Cholinergic dysfunction in isolated rapid eye movement sleep behaviour disorder links to impending phenoconversion
Abstract
Background and purpose
Most patients with isolated rapid eye movement sleep behaviour disorder (iRBD) progress to a parkinsonian alpha-synucleinopathy. However, time to phenoconversion shows great variation. The aim of this study was to investigate whether cholinergic and dopaminergic dysfunction in iRBD patients was associated with impending phenoconversion.
Methods
Twenty-one polysomnography-confirmed iRBD patients underwent baseline 11C-donepezil and 6-Fluoro-(18F)-l-3,4-dihydroxyphenylalanine (18F-DOPA) positron emission tomography (PET). Potential phenoconversion was monitored for up to 8 years. PET images were analysed according to patients' diagnoses after 3 and 8 years using linear regression. Time-to-event analysis was made with Cox regression, dividing patients into low and high tracer uptake groups.
Results
Follow-up was accomplished in 17 patients. Eight patients progressed to either Parkinson's disease (n = 4) or dementia with Lewy bodies (n = 4), while nine remained non-phenoconverters. Compared with non-phenoconverters, 8-year phenoconverters had lower mean 11C-donepezil uptake in the parietal (p = 0.032) and frontal cortex (p = 0.042), whereas mean 11C-donepezil uptake in 3-year phenoconverters was lower in the parietal cortex (p = 0.005), frontal cortex (p = 0.025), thalamus (p = 0.043) and putamen (p = 0.049). Phenoconverters within 3 years and 8 years had lower 18F-DOPA uptake in the putamen (p < 0.001). iRBD patients with low parietal 11C-donepezil uptake had a 13.46 (95% confidence interval 1.42;127.21) times higher rate of phenoconversion compared with those with higher uptake (p = 0.023). iRBD patients with low 18F-DOPA uptake in the most affected putamen were all phenoconverters with higher rate of phenoconversion (p = 0.0002).
Conclusions
These findings suggest that cortical cholinergic dysfunction, particularly within the parietal cortex, could be a biomarker candidate for predicting short-term phenoconversion in iRBD patients. This study aligns with previous reports suggesting dopaminergic dysfunction is associated with forthcoming phenoconversion.
INTRODUCTION
Rapid eye movement (REM) sleep behaviour disorder (RBD) is a parasomnia characterised by absence of normal muscle atonia during REM sleep, leading to the enactment of dreams [1]. This parasomnia is commonly reported in patients with synucleinopathies [2] and, when it occurs before the onset of motor and cognitive symptoms, isolated RBD (iRBD) is considered a prodromal or early phenotype of these conditions, with up to 91% of iRBD patients eventually converting to either Parkinson's disease (PD) [3], dementia with Lewy bodies (DLB) [4] or the rarer multiple system atrophy (MSA) after 15 years of follow-up [1, 5-7].
Dopaminergic dysfunction in the basal ganglia is one of the hallmarks of PD pathology and plays a major role in causing motor symptoms of the disease. Dopaminergic dysfunction has also been demonstrated in iRBD patients with motor complaints not yet sufficient to fulfil consensus criteria for a parkinsonian diagnosis [8-11]. Conversely, the involvement of other neurotransmitter systems is considered responsible for the onset of other symptoms of PD. In particular, neuroimaging studies employing positron emission tomography (PET) have linked cholinergic dysfunction to cognitive impairment and gait disturbances in parkinsonian diseases [12].
Previously, we have identified potential alterations of the striatal dopaminergic system in iRBD patients using 6-Fluoro-(18F)-l-3,4-dihydroxyphenylalanine (18F-DOPA) PET [11] as well as the cortical cholinergic system using 11C-donepezil PET [13]. In addition, we observed a progressive worsening of striatal dopaminergic dysfunction [14] as well as cholinergic dysfunction spreading to subcortical structures over 3 years [15]. However, the predictive value for future disease progression of these PET tracers remains to be established.
The objective of this study was to examine whether dysfunction of the cholinergic and dopaminergic systems in patients with iRBD were associated with impending phenoconversion to parkinsonian alpha-synucleinopathies. We accomplished this by performing an 8-year clinical follow-up of iRBD patients who had received 11C-donepezil and 18F-DOPA brain PET at baseline.
MATERIALS AND METHODS
Study population
Between March 2015 and October 2016, a cohort of 21 patients with polysomnography-confirmed iRBD were recruited for baseline assessments from sleep clinics at Aarhus University Hospital, Denmark (n = 11) and Hospital Clinic Barcelona, Spain (n = 10) [13]. Patients presenting with parkinsonism, dementia and other neurological conditions were excluded prior to inclusion based on established criteria [3-5, 16, 17]. At baseline, all participants underwent clinical assessments and had PET scans with 11C-donepezil and 18F-DOPA tracers to investigate the cholinergic system and nigrostriatal dopaminergic system function, respectively. None of the patients were receiving medication that would impact the cholinergic and the dopaminergic neurotransmitter systems [13]. Subsequently, all participants were invited to participate in a long-term follow-up to capture phenoconversion to a parkinsonian alpha-synucleinopathy. For individual participants, follow-up stopped once a final diagnosis was reached. All participants maintained regular appointments with the neurologist-led sleep clinics to monitor any signs of possible phenoconversion, with diligent records of diagnosis timing meticulously maintained. Parkinsonian phenoconversion was recognised according to the UK Brain Bank criteria for PD [3], the revised McKeith consensus criteria of the fourth report of the DLB consortium for probable DLB [4], and the criteria of the second consensus statement on the diagnosis of MSA atrophy for possible MSA [5].
At baseline, age-matched, healthy controls were recruited for 11C-donepezil PET (n = 9) and 18F-DOPA (n = 9) PET, respectively (details on recruitment previously published) [11, 13].
All participants provided informed consent in accordance with the principles of the Declaration of Helsinki before their enrolment in the study. The study protocol was approved by the Central Denmark Region Committee on Health Research (M-2014-397-14) and the Ethics Committee of the Hospital Clinic Barcelona (HCB/2015/0186).
Imaging techniques
Comprehensive information on the scanners used and the specific procedures employed has previously been published [11, 13]. Briefly, all baseline PET scans were performed with a Siemens High-Resolution Research Tomograph (ECAT HRRT; CTI/Siemens, Knoxwille, TN, USA) at the Department of Nuclear Medicine and PET Centre, Aarhus University Hospital, Denmark. 11C-donepezil, which binds to acetylcholinesterase, enabled the visualisation of cholinergic function with PET, while 18F-DOPA, which is metabolised by aromatic L-amino acid decarboxylase, was used to visualise striatal, dopamine storage. Additionally, to facilitate co-registration, high-resolution T1-weighted magnetic resonance imaging (with 3-T MAGNETOM Skyra; Siemens Healthcare, Erlangen, Germany) was performed for all participants.
Imaging analysis
Comprehensive details on imaging analyses are provided in our previous publications [11, 13]. To summarise briefly, brain scans were transformed into Montreal Neurological Institute space using PMOD software (version 3.6; PMOD Technologies Ltd., Zürich, Switzerland). Regions of interest (ROIs) were predefined, and kinetic modelling of tracer time–activity curves were analysed. To quantify the distribution volume ratio (DVR) of the 11C-donepezil PET, the Logan reference tissue graphical model was employed [18]. White matter in the centrum semiovale was chosen as the reference region. Cholinergic ROIs were the averaged frontal, parietal, temporal, and occipital cortical regions, as well as the thalamus, putamen, caudate, insula, posterior cingulate cortex, and cerebellum. In a secondary comparison, the 11C-donepezil uptake of the entire neocortex was compared between iRBD patients and healthy controls. To quantify the 18F-DOPA influx constant (Ki), grey matter of the occipital lobe was used for the reference tissue input function with the Patlak graphical approach [19]. Dopaminergic ROIs were the putamen and caudate of the most and least affected hemisphere.
Clinical assessments
Patients were examined at least once a year for possible phenoconversion, and time of recognised phenoconversion was documented. At baseline and 8-year study visits, participants underwent comprehensive evaluations for motor and non-motor symptoms using the Movement Disorder Society Unified Parkinson's Disease Rating Scale (MDS-UPDRS) part III [20], the Non-Motor Symptoms Scale for Parkinson's Disease (NMSS) [21], the Parkinson's Disease Non-Motor Symptom Questionnaire (PDNMS) [22], the Scale for Outcomes in Parkinson's disease for Autonomic symptoms (SCOPA-AUT) [23], and the Major Depression Inventory (MDI) [23]. Cognitive functioning was assessed using the Montreal Cognitive Assessment (MoCA) [24]. Sleep quality was evaluated using the Parkinson's Disease Sleep Scale (PDSS-2) [25]. The University of Pennsylvania Smell Identification Test (UPSIT, MediSense, The Netherlands) was only administered during the baseline assessment.
Statistical analysis
Statistical analyses and graphical presentations were conducted using StataBE 17.0 (StataCorp LP, College Station, TX, USA) and Prism 10 (GraphPad Software, La Jolla, CA, USA).
Group comparisons
The iRBD cohort was first divided into two groups, 8-year phenoconverters and 8-year non-phenoconverters, based on their status at the total follow-up time of this study. An ideal biomarker for future disease modification trials would be predictive of a short time to phenoconversion [10], so an additional group comparison based on the 3-year status, 3-year phenoconverters and 3-year non-phenoconverters, was also performed. Analyses of PET uptake values across groups were made using a multiple regression model adjusting for age. Furthermore, the PET uptake values for healthy controls were compared to the 8-year phenoconverters and 8-year non-phenoconverters. The model was checked using diagnostic plots of the residuals.
The clinical baseline assessments were compared between phenoconverters and non-phenoconverters using a regression model adjusting for age. Furthermore, baseline clinical assessments of patients with iRBD, PD and DLB were interrogated with analyses of variance (ANOVA) with Bonferroni post hoc analysis.
Assumptions of normality were verified through Q–Q plots. Estimates are presented with 95% confidence intervals (CIs).
Analyses of time to phenoconversion
Recognised phenoconversion was analysed with number of years since baseline PET scans as the time scale. Follow-up was censored at the time of the patient's last completed visit with the study investigators. Recognised phenoconversion was estimated by Kaplan–Meier curves and differences between high and low tracer uptake by age-adjusted Cox proportional hazard model in each ROI. High tracer uptake was defined as values higher than the mean uptake value of all iRBD patients in each ROI, while low tracer uptake was defined as values below the mean. The proportionality was validated by observed and predicted phenoconversion plots. As the most affected putamen did not meet this assumption, comparisons between high and low 18F-DOPA Ki groups in this ROI were performed using the log-rank test. All estimates are presented with 95% CIs.
Paired analyses of non-phenoconverters' progression
In non-phenoconverters, comparisons of clinical scores from baseline to the 8-year follow-up were performed using Student's paired, two-sided t-tests. Assumption of normality was verified through Q–Q plots. Estimates are presented with 95% CIs.
RESULTS
Nineteen iRBD patients who had received at least one of the two baseline PET scans completed the follow-up study. All of them had a baseline 18F-DOPA PET and 16 had a successful baseline 11C-donepezil PET.
Baseline characteristics of all iRBD participants in the follow-up are presented in Table 1. Nine participants (63.4 ± 6.6 years old at baseline, all male) maintained their iRBD diagnosis during the 8-year follow-up (7.3 ± 0.5 years, range: 6.2–8.1 years) and are referred to hereafter as 8-year non-phenoconverters. Eight participants (67.1 ± 7.2 years old at baseline, two female) had progressed to either PD (n = 4) or DLB (n = 4). Six of these phenoconverters received their diagnoses by 3 years from baseline (0.5, 1.0, 1.3, 1.6, 2.4 and 3.0 years, respectively), while two phenoconverters were diagnosed at 6.2 and 7.6 years after baseline. Thus, all phenoconverters in the secondary comparison analyses of 3-year diagnosis status progressed to PD or DLB after no more than 3 years.
| Characteristics | iRBD participants |
|---|---|
| Number of participants | 17 |
| Female sex, number of observations | 2 |
| Age, yearsa | 65.2 ± 6.9 |
| iRBD disease duration, yearsa | 4.0 ± 3.6 |
| PDNMSa | 7.6 ± 4.6 |
| NMSSa | 31.8 ± 19.2 |
| MoCAa | 24.9 ± 2.2 |
| MDS-UPDRS part III motor score | 3.3 ± 2.4 |
| SCOPA-AUT | 17.1 ± 8.1 |
| MDI | 3.6 ± 3.6 |
| PDSS-2 | 11.6 ± 8.9 |
| UPSIT | 19.1 ± 7.4 |
- Note: Baseline characteristics of all iRBD participants of the follow-up (n = 17).
- Abbreviations: iRBD, isolated rapid eye movement sleep behaviour disorder; MDI, Major Depression Inventory; MDS-UPDRS, Movement Disorder Society Unified Parkinson's Disease Rating Scale; MoCA, Montreal Cognitive Assessment; NMSS, Non-Motor Symptoms Scale for Parkinson's Disease; PDNMS, Parkinson's Disease Non-Motor Symptom Questionnaire; PDSS-2, Parkinon's Disease Sleep Scale; SCOPA-AUT, Scale for Outcomes in Parkinson's Disease for Autonomic Symptoms; UPSIT, University of Pennsylvania Smell Identification Test.
- a Mean ± SD.
The remaining two participants were excluded from the subsequent analyses as they developed comorbidities during the follow-up: one participant received an additional diagnosis of Alzheimer's disease after 7 years, while the other developed normal pressure hydrocephalus after 6 years (Data S1, Figure S1). Regrettably, due to technical problems, 11C-donepezil PET scans were not acquired at baseline for two individuals who subsequently developed PD. Thus, the cohort available for baseline 11C-donepezil DVR analyses comprised 15 individuals (nine 8-year non-phenoconverters vs. six 8-year phenoconverters, and ten 3-year non-phenoconverters vs. five 3-year phenoconverters), while the cohort available for 18F-DOPA and clinical assessments comprised 17 individuals (nine 8-year non-phenoconverters vs. eight 8-year phenoconverters, and eleven 3-year non-phenoconverters vs. six 3-year phenoconverters).
For comparison, nine healthy controls (63.7 ± 6.9 years old, all male) had 11C-donepezil PET at baseline and nine healthy controls (64.2 ± 3.8 years old, all male) had 18F-DOPA PET.
Comparison of clinical and imaging baseline characteristics
Clinical baseline characteristics
The 8-year phenoconverters had a significantly higher mean score on the PDNMS (p = 0.030) compared with 8-year non-phenoconverters at baseline, but the two groups did not differ significantly in iRBD disease duration, SCOPA-AUT score, MDS-UPDRS III motor score, MoCA score, MDI score, PDSS-2 score, or UPSIT score (Table S1A). Age also did not differ significantly between the two groups. However, there was a 4-year difference in the mean age of 8-year phenoconverters compared with 8-year non-phenoconverters. Therefore, all analyses were performed adjusting for age.
A comparison of the 8-year non-phenoconverters and 8-year phenoconverters divided into patients diagnosed with PD or DLB, respectively, showed significant variance only in MDI score (ANOVA, p = 0.004), while the variance in non-motor scales did not meet significance in this analysis (Table S1B). Post hoc analysis showed that the patients diagnosed with PD scored significantly higher on the MDI compared to the 8-year non-phenoconverters (p = 0.003) and DLB patients (p = 0.044).
Compared with the 3-year non-phenoconverters, 3-year phenoconverters did not differ significantly in any clinical assessments at baseline (Table S2A). ANOVA comparing 3-year non-phenoconverters and 3-year phenoconverters divided into PD or DLB groups showed significant variance only in MDI score (p = 0.026, Table S2B). Furthermore, post hoc analysis showed that this variance was driven by a higher mean score of the PD patients compared to the 8-year non-phenoconverters (p = 0.027).
Baseline 11C-donepezil PET uptake
Compared with 8-year non-phenoconverters, 8-year phenoconverters (PD: n = 2, DLB: n = 4) had significantly lower mean 11C-donepezil uptake in the parietal cortex (−0.12 [−0.2296;–0.0119]; p = 0.032) and frontal cortex (−0.1256 [−0.2460; −0.0051]; p = 0.042). No differences were found in the remaining cholinergic ROI (Table S3A). Compared with healthy controls, the mean 11C-donepezil uptake of the 8-year phenoconverters was significantly lower in all cortical ROIs, including the posterior cingulate cortex and insula as well as in the caudate (Figure 1). Meanwhile, the mean 11C-donepezil uptake of the 8-year non-phenoconverters only differed from the healthy control mean in the caudate and insula.
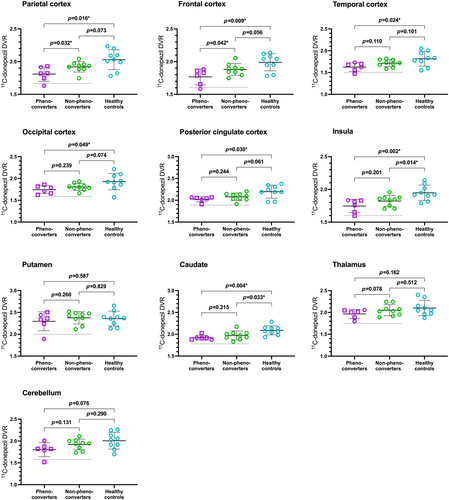
Within the neocortex, two of the iRBD patients had baseline 11C-donepezil uptake values below 2 standard deviations (SDs) from the healthy control mean and both patients phenoconverted within 3 years of their cholinergic PET scan (PD: n = 1, DLB: n = 1).
Finally, 3-year phenoconverters (PD: n = 2, DLB: n = 3) had significantly lower mean 11C-donepezil uptake in the parietal cortex (p = 0.005) and frontal cortex (p = 0.025) as well as in the thalamus (p = 0.043) and putamen (p = 0.049) compared with 3-year non-phenoconverters at baseline (Table S3B).
Baseline 18F-DOPA PET uptake
18F-DOPA uptake by the putamen was significantly lower in 8-year phenoconverters (PD: n = 4, DLB: n = 4) compared to 8-year non-phenoconverters in both the most affected hemisphere (mean Ki difference of −0.0032 [−0.0047; −0.0017]; p < 0.001) and the least affected hemisphere (mean Ki difference of −0.0027 [−0.0042; −0.0012]; p < 0.001).
Interestingly, 8-year phenoconverters with putaminal 18F-DOPA Ki values below 2 SDs from the entire iRBD group mean were all diagnosed with PD or DLB no later than 1.5 years after their scan, while 8-year phenoconverters with phenoconversion more than 5 years after their scan had the highest putaminal 18F-DOPA uptake values within the phenoconverters group.
In the putamen, the means of both the 8-year phenoconverters and 8-year non-phenoconverters were significantly lower compared to the healthy controls in both the most and least affected hemispheres (p < 0.001 in all age-adjusted comparisons [Figure 2]).
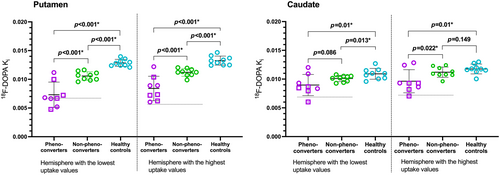
18F-DOPA uptake by the caudate was significantly lower in the least affected hemisphere of 8-year phenoconverters (−0.0017 [−0.0031; −0.0002]; p = 0.022) compared to the 8-year non-phenoconverters, while the mean difference in the most affected hemisphere was not significant (−0.0011 [−0.0023; 0.00002]; p = 0.086).
The mean 18F-DOPA uptake of the DLB phenoconverters (n = 4) was significantly lower compared with the mean uptake of all other iRBD participants at baseline (non-phenoconverters pooled with PD phenoconverters, n = 13) in both the most (p = 0.001) and least (p < 0.001) affected caudate.
Compared with healthy controls, 8-year phenoconverters had significantly lower mean 18F-DOPA uptake in the caudate, in both the most affected and least affected hemisphere (both p = 0.01). Conversely, mean caudate 18F-DOPA uptake was significantly lower only in the most affected hemisphere of 8-year non-phenoconverters compared with healthy controls (Figure 2).
Likewise, when we compared 18F-DOPA uptake in 3-year phenoconverters (PD: n = 3, DLB: n = 3) with the 3-year non-phenoconverters, we found a significant difference in the most affected putamen (p < 0.001), the least affected putamen (p < 0.001), and the least affected caudate (p = 0.022, details in Table S4).
Analyses of time to phenoconversion
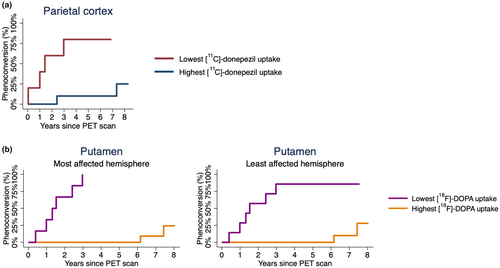
The estimated phenoconversion curves are shown in Figures 3 and S2. Cox regression analyses showed that the iRBD patients with baseline parietal 11C-donepezil PET uptake values below the group mean had a 13.46 (1.42;127.21; p = 0.023) times higher rate of phenoconversion compared with iRBD patients with uptake values above the group mean. Analyses of the remaining cholinergic ROIs did not reach significance (Table 2). iRBD patients with 18F-DOPA PET uptake values below the group mean in the most affected putamen had a significantly higher rate of phenoconversion (p = 0.0002), while those with low dopaminergic uptake values in the least affected putamen had a 10.06 (1.88;53.89; p = 0.007), times higher phenoconversion rate compared with iRBD patients with higher uptake values. Analyses of 18F-DOPA PET uptake values in the most and least affected caudate, showed a 5.30 (1.05;26.70; p = 0.043) and 7.91 (1.52;41.06; p = 0.014) times higher rate of phenoconversion, respectively.
| Regions of interest | Estimated age-adjusted HR (95% CI) | p values |
|---|---|---|
| 11C-donepezil | ||
| Parietal cortex | 13.46 (1.42; 127.21) | 0.023* |
| Frontal cortex | 4.54 (0.78; 26.43) | 0.092 |
| Cerebellum | 6.36 (0.72; 56.34) | 0.096 |
| Temporal cortex | 3.09 (0.51; 18.88) | 0.222 |
| Occipital cortex | 2.67 (0.52; 13.57) | 0.240 |
| Caudate | 3.62 (0.42; 31.42) | 0.243 |
| Putamen | 2.26 (0.45; 11.35) | 0.320 |
| Thalamus | 2.69 (0.48; 15.08) | 0.261 |
| Posterior cingulate cortex | 1.77 (0.32; 9.75) | 0.513 |
| Insula | 1.79 (0.36; 8.95) | 0.476 |
| 18F-DOPA | ||
| Putamen | ||
| Most affected sidea | 0.0002* | |
| Least affected side | 10.06 (1.88; 53.89) | 0.007* |
| Caudate | ||
| Most affected side | 5.30 (1.05; 26.70) | 0.043* |
| Least affected side | 7.91 (1.52; 41.06) | 0.014* |
- Note: PET uptake values below the group mean compared with higher values for regions of interest with tracers 11C-donepezil (n = 15) or 18F-DOPA (n = 17).
- Abbreviations: 18F-DOPA, 6-Fluoro-(18F)-l-3,4-dihydroxyphenylalanine; CI, confidence interval; HR, hazard ratio; PET, positron emission tomography.
- a Analysed using the log-rank test.
- * p < 0.05.
Clinical progression of non-phenoconverters
The 8-year non-phenoconverters did not meet the criteria for PD [3], DLB [4], MSA [5] or any other neurological condition during the follow-up period of this study. However, over the course of these 8 years, they showed a significant deterioration in MDS-UPDRS part III motor score from 2.4 ± 1.5 to 7.8 ± 4.5 (p = 0.0089) and in SCOPA-AUT total score from 14.6 ± 7.3 to 17.4 ± 9.4 (p = 0.032). Additionally, the group showed worsening in non-motor scores (NMSS, PD-NMS and MDI), although these changes did not reach statistical significance (Table 3).
| Outcomes | Baseline (mean ± SD) | Follow-up (mean ± SD) | p value |
|---|---|---|---|
| MDS-UPDRS part III motor score | 2.4 ± 1.5 | 7.8 ± 4.5 | 0.009* |
| SCOPA-AUT | 14.6 ± 7.3 | 17.4 ± 9.4 | 0.032* |
| MoCA | 24.8 ± 2.3 | 25.0 ± 2.7 | 0.898 |
| MDI | 2.6 ± 2.0 | 4.6 ± 2.8 | 0.070 |
| NMSS | 22.6 ± 15.5 | 34.3 ± 24.6 | 0.165 |
| PDNMS | 5.3 ± 3.4 | 6.1 ± 3.0 | 0.495 |
| PDSS-2 | 10.0 ± 7.4 | 8.0 ± 6.5 | 0.077 |
- Note: Paired t-test of baseline and 8-year follow-up clinical assessments of participants who maintained their isolated rapid eye movement sleep behaviour disorder diagnosis (n = 9; SCOPA-AUT, n = 8 as one participant permanently used intermittent catheterisation at follow-up).
- Abbreviations: MDI, Major Depression Inventory; MDS-UPDRS, Movement Disorder Society Unified Parkinson's Disease Rating Scale; MoCA, Montreal Cognitive Assessment; NMSS, Non-Motor Symptoms Scale for Parkinson's Disease; PDNMS, Parkinson's Disease Non-Motor Symptom Questionnaire; PDSS-2, Parkinson's Disease Sleep Scale; SCOPA-AUT, the Scale for Outcomes in Parkinson's Disease for Autonomic symptoms.
- * p < 0.05.
DISCUSSION
Even though an iRBD diagnosis may be considered an early stage of parkinsonian alpha-synucleinopathy, the variability in interval from iRBD onset to parkinsonian phenoconversion [7] poses a significant challenge, impacting not only individuals coping with the condition but also the feasibility of clinical trials. This study showed novel in vivo findings in patients with iRBD suggesting that the presence of baseline cholinergic dysfunction in the parietal cortex is associated with short-term disease progression to PD or DLB. In our cohort, 47% of polysomnography-confirmed iRBD patients developed a parkinsonian syndrome during the mean (range) study time of 7.5 (6.9–8.2) years (n = 17).
We found that on their baseline scan, iRBD patients with a forthcoming phenoconversion had lower 11C-donepezil PET uptake in the parietal and frontal cortex compared with patients who maintained their iRBD diagnosis. However, it should be noted that in this analysis, all our converters except one received a final diagnosis of a parkinsonian disorder within only 3 years, and their baseline 11C-donepezil PET as a group showed significantly lower uptake not only in the parietal and frontal cortex, but also in the thalamus and putamen. Time-to-phenoconversion analyses of the baseline cholinergic PET imaging suggested that low DVR uptake values in the parietal cortex could predict a higher rate of phenoconversion. Furthermore, both patients with baseline cholinergic PET uptake in the neocortex below 2 SDs from the healthy control mean phenoconverted within 3 years of their scan. Collectively, these findings suggest that cholinergic dysfunction in these cortical areas is indeed associated with impending phenoconversion of iRBD.
Additionally, we demonstrated that striatal, dopaminergic dysfunction, especially in the putamen, was also associated with phenoconversion. In the putamen, patients with 18F-DOPA uptake values lower than 2 SDs from the iRBD patients' overall mean (n = 4) phenoconverted within 1.5 years after their scans. Furthermore, the rate of phenoconversion was significantly higher in those iRBD patients with 18F-DOPA uptake values below the group mean in both the most and least affected putamen. The 8-year phenoconverters' mean 18F-DOPA Ki value in the caudate was significantly lower in the least affected hemisphere compared to 8-year non-phenoconverters', and furthermore, iRBD patients with low uptake values there had a rate of phenoconversion that was seven times greater than those who had higher Ki values. These findings align with previous studies that found striatal dopaminergic dysfunction to be associated with future phenoconversion using single-photon emission computed tomography to image dopamine transporter (DAT-SPECT) binding and had a clinical mean follow-up time of 2.5 years [8], 4.7 years [9] and 5.7 years [10], respectively. The robust results of the present study confirm these previous findings with a longer follow-up time, although within a smaller cohort. Furthermore, this study used neuroimaging targeting the aromatic L-amino acid decarboxylase, which unlike the dopamine transporter, is not downregulated in early PD and so does not overestimate disease impact.
When comparing the baseline caudate 18F-DOPA uptake by all phenoconverters with non-phenoconverters, we did not find any significant difference, which may be because the caudate dopamine terminals are relatively preserved in early PD compared to those in the putamen nucleus [26]. However, those individuals who underwent DLB phenoconversion showed significant baseline reduction in caudate dopaminergic function compared to both non-phenoconverters and those participants who phenoconverted to PD. This suggests that the more global striatal dysfunction seen in DLB [27] can be present in early disease stages and this may provide a marker of the specific type of phenoconversion in iRBD patients.
Phenoconverters to PD scored significantly higher on the MDI screening for depressive symptoms at baseline compared to both non-phenoconverters and DLB phenoconverters. Another study also found both baseline olfactory dysfunction and MDS-UPDRS motor score to be amongst the strongest clinical predictors of phenoconversion [9]. While the mean scores presented here show a similar trend, the lack of significance between these clinical scores is probably attributable to the limitation of the small cohort size. Furthermore, the baseline olfaction function was below the 5th percentile for age and sex for all our iRBD patients except one (an 8-year non-phenoconverter).
The majority of iRBD patients in our cohort converted within 3 years of enrolment in the study and, therefore, the results presented here may be more representative of the very short-term phenoconverters than a general iRBD population.
The limitations of this study include the relatively small cohort size. While the original cohort of 21 participants was appropriate for a cross-sectional PET-imaging study of a rare condition, exclusions, dropouts, and technical difficulties obtaining scans made the cohorts available for these retrospective analyses relatively small. Furthermore, this study included a relatively small number of female participants, which may affect the generalisability of the findings across gender; thus, our findings need to be confirmed in larger cohorts. Finally, as this study was originally designed to assess in vivo brain changes in iRBD patients, a full neurophysiological assessment was not performed. We were therefore unable to correlate PET findings with detailed cognitive scores. This important point needs to be clarified in future studies specifically designed for this aim and with appropriate sample sizes.
The study population has been comprehensively phenotyped with multiple clinical assessments and included only polysomnography-confirmed iRBD patients for PET imaging, which targeted both the dopaminergic and the cholinergic system. Participants have been followed up closely by the collaborating sleep units between study visits and the time of recognised phenoconversion meticulously noted. To investigate cholinergic function, this study used the 11C-donepezil PET tracer which binds to acetylcholinesterase. However, acetylcholinesterase can also be found in non-cholinergic synapses, and furthermore, the tracer also binds with high affinity to the sigma-1 receptor located in the endoplasmic reticulum [28, 29]. Thus, our results need confirmation in future studies with cholinergic tracers targeting other sites of the cholinergic synapse such as the vesicular acetylcholine transporter.
In conclusion, the results of this study suggest that cholinergic dysfunction, especially in the parietal cortex of iRBD patients, is associated with impending phenoconversion to PD or DLB. This study also confirms previous reports of striatal, dopaminergic dysfunction as a possible predictor of forthcoming phenoconversion using 18F-DOPA PET or DAT-SPECT. Furthermore, this study suggests that early loss of the caudate dopaminergic function could be predictive of future progression to DLB. Predicting the course of disease progression in iRBD would not only be meaningful in daily clinical practice but would especially become useful in selection of study populations in future trials of neuroprotective treatments. These results need replication in larger cohorts to assess whether the neuroimaging predictors that we observed can be generalised. Further follow-up of our Aarhus-Barcelona iRBD cohort is ongoing.
AUTHOR CONTRIBUTIONS
Miriam H. Terkelsen: Data curation; formal analysis; investigation; methodology; project administration; visualization; writing – original draft. Alex Iranzo: Conceptualization; funding acquisition; investigation; supervision; writing – review and editing. Mónica Serradell: Data curation; investigation; project administration; writing – review and editing. Andreas M. Baun: Writing – review and editing; formal analysis. Morten G. Stokholm: Data curation; investigation; project administration; writing – review and editing. Erik Hvid Danielsen: Investigation; writing – review and editing. Karen Østergaard: Conceptualization; funding acquisition; writing – review and editing. Marit Otto: Investigation; writing – review and editing. Kristina B. Svendsen: Investigation; writing – review and editing. Mette Møller: Writing – review and editing; supervision. Erik L. Johnsen: Supervision; writing – review and editing. Alicia Garrido: Investigation; writing – review and editing. Dolores Vilas: Writing – review and editing; investigation. Joan Santamaria: Writing – review and editing; investigation. Arne Møller: Conceptualization; writing – review and editing. Carles Gaig: Writing – review and editing; investigation. David J. Brooks: Writing – review and editing. Per Borghammer: Writing – review and editing; methodology. Eduardo Tolosa: Conceptualization; funding acquisition; writing – review and editing. Nicola Pavese: Conceptualization; funding acquisition; writing – review and editing; supervision.
ACKNOWLEDGEMENTS
The authors thank the participants and their families for their time and cooperation. We also thank the study coordinators, cyclotron operators, chemists, and imaging technologists who collaborated on this study. Figure S1 was made with Biorender.com.
FUNDING INFORMATION
The work presented here was supported by the Danish Council for Independent Research (Denmark; 4004-00480B, 8020-00260B), the Instituto de Salud Carlos III (Spain; CB06/05/0018), the Danish Parkinson's Association (Denmark; R4-A104-B60), and the Augustinus Fond (Denmark; 23-0916). Funders had no role in the design and conduct of the study.
CONFLICT OF INTEREST STATEMENT
The authors report no competing interests.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




