onset latency, ms | 315.7 ± 88.1 | 315.6 ± 81.1 | 295.0 ± 56.1 |
| Peak velocity, °/s | 48.7 ± 21.4 | 69.5 ± 24.4a | 65.7 ± 19.0b |
| Duration, ms | 39.5 ± 10.6 | 44.7 ± 13.1a | 40.2 ± 14.3 |
- Note: Data are presented as mean ± SD. Kruskal–Wallis pairwise comparison was performed.
- Abbreviations: DM1, myotonic dystrophy type 1; DM2, myotonic dystrophy type 2; NC, normal control; VOR, vestibulo-ocular reflex.
- a Significant difference between DM1 and NC groups.
- b Significant difference between DM2 and NC groups.
| DM1 | DM2 | |||||
|---|---|---|---|---|---|---|
| HC | AC | PC | HC | AC | PC | |
| Unilateral | 0 | 0 | 4 | 0 | 4 | 3 |
| Bilateral | 6 | 1 | 2 | 3 | 0 | 2 |
- Abbreviations: AC, anterior canal; DM1, myotonic dystrophy type 1; DM2, myotonic dystrophy type 2; HC, horizontal canal; PC, posterior canal.
| HC vHIT abnormality | DM1 | DM2 |
|---|---|---|
| Reduced VOR gain, <0.83 | 37.5 | 18.7 |
| Negative VOR AR, < −13 | 75 | 25 |
| Increased saccade prevalence, >87.4 per 100 impulses | 56.2 | 28.1 |
| 1st saccade | ||
| Increased amplitude, >1.0° | 56.2 | 40.6 |
| Increased peak velocity, >76.3°/s | 43.7 | 59.4 |
| Delayed onset latency, >286.4 ms | 65.6 | 68.7 |
- Note: Cutoff values were determined from normative data (mean ± 2 SD) including 80 subjects published by Pogson et al. [14]. Reference norms were as follows: HC gain = 0.94 ± 0.1, VOR AR = −4.7 ± 8, saccade prevalence = 82.1 ± 5.3, 1st saccade amplitude = 0.95 ± 0.12°, peak velocity = 70.4 ± 5.9°/s, and onset latency = 271.1 ± 13.5 ms.
- Abbreviations: AR, asymmetry ratio; DM1, myotonic dystrophy type 1; DM2, myotonic dystrophy type 2; HC, horizontal canal; vHIT, video head impulse test; VOR, vestibulo-ocular reflex.
For DM1 patients, there was no significant relationship between age at onset, disease duration, severity of muscle involvement as assessed by MIRS and MRC scores, and VOR impairment. We found that HC VOR gain was lower in DM1 patients with disease onset before age 25 years (0.76 ± 0.2 vs. 0.95 ± 0.2, p = 0.018), and we postulate that this observation may be because of a higher number of triple repeats in these patients. In all three canal planes, the VOR gain was significantly lower in patients with moderate/severe ptosis compared to patients with mild or no ptosis (HC, 0.74 vs. 0.96; AC, 0.73 vs. 0.96; PC, 0.50 vs. 0.78, p = 0.001–0.003). HC VOR gain was lower in patients with reported gait imbalance and falls compared to the patients without gait imbalance and falls (0.78 vs. 1.01, p = 0.001). There was a negative correlation between gait imbalance and falls and HC VOR gain (r = −0.403, p = 0.022 [Spearman correlation]). No significant correlations were present for AC and PC gains.
VOR gain in DM2
The mean VOR gain for HCs, ACs, and PCs were 0.96 ± 0.2, 0.84 ± 0.1, and 0.75 ± 0.2 in DM2 and 0.98 ± 0.1, 0.90 ± 0.1, and 0.83 ± 0.1 in controls (Table 1). VOR gain was significantly smaller than in controls for the PC only. Gains fell below normal range in 37.5% of patients. Table 2 shows distribution of SCC involvement in patients with DM2. Figure 1g–i illustrates reduced HC VOR gain in DM2 patients. No lag in the eye velocity was observed in patients with DM2.
There was a trend towards higher age at disease onset and VOR impairment (p = 0.051) and no significant differences in disease duration and MRC score in patients with VOR impairment (p = 0.718, p = 0.543). In DM2, VOR gain values were not significantly different in patients with and without ptosis and there was no relationship between gain and history of gait imbalance/falls.
Refixation saccade characteristics
Table 1 summarizes the VOR gain and refixation saccade characteristics for controls, DM1, and DM2.
DM1 versus NCs
As shown in Table 1, for HC and PC planes, the refixation saccade prevalence in DM1 was double that of healthy controls. In the HC plane, the first saccade amplitude was significantly larger, onset latency was shorter, peak velocity was faster, and saccade duration was longer in DM1 compared to controls (Table 1).
DM2 versus NCs
As shown in Table 2, for HC and AC planes, saccade prevalence, first saccade amplitude and peak velocity were significantly greater in DM2 compared to controls.
VOR profile in DM1 and DM2
Visual inspection of the eye velocity response in DM1 revealed a rightward skewed velocity profile compared with healthy controls (Figure 3).
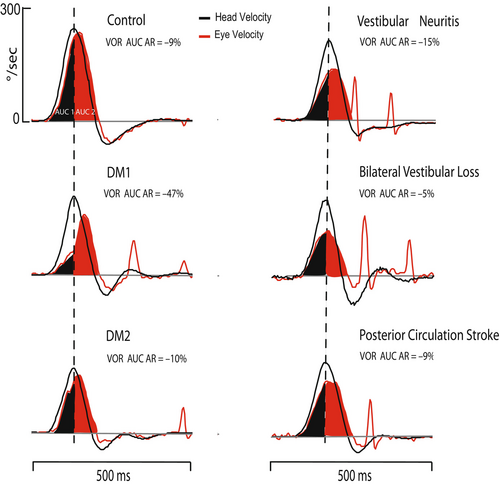
The mean ARs for the HC VOR in DM1, DM2, and controls were −22.5 ± 17%, −2.0 ± 16%, and −4.7 ± 8%, respectively. VOR asymmetry was larger and negative in patients with DM1 compared to controls (p < 0.001). There was no difference in VOR asymmetry between DM2 and controls (p = 0.979). HC VOR asymmetry in patients with DM1 was more negative in patients with moderate/severe ptosis compared to patients with mild or no ptosis (−33.3 ± 10% vs. −18.0 ± 18, p = 0.017) and in patients with reported gait imbalance and falls compared to the patients without gait imbalance and falls (−32.6 ± 10% vs. −14.7 ± 18%, p = 0.003).
Saccade characteristics of DM1 compared against peripheral and central vestibular loss
We next sought to explore the origins of VOR deficits in DM1. Considering the possibility of peripheral vestibular, central, or neuromuscular (oculomotor) causes for the VOR abnormalities in DM1, we compared it against VN, BVL (representing peripheral vestibulopathy), and PCS (representing central vestibulopathy). VN, BVL, and PCS patients with average vHIT gains similar to the DM1 group were chosen. As shown in Table 4, in DM1 patients whose VOR gain was abnormal, the first saccade amplitude was significantly smaller, onset latency was more prolonged, and peak velocity was lower when compared to VN and BVL (p = 0.001–0.033). There was no difference in the first saccade duration. DM1 VOR asymmetry was significantly different and negative when compared to the AR in patients with VN and BVL (Table 4). On comparison of DM1 and PCS, first saccade amplitudes, latencies, and durations were not significantly different (p = 0.06–0.713). However, peak saccade velocity was significantly lower in DM1 than in PCS (p = 0.044).
| DM1 | VN | BVL | PCS | |
|---|---|---|---|---|
| VOR AR% | −29 ± 11 | −4.0 ± 13a | −8.0 ± 14a | −2.3 ± 16a |
| First saccade | ||||
| Amplitude, ° | 2.04 ± 0.5 | 3.6 ± 2.1a | 3.4 ± 1.4a | 2.8 ± 2.1 |
| Onset latency, ms | 273.3 ± 50.1 | 211.4 ± 37.8a | 222.5 ± 37.4a | 220.5 ± 74.6 |
| Peak velocity, °/ms | 90.3 ± 18.0 | 182.3 ± 61.4a | 165.7 ± 54.7a | 139.4 ± 57.3a |
| Duration, ms | 43.2 ± 8.7 | 38 ± 8.6 | 38.7 ± 6.0 | 40.5 ± 9.6 |
- Note: Data are presented as mean ± SD.
- Abbreviations: AR, asymmetry ratio; BVL, bilateral vestibular loss; DM1, myotonic dystrophy type 1; PCS, posterior circulation stroke; VN, vestibular neuritis; VOR, vestibulo-ocular reflex.
- a Indicates significant difference between DM1 and VN, BVL, and PCS groups.
There were no significant differences in saccade parameters between patients with DM2 with reduced VOR gain and matched VOR gain in patients with VN, BVL, and PCS.
DISCUSSION
This study found VOR abnormalities to be common in DM1 and DM2. Our patients with DM1 demonstrated a lag in the eye velocity (acceleration phase) with respect to the head velocity, which we expressed as a negative asymmetry ratio between the first and second halves of the VOR. These patients also had refixation saccades that were relatively smaller, more delayed, and with lower peak velocity when compared with patients with peripheral vestibular disorders (VN and BVL) but of similar amplitude to saccades measured in PCS with comparable VOR deficits, implicating that saccade characteristics in DM1 were closer to those of central vestibulopathy. In DM1 patients, presence of moderate–severe ptosis was associated with lower VOR gain in all canals. HC VOR gain asymmetry ratio in DM1 was significantly negative compared with central and peripheral vestibular disorders. These features imply an additional contribution to the VOR deficits from ocular myopathy.
A history of recurrent falls/imbalance was associated with lower VOR gain in HC in patients with DM1. There was a lack of correlation between age at onset/disease duration and VOR impairment in patients with DM1 and DM2, suggesting that VOR impairment may be present anytime during the disease course, even at an early stage when other symptoms and signs are not easily detected.
VOR impairment and clinical severity
In DM1 and DM2, different organ systems demonstrate a separate disease course in keeping with somatic mosaicism (i.e., presence of a different number of CTG/CCTG repeats in different tissues) [1]. For example, the average allele length in skeletal muscle, the primary affected tissue in DM1, is always larger than that observed in circulating lymphocytes [17]. Prior studies showed that cardiac conduction defects may not always correlate with the disease duration and the degree of muscle weakness [18-20]. The absence of correlation between VOR impairment and disease duration and/or clinical severity suggests that it remains unpredictable when patients with DM would develop VOR dysfunction. Muscle contractile function, including oculomotor function, is known to be abnormal in DM. Saccades are abnormal, with reduced peak velocities [21]. Patients show prolonged relaxation times, independent of myotonia [22], which may partly explain the VOR profile found in the present study. Muscle strength is reduced, as are doublet responses [22, 23], meaning a given level of neural activity generates less force than in normal subjects. It is likely that reflex function in general will have reduced effectiveness.
Comprehensive imaging studies in myotonic dystrophies have demonstrated the presence of white matter lesions in both conditions, with microstructural brain changes observed in multiple brain regions, including infratentorial structures [24, 25].
Comparison with previous studies
The vestibular system has not been fully studied in patients with DM. Like us, Verhagen et al. [2] found impairment in the VOR as measured by rotatory chair in 46.1% (6/13) of patients with DM1. Three patients had hyperreflexia with increased gain, whereas three patients had hyporeflexia. Electro-oculographic recordings showed saccadic slowing in 77% (10/13) and reduction in peak velocity in 54% (7/13) of patients. Possible reasons for saccade slowing in DM include central dysfunction and apparent dysfunction due to extraocular muscle involvement [2]. In one case–control study of DM1 patients, a negative correlation was found between saccadic velocity and number of CTG repeats [26]. Studies indicate that small refixation saccades are observed even in healthy controls [14]. Corrective saccade prevalence in HC and PC was higher in both conditions compared to NCs. In DM1 patients, all first saccade parameters were different from NC, in the HC plane. For the PC, all saccade parameters excepting onset latency were different from controls.
VOR profile in DM1 and DM2
The apparent lag in the eye velocity in the HC vHIT as evidenced in an asymmetric VOR gain profile (Figure 3) in DM1 combined with slow velocity and smaller than expected amplitude of refixation saccades suggests that extraocular muscle performance contributes to this VOR pattern more than does the vestibular drive.
Conversely, in diffuse cerebellar diseases, the opposite pattern of early acceleration in the eye velocity, reaching its peak ahead of head velocity and followed by premature deceleration of the VOR, was described in the HC plane [27]. In our cohort, we did not observe this central pattern.
Studying the vHIT eye velocity profile may help separate vestibular from neuromuscular causes of VOR impairment and may even enable identification of disorders where VOR suppression is impaired.
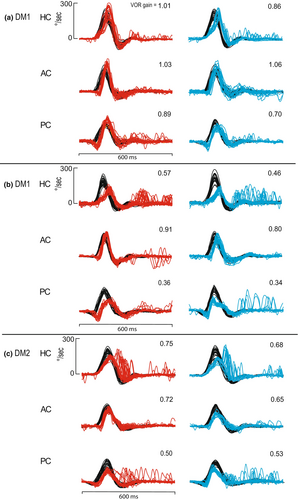
Our observation in one patient with DM1 with a long disease duration (Figure 2b) indicates that degree of the VOR impairment is not adequately compensated with relatively prolonged onset, moderate amplitude overt refixation saccades. In one patient with DM2, refixation saccades occurred early during the head movement (“covert saccade”), consistent with an adequate compensatory response for the degree of VOR deficit (Figure 2c). This observation may reflect a different, possibly peripheral vestibular mechanism for the deficits observed in DM2. At this stage, the underlying mechanism of the VOR impairment remains unknown, and the presence of ptosis may be a marker of greater impairment.
Falls and VOR impairment
A previous cross-sectional survey study identified poor balance, not only muscle weakness, as one of the factors increasing the risk of falls in patients with DM disease [28]. We found that in patients with DM1 the presence of recurrent falls was associated with reduced VOR gain. Although the peripheral vestibular end organs may not be the site of lesion, inability to stabilize gaze during head movements could still result in imbalance. Performing a noninvasive vHIT in patients with DM may allow early identification of VOR impairment, and this in turn may lead to timely initiation of VOR habituation exercises to promote gaze stabilization.
Study limitations
This preliminary study was conducted on a small sample and merits confirmation in a larger study series. Caloric testing was not done, thus we may have missed opportunities to detect HC hypofunction using an alternate method. Focusing solely on the SCC function, we may have missed coexisting or isolated abnormalities of the otolith ocular pathways identifiable by ocular vestibular evoked myogenic potential testing. Quantitative analysis of saccades and smooth pursuit eye movements in the same patient group would have provided a better understanding of the true origins of the apparent VOR deficits. We did not have data of nucleotide repeat numbers, thus we were unable to examine the link between VOR deficits and number of CTG and CCTG repeats. Clinical testing for oscillopsia was not performed, and its presence would indicate underlying functional impairment in patients with myotonic dystrophies.
CONCLUSIONS
Gait imbalance and falls are common in patients with myotonic dystrophies. Bedside vHIT could reveal VOR deficits and provide a better understanding of the mechanisms of imbalance in DM. Patients with vestibular impairment may derive additional benefits from the incorporation of vestibular rehabilitation therapies into conventional physiotherapy interventions in patients with DM1.
AUTHOR CONTRIBUTIONS
Zeljka Calic: Investigation; writing – original draft; conceptualization; formal analysis; data curation; methodology; writing – review and editing. Stojan Peric: Conceptualization; investigation; writing – review and editing. Milorad Vujnic: Writing – review and editing; investigation. Bogdan Bjelica: Conceptualization; investigation; writing – review and editing. Ivo Bozovic: Writing – review and editing; conceptualization. Vidosava Rakocevic-Stojanovic: Writing – review and editing; conceptualization. Andrew Bradshaw: Software; formal analysis; methodology. James G. Colebatch: Writing – review and editing. Miriam S. Welgampola: Writing – review and editing; supervision; methodology; conceptualization; investigation; formal analysis.
ACKNOWLEDGMENTS
Open access publishing facilitated by The University of Sydney, as part of the Wiley - The University of Sydney agreement via the Council of Australian University Librarians.
FUNDING INFORMATION
M.S.W. received a Garnett Passe and Rodney Williams Memorial Foundation Conjoint Grant during the period of this study.
CONFLICT OF INTEREST STATEMENT
The authors declare no financial or other conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




