Economic and societal burden of myasthenia gravis in Denmark, Finland, and Sweden: A population-based registry study
Abstract
Background and purpose
Health care resource utilization (HCRU) and the economic burden of myasthenia gravis (MG) are significant, but existing studies rarely include comprehensive nationwide data. We examined HCRU and direct and indirect costs associated with MG overall and by disease severity in Denmark, Finland, and Sweden.
Methods
Data were collected retrospectively from nationwide health and social care registries. All individuals ≥18 years of age with ≥2 International Classification of Diseases diagnoses of MG between 2000 and 2020 were included. HCRU, direct (inpatient and outpatient contacts, medication) and indirect costs (early retirement, sick leave, death), and associated factors were calculated.
Results
The full study cohort comprised 8622 people with MG (pwMG). Mean annual numbers of all-cause secondary health care contacts for pwMG were 3.4 (SD = 8.3), 7.0 (SD = 12.3), and 2.9 (SD = 3.9), with mean annual total costs of €12,185, €9036, and €5997 per person in Denmark, Finland, and Sweden, respectively. Inpatient periods, involving 77%–89% of study participants in the three countries, contributed most to direct costs, whereas the majority of indirect costs resulted from early retirement in Denmark and Finland, and sick leave periods in Sweden. Mean annual total costs were highest with very severe MG (€19,570–€33,495 per person across the three countries). Female sex and comorbidities, such as mental and behavioral disorders and severe infections, were also associated with higher total costs.
Conclusions
This population-based study shows a high level of HCRU and a significant direct and indirect economic burden of MG across three Nordic countries, especially for severe forms of MG.
INTRODUCTION
Myasthenia gravis (MG) is a rare chronic autoimmune disease causing fluctuating muscle fatigue in ocular, bulbar, axial, and extremity skeletal muscle groups and, in rare cases, life-threatening respiratory insufficiency [1]. MG is associated with antibodies directed against postsynaptic antigens of the neuromuscular junction, most commonly targeting the acetylcholine receptor or muscle-specific tyrosine kinase protein [2]. We have recently reported an incidence of MG of 1.3–1.7 per 100,000 individuals, a prevalence of 18.6–23.4 per 100,000 individuals, and a standardized mortality ratio of 1.2–1.3 in Denmark, Finland, and Sweden based on nationwide population-based data [3].
Current MG treatment guidelines include acetylcholinesterase inhibitors, corticosteroids, immunosuppressive therapy, intravenous immunoglobulin (IVIg), plasma exchange (PLEX), and thymectomy, depending on the type, phase, and severity of the disease combined with individual risk/tolerability profiles [4, 5]. Despite several available treatment options, the burden of MG remains significant, in terms of both inadequate disease control and potential treatment side effects [6]. Collectively, this translates into high health care resource utilization (HCRU) and health care costs in people with MG (pwMG) compared with the general population [7-9]. Additionally, existing literature indicates a significant productivity loss due to early retirement and sick leave [10-13]. However, important knowledge gaps remain regarding direct costs and especially indirect costs [14-16]. In addition, existing studies often assess restricted populations or geographical settings, in some cases use older data, and suffer from methodological variability and weaknesses that lead to wide variations in reported cost estimates.
The nationwide Nordic health and social care registries, covering a population base of approximately 22 million inhabitants (5.9, 5.6, and 10.5 million inhabitants in Denmark, Finland, and Sweden, respectively), offer a unique opportunity for population-based research on the economic burden of MG over extended periods [17-19]. All three countries have publicly funded, universal, and high-quality health care available to all citizens, as well as similar treatment practices [5]. All citizens are assigned a unique personal identification number at birth or immigration, which allows reliable linkage and sourcing of data from different registries [20]. The objective of this study was to analyze specialized care HCRU, direct costs (based on HCRU and medication costs), indirect costs (productivity loss due to sick leave, early retirement, and mortality), and total costs for pwMG in Denmark, Finland, and Sweden. An additional objective was to explore the association between disease severity and other factors driving the cost burden for pwMG in the Nordic countries.
METHODS
Study setting, population, and subgroups
This observational, population-based study used data sourced from nationwide Danish, Finnish, and Swedish health, social, and administrative registries with virtually complete population-wide coverage.
The study population comprised all pwMG ≥18 years of age with ≥2 diagnostic codes for MG (to minimize the risk of misclassification/inclusion of false positives; International Classification of Diseases [ICD]-10 codes G70.0*, ICD-9 codes 358.0*, ICD-8 code 73309, and/or ICD-7 code 74400) in the national patient registries during 1 January 2000–31 December 2020 (Table S1). The years 2019–2020 were excluded from the Danish cohort due to the introduction of Landspatientregisteret, version 3, which significantly impacted health care visit recording practices. The population included both incident cases (those with a first MG diagnosis during the study period) and prevalent cases (those with a first MG diagnosis prior to the study period).
Follow-up started from the date of the first MG diagnosis, 1 January 2000, or the date of the participant's 18th birthday, whichever came latest. Follow-up ended at death, emigration, or end of the study period, whichever came first. However, indirect costs (consisting of sick leave, early retirement and premature death but not, e.g., informal caregiver costs) were defined only until the participant turned 65 years of age. Additionally, indirect costs could accumulate after premature death (at <65 years of age), as calculated according to the Human Capital Approach (HCA) [21].
Analyses were performed in the full study cohort and stratified by disease severity (remission, mild, moderate, severe, or very severe; see study-specific definition in Table 1) or age (18–64 years or ≥65 years).
| Disease severity category | Criteria |
|---|---|
| (i) Remission | Person must fulfill all of the following criteria:
|
| (ii) Mild disease | Person must fulfill ≥1 of the following criteria:
|
| (iii) Moderate disease | Person must fulfill ≥1 of the following criteria:
|
| (iv) Severe disease | Person must fulfill only 1 of the following criteria:
|
| (v) Very severe disease | Person must fulfill ≥2 of the "severe" criteria listed under (iv). |
- Abbreviations: AChEI, acetylcholinesterase inhibitor; CS, corticosteroids; ER, emergency room; ICU, intensive care unit; IST, immunosuppressive therapy; IVIg, intravenous immunoglobulin; MG, myasthenia gravis; PLEX, plasma exchange.
Registry data collection
Individual-level data from several nationwide health, social, and administrative registries in Denmark, Finland, and Sweden were sourced and linked using personal identification numbers (Table S1).
Outcome measures and explanatory variables
The outcomes evaluated were: (i) annual specialized care HCRU (inpatient and outpatient contacts); (ii) annual direct costs associated with specialized care HCRU and medication; (iii) annual indirect costs associated with lost productivity due to sick leave, early retirement, and premature death; and (iv) annual total costs (direct and indirect costs).
In the models assessing factors associated with costs, the outcome measure was total or direct costs over the whole follow-up period.
The explanatory variables of interest, assessed at start of follow-up unless otherwise specified, were age, sex, time since diagnosis, disease severity, country of residence, and comorbidities (Table S2).
Statistical analyses
Characteristics and outcomes at the start of follow-up are presented as a number and percentage for categorical variables, and as mean (SD) and/or median (Q1, Q3) for continuous variables. All descriptive analyses were stratified by country.
Comorbidities were identified based on ICD-8, ICD-9, or ICD-10 codes utilizing all data before the start of follow-up.
Annual all-cause and MG-related specialized HCRU was assessed in categories of in- and outpatient contacts, also calculating the percentage of pwMG with inpatient periods. HCRU contacts of the same type that occurred on the same day were considered to be part of the same contact, that is, counted as one contact. In HCRU analyses, pwMG were included in the populations aged 18–64 or ≥65 years if they were of the respective age at any time during follow-up.
Direct (all-cause and MG-related), indirect (all-cause and for Finland and Sweden MG-related), and total costs (all-cause) were assessed over the whole follow-up. In cost analyses, pwMG were included in the population aged 18–64 or ≥65 years if they were of the respective age at the start of follow-up or any time during follow-up, respectively. To harmonize the data across the three countries, specialized HCRU costs were calculated based on the median costs observed in all data across the three studied countries for each HCRU type. Medication costs included only MG-related reimbursable prescription medications (Table S3). Costs for hospital-administered drugs were included in the costs of inpatient periods but not in medication costs. Indirect costs were estimated based on lost productivity due to long periods of sick leave (>14 days in Denmark and Sweden; >10 days in Finland), early retirement, and premature death, calculated based on the average wage per age and calendar year in each country, using the HCA and the mean annual gross wages in each country [21]. Shorter periods of sick leave were not available in the social care registries. For each longer sick leave period, the initial 14 (Denmark and Sweden) or 10 working days (Finland) were added to the cost. To adjust for inflation, costs were calibrated to the cost level of 2019 using an interest rate of 3% per annum and fixed exchange rates (1 Danish krone = €0.13, 1 Swedish krona = €0.098).
Proportions of MG-related health care contacts and costs were assessed based on recordings of MG as the primary or secondary diagnosis. MG-related indirect costs could not be assessed for Denmark, where diagnosis codes are not recorded for sick leave or early retirement.
A negative binomial regression model was performed to estimate the association between total or direct costs and the explanatory variables.
Disease severity was analyzed dynamically in a time-dependent manner (Table 1). The severity was estimated separately for each pwMG and for each follow-up year. Subsequently, the follow-up years were categorized, driven by data (0–1.99 or 2–19.99 years after MG diagnosis).
The analyses were conducted using R v4.2.2. Concealing identifiable information ensured participants' privacy and confidentiality.
Ethical considerations
This study was approved by Statistics Denmark (708396), the Finnish Data Permit Authority, Findata (THL/1010/14.02.00/2021), Statistics Finland (TK/2457/07.03.00/2021), and the Swedish National Board of Health & Welfare (Socialstyrelsen; Dnr 16836/2021). Ethical approval was obtained by the Swedish Ethics Review Authority (2021–00858). Ethical approval was not required according to Finnish and Danish legislation, given that the study was observational and based solely on pseudonymized registry data.
RESULTS
Patient characteristics of the study population
A total of 8622 pwMG were identified during the study period (Denmark n = 1971, Finland n = 2248, Sweden n = 4403), constituting the full study cohort (Table 2). From this cohort, 4344 pwMG (50.4%) were included in the population aged 18–64 years (Denmark n = 1069, Finland n = 1156, Sweden n = 2119; Table S4) and 4278 pwMG (49.6%) in the population aged ≥65 years (Denmark n = 902, Finland n = 1092, Sweden n = 2284; Table S5). The distribution of pwMG according to disease severity is summarized in Figure 1 and Table S6.
| Variable | Denmark, n = 1971 | Finland, n = 2248 | Sweden, n = 4403 |
|---|---|---|---|
| Age at start of follow-up, years | |||
| Mean (SD) | 59.7 (18.4) | 61.3 (16.8) | 62.5 (17.9) |
| Age at end of follow-up, years | |||
| Mean (SD) | 68.8 (16.5) | 71.3 (15.0) | 72.7 (15.9) |
| Sex, n (%) | |||
| Female | 1062 (53.9%) | 1158 (51.5%) | 2309 (52.4%) |
| Duration of follow-up, years | |||
| Mean (SD) | 9.1 (6.5) | 9.9 (6.6) | 10.2 (6.9) |
| Time since MG diagnosis, n (%) | |||
| 0.00–1.99 years | 1390 (70.5%) | 2071 (92.1%) | 3151 (71.6%) |
| ≥2.00 years | 581 (29.5%) | 177 (7.9%) | 1252 (28.4%) |
| Missing | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Age at MG diagnosis, n (%) | |||
| 18.0–29.9 years | 259 (13.1%) | 151 (6.7%) | 541 (12.3%) |
| 30.0–49.9 years | 451 (22.9%) | 419 (18.6%) | 876 (19.9%) |
| 50.0–64.9 years | 442 (22.4%) | 600 (26.7%) | 922 (20.9%) |
| ≥65.0 years | 819 (41.6%) | 1078 (48.0%) | 2064 (46.9%) |
| Missing | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Disease severity, n (%) | |||
| Remission | 462 (23.4%) | 196 (8.7%) | 1444 (32.8%) |
| Mild | 413 (21.0%) | 681 (30.3%) | 735 (16.7%) |
| Moderate | 793 (40.2%) | 909 (40.4%) | 1386 (31.5%) |
| Severe | 271 (13.7%) | 358 (15.9%) | 724 (16.4%) |
| Very severe | 32 (1.6%) | 104 (4.6%) | 114 (2.6%) |
| Civil status, n (%) | |||
| Married | 1102 (68.9%) | 1271 (56.5%) | 2274 (52.4%) |
| Unmarried | 498 (31.1%) | 977 (43.5%) | 2064 (47.6%) |
| Missing | 371 | 0 | 65 |
| Employment status, n (%) | |||
| Working | 603 (33.9%) | 662 (29.4%) | 1416 (32.6%) |
| Not working | 294 (16.5%) | 494 (22.0%) | 688 (15.9%) |
| Retired | 882 (49.6%) | 1092 (48.6%) | 2234 (51.5%) |
| Missing | 192 | 0 | 65 |
| Comorbidities, n (%) | |||
| Autoimmune diseases | 263 (13.3%) | 282 (12.5%) | 364 (8.3%) |
| Blood diseases | 70 (3.6%) | 95 (4.2%) | 114 (2.6%) |
| Circulatory diseases | 386 (19.6%) | 570 (25.4%) | 826 (18.8%) |
| Connective diseases | 88 (4.5%) | 18 (0.8%) | 47 (1.1%) |
| Endocrine diseases | 219 (11.1%) | 252 (11.2%) | 398 (9.0%) |
| Eye diseases | 216 (11.0%) | 331 (14.7%) | 504 (11.4%) |
| Mental diseases | 103 (5.2%) | 140 (6.2%) | 129 (2.9%) |
| Neoplasm diseases except thymoma | 139 (7.1%) | 217 (9.7%) | 312 (7.1%) |
| Respiratory diseases | 130 (6.6%) | 190 (8.5%) | 132 (3.0%) |
| Severe infections | 31 (1.6%) | 53 (2.4%) | 43 (1.0%) |
| Thymoma | 20 (1.0%) | 7 (0.3%) | 18 (0.4%) |
- Abbreviation: MG, myasthenia gravis.
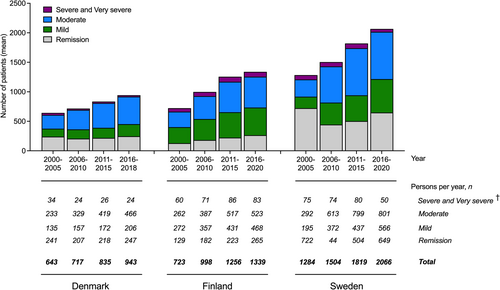
In the full study cohort, the mean age at the start of follow-up was 59.7 years (SD = 18.4) in Denmark, 61.3 years (SD = 16.8) in Finland, and 62.5 years (SD = 17.9) in Sweden (Table 2). Higher mean age was associated with worsening disease severity (data not shown). Women constituted the majority in all countries (53.9% in Denmark, 51.5% in Finland, and 52.4% in Sweden).
Health care resource utilization
In the full study cohort, mean annual numbers of all-cause contacts in specialized care per person during the follow-up were 3.4 (SD = 8.3), 7.0 (SD = 12.3), and 2.9 (SD = 3.9) in Denmark, Finland, and Sweden, respectively (Table S7). Of all contacts, 35.0% in Denmark, 41.1% in Finland, and 60.6% in Sweden were MG-related. Mean annual outpatient contacts ranged from 1.7 (Denmark) to 5.7 (Finland). Altogether, 87.0% of pwMG in Denmark, 88.9% in Finland, and 77.1% in Sweden had inpatient periods during the follow-up, with mean annual numbers ranging from 0.8 (Sweden) to 1.7 (Denmark). The mean annual duration of inpatient periods ranged from 9.1 days (Sweden) to 14.9 days (Finland). HCRU stratified by disease severity and age is detailed in Tables S8–S12.
Direct, indirect, and total costs in pwMG
Costs in full study cohort
In the full study cohort, mean annual total costs per person (all-cause) over the follow-up were €12,185, €9036, and €5997 in Denmark, Finland, and Sweden, respectively (Table 3, Table S13). In general, mean costs were higher than median costs, indicating that some pwMG contributed disproportionally to the economic burden.
| Denmark, n = 1971 | Finland, n = 2248 | Sweden, n = 4403 | |
|---|---|---|---|
| Total costs, € | |||
| Mean (SD) | 12,185 (19,261) | 9036 (14,915) | 5997 (9587) |
| Direct costs, € | |||
| Mean (SD) | 6721 (16,243) | 6360 (13,585) | 3318 (7046) |
| Indirect costs, €a | |||
| Mean (SD) | 5463 (9917) | 2676 (5869) | 2679 (6490) |
- a For people aged ≥65 years, indirect costs were estimated as €0, as indirect costs consisted of sick leave, early retirement, and premature death and did not include, for example, informal caregiver costs.
Most of the annual total costs per person were contributed by direct costs (€6721, 55.2% in Denmark; €6360, 70.4% in Finland; and €3318, 55.3% in Sweden; Table 3, Table S13). Inpatient periods drove the direct costs in all three countries (74.6% in Denmark, 65.3% in Finland, and 68.2% in Sweden). Of the direct costs, 44.8% in Denmark, 47.2% in Finland, and 56.7% in Sweden were MG-related (data not shown).
The mean annual indirect costs per person were €5463, €2676, and €2679 in Denmark, Finland, and Sweden, respectively (Table 3, Table S13). Early retirement drove the indirect costs in Denmark (78.8%) and Finland (75.0%). In contrast, sick leave periods were the main contributor in Sweden (51.5%). Of the indirect costs, 33.3% in Finland and 42.4% in Sweden were MG-related (data not shown).
Costs stratified by disease severity
Stratified by disease severity, the mean annual total costs per person during the whole follow-up were highest with very severe MG: €33,495 in Denmark, €22,761 in Finland, and €19,570 in Sweden. The same pattern persisted regardless of how many years had passed since MG diagnosis (Tables 4, S14–S16). However, in very severe MG, the highest mean annual total costs occurred within the first 2 years after diagnosis in Finland (€30,780) and Sweden (€22,887; Table S15). In Denmark, the highest total costs occurred 2–20 years after diagnosis (€39,358; Table S16). Indirect costs were in the same range in all severity-stratified populations in all three countries, whereas the direct costs increased with worsening disease severity, with the largest differences between disease severity levels observed for inpatient period costs and medication costs.
| Denmark | Finland | Sweden | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Remission | Mild | Moderate | Severe | Very severe | Remission | Mild | Moderate | Severe | Very severe | Remission | Mild | Moderate | Severe | Very severe | |
| Patients, n (%)a | 2408 (19.9%) | 2849 (23.5%) | 6299 (52.0%) | 519 (4.3%) | 48 (0.4%) | 3472 (16.1%) | 7917 (36.6%) | 8901 (41.2%) | 1088 (5.0%) | 227 (1.1%) | 9550 (31.5%) | 6851 (22.6%) | 12,385 (40.9%) | 1330 (4.4%) | 188 (0.6%) |
| Total costs, mean (SD) | 7495 (11,574) | 7309 (10,681) | 8812 (13,120) | 19,405 (19,902) | 33,495 (28,071) | 4184 (8044) | 5479 (8584) | 7484 (9631) | 14,835 (13,751) | 22,761 (16,051) | 4083 (8119) | 4430 (7824) | 6502 (9943) | 12,209 (11,368) | 19,570 (13,500) |
| Direct costs, mean (SD) | 1776 (3303) | 2684 (3538) | 4067 (7290) | 13,804 (14,768) | 26,371 (22,078) | 1983 (4433) | 2609 (4199) | 4539 (6123) | 10,774 (9670) | 19,481 (13,252) | 987 (2675) | 1776 (2500) | 3040 (4192) | 7565 (5676) | 14,005 (8055) |
| Indirect costs, mean (SD) | 5719 (10,737) | 4625 (9992) | 4745 (10,462) | 5602 (11,471) | 7124 (13,366) | 2201 (6316) | 2871 (7051) | 2945 (7114) | 4061 (8214) | 3280 (7567) | 3097 (7532) | 2655 (7294) | 3463 (8581) | 4644 (9315) | 5565 (10,926) |
- a Disease severity was estimated separately for each person and for each follow-up year. Thus, one person can contribute to the results several times.
Costs stratified by age
For pwMG aged 18–64 years, annual total costs per person were in the same range as in the full study cohort: €15,760 in Denmark, €11,121 in Finland, and €9180 in Sweden (Table S17). Indirect costs exceeded the direct cost (56%–74% of total costs) in all three countries. Details on the costs stratified by age are presented in Tables S17 and S18.
Total costs per country
Accumulated annual total costs for pwMG increased by calendar year during follow-up in all three countries, reaching >€11 million in Denmark by end of follow-up (year 2018), >€8 million in Finland (year 2020), and >€13 million in Sweden (year 2020; Table 5).
| Mean annual total costs, € | |||
|---|---|---|---|
| Year | Denmark | Finland | Sweden |
| 2000 | 6,385,000 | 4,356,000 | 1,681,000a |
| 2001 | 6,769,000 | 4,254,000 | 3,302,000a |
| 2002 | 7,088,000 | 4,790,000 | 5,013,000a |
| 2003 | 7,353,000 | 5,515,000 | 5,823,000a |
| 2004 | 7,700,000 | 6,202,000 | 6,773,000a |
| 2005 | 7,860,000 | 6,421,000 | 8,387,000a |
| 2006 | 7,660,000 | 6,442,000 | 9,749,000 |
| 2007 | 8,075,000 | 6,551,000 | 9,292,000 |
| 2008 | 8,164,000 | 6,238,000 | 9,829,000 |
| 2009 | 8,334,000 | 6,216,000 | 9,409,000 |
| 2010 | 9,792,000 | 6,278,000 | 9,728,000 |
| 2011 | 9,944,000 | 7,119,000 | 10,958,000 |
| 2012 | 10,183,000 | 7,190,000 | 10,819,000 |
| 2013 | 10,587,000 | 7,159,000 | 10,948,000 |
| 2014 | 10,545,000 | 7,009,000 | 11,899,000 |
| 2015 | 10,874,000 | 7,400,000 | 12,304,000 |
| 2016 | 11,465,000 | 8,001,000 | 12,902,000 |
| 2017 | 11,394,000 | 8,307,000 | 12,894,000 |
| 2018 | 11,763,000 | 8,175,000 | 13,206,000 |
| 2019 | NA | 8,434,000 | 13,037,000 |
- Abbreviations: MG, myasthenia gravis; NA, not available.
- a In Sweden, outpatient data were only available from 2001, and prescription data were only available from 1 July 2005.
Factors associated with costs in pwMG
Based on a negative binomial regression model analysis, being female was associated with higher total costs than being male in pwMG aged 18–64 years (effect size [ES] = 1.16, 95% confidence interval [CI] = 1.12–1.19; Figure 2a), but there was no difference in direct costs (Figure 2b). Time of 2 years or more since diagnosis was associated with lower total and direct costs (Figure 2a,b).
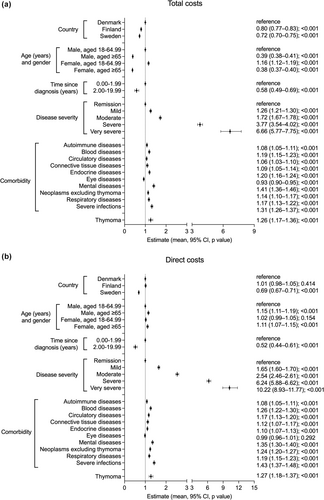
Total and direct costs were both associated with disease severity, with the highest costs observed in very severe MG (for total costs: ES = 6.66, 95% CI = 5.77–7.75; for direct costs: ES = 10.22, 95% CI = 8.93–11.77). As expected, pwMG in remission showed the lowest total age-adjusted costs (Figure 2a), although in the case of Denmark, raw, unadjusted data showed higher total costs for pwMG in remission compared with mild MG (Table 4).
Comorbidities with the strongest association with higher total costs included mental and behavioral disorders (ES = 1.41, 95% CI 1.36–1.46) and severe infections (ES = 1.31, 95% CI = 1.26–1.37; Figure 2a).
DISCUSSION
Existing data on the economic burden of MG are limited and complicated by differences in geographic settings, health care systems, and types of costs examined. In this nationwide, population-based study covering three Nordic countries, we examined HCRU and direct and indirect costs in adult pwMG in Denmark, Finland, and Sweden in the years 2000–2020, also stratified by disease severity. The mean annual total costs per person in pwMG aged ≥18 years were €12,185 in Denmark, €9036 in Finland, and €5997 in Sweden. Persons with very severe MG constituted only 1.6%–4.6% of the cohort but showed 2–3-fold higher annual total costs than the average (€33,495 per person in Denmark, €22,761 in Finland, and €19,570 in Sweden). The costs in pwMG overall were higher during the first 2 years following diagnosis, as also reported in Sweden [16, 22].
The mean annual direct per-person costs ranged between €3318 and €6721 across the three countries and were based on the standard costs of all-cause specialized HCRU and MG-related prescription medications. This overlaps with studies from other European countries: €3952 in Norway [15], €1366 in Bulgaria [10], and €3771 in Italy [7]. Not surprisingly, a worldwide meta-analysis estimated a much broader range for mean annual direct costs (US $760–$28,780) [14], but the emphasis was on North American and Asian countries, where health care systems differ from the Nordic countries.
In our study, the largest component of direct costs in all three countries was inpatient periods (65%–75% of total costs). The duration of annual all-cause inpatient periods aligned with a UK study [23], but the proportion of pwMG with inpatient periods (77%–89%) was higher than in studies from the USA (48%) [8] and Germany (11.5%) [24], which included only MG-related hospitalizations. In general, pwMG have been reported to incur a higher risk of HCRU compared with the general population, with a 3.5-fold higher risk for hospitalization for any cause [8].
MG is associated with increased sick leave, lower employment rates, and increased mortality compared with the general population [13, 25-27], but studies have rarely evaluated the associated indirect costs [10-12, 15]. We estimated indirect costs by HCA [21], using data on long sick leaves, early retirement, or premature death. The mean annual indirect cost ranged between €6268 and €10,321 and exceeded the direct cost (56%–74% of total costs) in pwMG aged 18–64 years in all three countries. The highest indirect cost component was early retirement in Denmark and Finland and sick leave in Sweden. Shorter sick leave periods were, however, excluded from the analysis. These findings align relatively well with a mean annual per-person indirect cost of €8666 estimated in Norway, consisting of sickness absence, disability, patient's time costs, and marginal costs of public funds [15], whereas a German study estimated the mean annual indirect cost at €2790 based on temporary disability, premature retirement, and informal care [11].
In our study, the mean costs were higher than the median costs, indicating that some pwMG contributed disproportionately to costs. Existing literature supports this [28, 29], and we set out to explore this by examining disease severity. Although data on the association between costs and disease severity are limited, pwMG requiring assistance have shown markedly higher costs [11]. The key finding from this study relates to the linking of costs and severity. Those with severe and very severe MG accounted for the highest mean annual per-person costs, ranging between €19,570 and €33,945 across the three countries, with the largest differences between disease severity levels observed for costs of inpatient periods and medication. Direct costs drove the costs for severe and very severe MG, whereas indirect costs contributed most of the costs for remission and mild MG. The direct costs were 10-fold higher in people with very severe MG than in pwMG in remission, whereas the difference in indirect costs between these populations was far less. The distribution of cost was strongly skewed, with pwMG in remission and mild and moderate groups summing up to approximately 95% of the population while showing lower-than-average costs. This indicates that 5% of the studied population have a high burden of disease and high treatment-related costs.
Regarding further factors associated with costs, female sex was here associated with higher total costs but not direct costs, indicating higher indirect costs for women with MG. Certain comorbidities, especially mental and behavioral disorders, and severe infections were associated with higher total and direct costs. Comorbidities being associated with increased costs in MG represent another novel finding compared with existing literature. On the other hand, IVIg, PLEX, myasthenic crisis, and mechanical ventilatory support have also been shown to be associated with increased direct costs in MG [14] but were not available with sufficient robustness in our dataset.
That we leveraged nationwide Nordic health and social care registry data for all citizens regardless of social status, income, or insurance constitutes a strength of this study, because it is population-based with negligible selection bias. Furthermore, all Nordic countries have a tax-funded health care system providing citizens universal access to health care, and detailed data on individuals, treatment (e.g., health care visits and drug purchases), and social benefits are available in nationwide registries. However, the data sources and data recording practices differ to some degree across the three countries, and full harmonization of the data is challenging. Generally, a broader spectrum of HCRU contact types and therefore more outpatient contacts are recorded in Finland than in Denmark or Sweden [30, 31]. Therefore, data were analyzed per country. Furthermore, this study is limited by the lack of a general population control group and the lack of data on primary care contacts. Data on some hospital-administered treatments, such as IVIg, PLEX, biologicals such as rituximab, and mechanical ventilatory support, are lacking as well in all three countries to varying extents, and due to unavailability, the Swedish data should be interpreted with caution regarding medication data before July 2005 and outpatient data before 2001. Assessment of proportions of MG-related health care contacts and costs should also be considered conservatively, as recording practices may vary. Defining disease severity for a study using data from national registries that lack disease-specific information posed a further challenge, and although we opted for the best possible proxy, the chosen, nonstandardized criteria include, for example, costly inpatient periods, which can partly explain the higher costs observed in people with severe disease. Danish pwMG in remission showing higher costs than mild disease can also be due to assessing severity via proxy instead of clinical measures. Finally, real-life indirect costs cover broader productivity losses than analyzed here and other elements such as presenteeism, short sick leave periods, caregiver costs, social benefits, and lost quality of life. Because these data were not captured, and also younger people still studying can be missing from the estimations, the full societal economic impact of MG is even higher than presented here.
In conclusion, we have shown a high level of HCRU and a significant societal economic burden among pwMG, in particular with severe and very severe MG. Compared with other chronic autoimmune diseases, the total per-person costs of MG were estimated here as slightly higher than those reported for, for example, asthma [32, 33], but lower than the costs for, for example, multiple sclerosis [34-36] or rheumatic arthritis [37, 38]. Overall, more data are needed to fully understand the cost structure of MG and its evolution at the population and individual levels, as the evidence remains limited compared with many other diseases with similar levels of clinical burden.
AUTHOR CONTRIBUTIONS
Fredrik Piehl: Conceptualization; writing–review & editing. John Vissing: Conceptualization; writing–review & editing. Juha Mehtälä: Conceptualization; data curation; formal analysis; investigation; methodology; software; visualization; writing–original draft preparation; writing–review & editing. Fredrik Berggren: Conceptualization; funding acquisition; investigation; methodology; supervision; writing–original draft preparation; writing–review & editing. Ingrid Lindberg-Schager: Conceptualization; funding acquisition; investigation; methodology; project administration; supervision; writing–original draft preparation; writing–review & editing. Didier Pitsi: Conceptualization; investigation; writing–review & editing. Evangelos Tsitlakidis: Conceptualization; project administration; writing–original draft preparation; writing–review & editing. Aino Vesikansa: Conceptualization; investigation; methodology; writing–original draft preparation; writing–review & editing. Riina-Minna Väänänen: Project administration; visualization; writing–original draft preparation; writing–review & editing. Tero Ylisaukko-oja: Conceptualization; supervision; writing–original draft preparation; writing–review & editing. Sari Atula: Conceptualization; writing–review & editing.
ACKNOWLEDGMENTS
The authors would like to thank Laila Mehkri, Karoline Doser, Simone Møller Hede, and Tina Bech Olesen (all MedEngine DK ApS) and Mirkka Koivusalo (MedEngine Oy) for project management and other support in the study conduct, and Harlan Barker (MedEngine Oy) for language review.
FUNDING INFORMATION
The study was funded by UCB Pharma.
CONFLICT OF INTEREST STATEMENT
F.P. has received funds from Janssen, Merck KGaA, UCB, Chugai, Lundbeck, Roche, and Novartis. J.V. has received funds from Roche, Sanofi Genzyme, Sarepta Therapeutics, Novartis Pharma, Fulcrum Therapeutics, Biogen, Lupin, Amicus, Regeneron, Argenx, UCB Biopharma, ML Biopharma, Atamyo, Horizon Therapeutics, Dyne Therapeutics Research, Alexion Pharmaceuticals, Edgewise Therapeutics, Genethon, and Janssen Pharmaceutical. J.M. is an employee of MedEngine. F.B. is an employee and stockholder of UCB Pharma, Copenhagen, Denmark. I.L.-S. is an employee of UCB Pharma, Stockholm, Sweden. D.P. was an employee of UCB Pharma, Brussels, Belgium. E.T. was employed by Significance Consulting and contracted by UCB Pharma, Copenhagen, Denmark, at the time of the study. A.V. is an employee of MedEngine. R.-M.V. is an employee of MedEngine. T.Y. is an employee and stockholder of MedEngine and MedEngine DK. S.A. has received funds from Merck, Roche, Biogen, Novartis, UCB Pharma, and Lundbeck.
Open Research
DATA AVAILABILITY STATEMENT
According to the local legislation in the participating countries, access to individual-level data is restricted only to individuals named in the study permit. The study protocol is available upon request from the corresponding author.




