Relationship between migraine and epilepsy in a large population-based cohort: The HUNT Study
Abstract
Background and Purpose
Several studies have reported substantial comorbidity between epilepsy and migraine. Most of these were based on clinical cohorts or used unvalidated diagnostic instruments. Our study re-examined this association in a large general population cohort using validated diagnoses for both disorders.
Methods
A total of 65,407 participants (≥20 years old) from HUNT (the Trøndelag Health Study) were classified for migraine and nonmigraine headache using a validated questionnaire. Medical record review was used to validate and classify epilepsy in 364 participants (cases), who were compared with 63,298 participants without epilepsy (controls). The association between epilepsy and migraine was analysed using logistic regression adjusted for sex and age.
Results
Patients with epilepsy had no increased prevalence of migraine (odds ratio [OR] = 0.95, 95% confidence interval [CI] = 0.68–1.33) or nonmigraine headache (OR = 1.18, 95% CI = 0.93–1.50) compared to controls. When stratified by headache frequency, epilepsy was associated with a higher prevalence of migraine with highly frequent headache (≥7 days/month; OR = 1.73, 95% CI = 1.08–2.78).
Conclusions
Migraine was equally common in people with and without epilepsy. Patients with epilepsy who suffered from migraine were more prone to having highly frequent migraine.
INTRODUCTION
The comorbidity between migraine and epilepsy has been assessed in several studies. A meta-analysis of population-based studies published between 1996 and 2013 reported a 79% overall increased prevalence of epilepsy in people with migraine and a 52% overall increased prevalence of migraine in people with epilepsy [1]. A large number of clinic-based studies report a higher prevalence of migraine in epilepsy populations [1-4].
Although headache can occur in relation to seizures, seizures and migraine attacks do not usually appear to be temporally related in individual patients [4], and no clear association has been found between migraine and epileptic focus location, seizure type, seizure frequency, or use of antiseizure medication [5, 6]. Various mechanisms have been proposed to explain the observed comorbidity, including a common tendency to neuronal excitation and the possibility of shared genetic factors [4, 7].
Although a large body of literature appears to support the co-occurrence of epilepsy and migraine, it is notable that nearly all studies to date have either (i) not been population-based, but instead examined the prevalence of migraine in epilepsy patient groups or (ii) used unvalidated instruments to diagnose epilepsy and/or migraine [1, 2, 4]. When the meta-analysis of population-based studies [1] was restricted to studies in which cases of epilepsy and migraine were identified by physician assessment, no association was found. Lastly, several of the population-based studies did not adjust for age and sex as potential confounders for an observed comorbidity [1, 3].
The aim of the present study was to re-examine the association between migraine and epilepsy in a large representative cohort of the general population using validated diagnoses for migraine and epilepsy and comparing the prevalence of migraine and nonmigraine headache among participants with and without epilepsy.
MATERIALS AND METHODS
Study population
We used a case–control design to compare the prevalence of migraine between cases with epilepsy and controls without epilepsy in the population-based Trøndelag Health Study (HUNT) [8]. HUNT is one of the largest epidemiologic health studies conducted, providing a unique database with information from inhabitants of former Nord-Trøndelag County in Norway. All inhabitants aged 20 years or older were invited to participate in the HUNT2 (inclusion 1995–1997) and HUNT3 (inclusion 2006–2008) studies, which included questions on headache and migraine. The health care system in Norway is publicly funded. To identify patients with current or previous epilepsy, we first obtained epilepsy diagnosis codes according to the International Classification of Diseases (ICD-9 and ICD-10) from hospital registries for all inpatient and outpatient contacts between 1987 and 2019 for all HUNT participants, and subsequently validated epilepsy diagnoses by review of medical records as described in detail below. Of the 78,962 people who participated in either HUNT2 or HUNT3, 65,407 answered the headache questions and were eligible for this study. To limit control group bias, a total of 1745 participants without validated epilepsy diagnoses were excluded. They had either (i) at least one hospital contact with a registered diagnosis of epilepsy (ICD-10G40.x or ICD-9348.x) or (ii) only self-reported epilepsy based on the HUNT questionnaires (in HUNT2, “Do you have or have you ever had: Epilepsy”; in HUNT3, “Have you had, or do you have any of the following diseases: Epilepsy”). The control group was defined as the remaining eligible population after removing participants with validated epilepsy or an unvalidated indication of epilepsy. This resulted in a final study population of 63,662 participants, of whom 63,298 were controls without epilepsy and 364 were cases with epilepsy. Whereas epilepsy case status was based on hospital records covering a wide time span (1987–2019), migraine was examined as 1-year prevalence at one point in time for each participant (in either HUNT2 or HUNT3). Information on the time of onset of migraine was not collected in HUNT, and for participants with both conditions the onset of migraine may therefore have been either before or after the onset of epilepsy. A flowchart of the cohorts and participation is given in Figure 1.
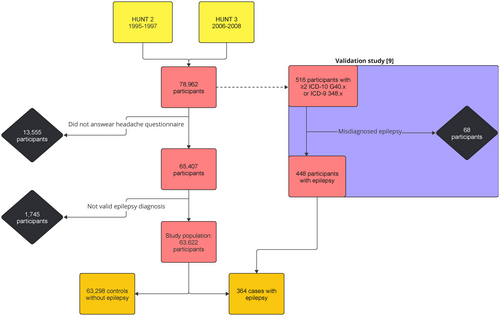
Epilepsy diagnoses
The validation of epilepsy diagnoses was carried out in 2021–2022 and was described in detail previously [9]. In Norway, epilepsy is diagnosed exclusively as part of follow-up care in hospital-based specialized health services. Briefly, individuals who had epilepsy diagnosis codes (ICD-10G40.x or ICD-9348.x) at two or more separate neurology or paediatric outpatient appointments during 1987–2019 were identified. For these patients, clinical information from medical records was reviewed to validate and classify seizure disorders according to the current definition of epilepsy and the revised classification of epilepsies by the International League Against Epilepsy [10, 11]. Hospital records dating back to at least 1987 were available for all participants selected for validation. The ascertainment procedure was supervised by an experienced clinical epileptologist (E.B.). A diagnosis of epilepsy was confirmed in 448 of 516 participants, of whom 364 had answered the headache questions and were included in the current study. Active epilepsy was defined as seizures within the past 5 years [12]. Epilepsy resolved was defined as seizure freedom for >10 years with no antiseizure medications for >5 years [10].
Migraine diagnoses
Headache diagnoses were ascertained using a validated questionnaire tool based on the International Classification of Headache Disorders II [13]. Individuals answering “Yes” to the question “Have you suffered from headache during the past 12 months” were classified as active headache sufferers. Those who answered “No” were classified as being headache-free. Based on the subsequent headache questions, migraine was defined in HUNT2 by the presence of the following three criteria: (i) headache attacks lasting 4–72 h (<4 h was accepted for those who reported commonly occurring visual disturbances before the headache); (ii) headache with at least one of the following characteristics: pulsating quality, unilateral location, or aggravation by physical activity; and (iii) during headache, at least one of nausea or photophobia and phonophobia. In addition, participants who self-reported suffering from migraine were included in the migraine group. Headache sufferers who did not meet the criteria for migraine were classified as having nonmigraine headache, and the diagnoses were mutually exclusive. The algorithm used in HUNT3 was similar, but for (i) a duration of ≤72 h was accepted; for (ii) two of the following four criteria were required: pulsating quality, unilateral location, moderate or severe pain intensity, or aggravation by physical activity; and for (iii) nausea was exchanged for nausea and/or vomiting. For individuals who had participated in both HUNT2 and HUNT3, demographic data and headache diagnoses from HUNT2 were used. These migraine diagnoses have been validated against clinical interviews by neurologists. In HUNT2, the sensitivity was 69% and the specificity 89% (κ = 0.59, 95% confidence interval [CI] = 0.47–0.71) [14]. In HUNT3, the sensitivity and specificity were 67% and 94%, respectively (κ = 0.58, 95% CI = 0.42–0.74) [15].
Statistical analyses
Stata software package, version 17.0 (StataCorp, 2021, Stata/SE 17.0 for Windows) was used for data analyses. The association between headache type and epilepsy status was analysed using binary logistic regression, adjusting for sex and age. Results were expressed as odds ratios (ORs) with 95% CIs for the respective headache type (migraine or nonmigraine headache) compared to headache-free controls. To examine the relationship between epilepsy and headache frequency, the analysis was repeated separately for subjects with low and high headache frequency (0–6 and ≥7 headache days per month, respectively). For the association between epilepsy and headache status, we also performed sensitivity analyses using two additional epilepsy definitions with decreasing stringency, first defining epilepsy as (i) validated epilepsy or any hospital contact with a recorded diagnosis of epilepsy (ICD-10G40.x or ICD-9348.x; n = 1593) and (ii) as for (i) or self-reported epilepsy in either HUNT2 or HUNT3 (n = 2109). Among participants with epilepsy, the association between epilepsy characteristics and headache type (no headache, migraine, or nonmigraine headache) was analysed using (i) chi-squared test for categorical variables, reporting adjusted residuals for significant differences; and (ii) the Kruskal–Wallis test for continuous variables. A p-value of <0.05 was considered significant.
Ethics
HUNT was approved by the Norwegian Data Protection Authority and the Regional Committee for Medical Research Ethics (REK #2018/1623 Helse Midt), and participation was based on informed consent.
RESULTS
The clinical characteristics of participants are presented in Table 1. Of the 63,662 individuals in the study population, 364 had a validated epilepsy diagnosis, corresponding to a prevalence of 0.57%.
| Characteristic | No epilepsy | Epilepsy |
|---|---|---|
| n | 63,298 | 364 |
| Discrete variables, n (%) | ||
| Sex, female | 34,400 (54.3) | 168 (46.2) |
| No headache | 38,537 (60.9) | 210 (57.7) |
| Migraine | 8669 (13.7) | 46 (12.6) |
| Nonmigraine headache | 16,092 (25.4) | 108 (29.7) |
| Headache frequency | ||
| 0–6 days/month | 18,541 (29.3) | 105 (28.8) |
| ≥7 days/month | 4802 (7.6) | 41 (11.3) |
| Continuous variables, mean (SD) | ||
| Age, years | 49.0 (17.1) | 44.9 (15.4) |
A total of 8715 participants (13.7%) had migraine, 16,200 (25.4%) had nonmigraine headache, and 38,747 were headache-free.
There was no association between epilepsy and migraine (OR = 0.95, 95% CI = 0.68–1.33, p = 0.78) or between epilepsy and nonmigraine headache (OR = 1.18, 95% CI = 0.93–1.50, p = 0.17) when adjusting for age and sex. Similar results were found when stratifying by focal and generalized epilepsy (Table 2).
| No headache, n | Migraine | Nonmigraine headache | |||
|---|---|---|---|---|---|
| n | OR (95% CI) p | n | OR (95% CI) p | ||
| No epilepsy | 38,537 | 8669 | 1.0 | 16,092 | 1.0 |
| All epilepsy | 210 | 46 | 0.95 (0.68–1.33) p = 0.78 | 108 | 1.18 (0.93–1.50) p = 0.17 |
| Focal epilepsya | 168 | 34 | 0.93 (0.63–1.36) p = 0.70 | 79 | 1.11 (0.85–1.46) p = 0.44 |
| Generalized epilepsya | 21 | 8 | 1.03 (0.44–2.42) p = 0.94 | 15 | 1.29 (0.65–2.53) p = 0.47 |
- Abbreviations: CI, confidence interval; OR, odds ratio.
- a Those with combined focal and generalized or unknown type of epilepsy were excluded from the analysis due to low sample sizes. Analyses were adjusted for sex and age.
To investigate how the inclusion of patients with less certain epilepsy diagnoses affects the observed association, we performed two sensitivity analyses. First, when all participants with one or more registered hospital ICD-10 code for epilepsy were included in the epilepsy group (resulting in 1593 epilepsy cases and 63,298 controls without epilepsy), no association was observed with migraine (OR = 1.11, 95% CI = 0.95–1.30, p = 0.19) or nonmigraine headache (OR = 1.08, 95% CI = 0.96–1.22, p = 0.20). Second, when participants with self-reported epilepsy were also included (resulting in 2109 epilepsy cases and 63,298 controls without epilepsy), there was a significant association with migraine (OR = 1.23, 95% CI = 1.08–1.40, p = 0.002) but not with nonmigraine headache (OR = 1.10, 95% CI = 0.99–1.23, p = 0.067).
Among patients with epilepsy, there was no association between headache status and specific epilepsy characteristics. Age at onset of epilepsy, seizure control, and the proportion of epilepsy types were similar in those who had migraine or nonmigraine headache, or who were headache free (Table 3).
| Characteristic | n | No headache | Migraine | Nonmigraine headache | p |
|---|---|---|---|---|---|
| Total, n (%) | 364 | 210 (57.7) | 46 (12.6) | 108 (29.7) | |
| Age, years, mean (SD) | 46.3 (16.4) | 40.4 (12.3) | 44.1 (14.4) | 0.104 | |
| Median epilepsy onset age, years, mean (SD)a | 35.8 (24.4) | 33.2 (21.5) | 30.1 (23.9) | 0.132 | |
| Distribution of epilepsy onset age, n (%) | |||||
| <18 years | 125 | 66 (52.8) | 14 (11.2) | 45 (36.0) | 0.36 |
| 18–39 years | 76 | 45 (59.2) | 10 (13.2) | 21 (27.6) | |
| 40–59 years | 68 | 39 (57.4) | 12 (17.6) | 17 (25.0) | |
| ≥60 years | 60 | 40 (66.7) | 5 (8.3) | 15 (25.0) | |
| Sex, n (%) [AR] | |||||
| Women | 168 | 81 (48.2) [−3.4] | 26 (15.5) | 61 (36.3) [2.6] | 0.003 |
| Epilepsy type, n (%) | |||||
| Focal | 281 | 168 (59.8) | 34 (12.1) | 79 (28.1) | 0.54 |
| Generalized | 44 | 21 (47.7) | 8 (18.2) | 15 (34.1) | |
| Combined | 3 | 1 (33.3) | 1 (33.3) | 1 (33.3) | |
| Unknown | 36 | 20 (55.6) | 3 (8.3) | 13 (36.1) | |
| Seizure control at last follow-up, n (%) | |||||
| Seizures past 5 years | 154 | 91 (59.1) | 16 (10.4) | 47 (30.5) | 0.81 |
| Seizures past year | 79 | 47 (59.5) | 6 (7.6) | 26 (32.9) | |
| Seizure-free >5 years | 168 | 96 (57.1) | 25 (14.9) | 47 (28.0) | |
| Epilepsy resolved | 59 | 31 (52.5) | 11 (18.6) | 17 (28.8) | |
| Unknown | 42 | 23 (54.8) | 5 (11.9) | 14 (33.3) | |
- Note: Each characteristic was compared between the headache groups using the chi-squared test for categorical variables and the Kruskal–Wallis test for continuous variables.
- Abbreviation: AR, adjusted residual.
- a Onset age was available in 329 of 364 patients: 190 of 210 for no headache; 98 of 108 for nonmigraine headache, and 41 of 46 for migraine.
When stratifying the analysis by headache frequency, epilepsy was associated with a higher prevalence of migraine with high headache frequency, whereas no association was found for migraine with low headache frequency or with nonmigraine headache (Table 4).
| No headache, n | All headache | Migraine | Nonmigraine headache | ||||
|---|---|---|---|---|---|---|---|
| n | OR (95% CI) p | n | OR (95% CI) p | n | OR (95% CI) p | ||
| 0–6 headache days/month | |||||||
| No epilepsy | 38,537 | 18,541 | 1.0 | 6177 | 1.0 | 12,364 | 1.0 |
| Epilepsy | 210 | 105 | 0.98 (0.77–1.25) p = 0.87 | 26 | 0.74 (0.48–1.12) p = 0.15 | 79 | 1.09 (0.84–1.42) p = 0.52 |
| ≥7 headache days/month | |||||||
| No epilepsy | 38,537 | 4802 | 1.0 | 2100 | 1.0 | 2702 | 1.0 |
| Epilepsy | 210 | 41 | 1.58 (1.22–2.22) p = 0.009 | 20 | 1.73 (1.08–2.78) p = 0.024 | 21 | 1.45 (0.92–2.28) p = 0.11 |
- Note: Analyses were adjusted for sex and age. The p-value in bold is the value that is statistically significant.
- Abbreviations: CI, confidence interval; OR, odds ratio.
DISCUSSION
We examined the association between epilepsy and migraine in a large sample of the adult general population. Using validated diagnoses and adjusting for age and sex, we found no association between epilepsy and migraine. This finding contrasts with recent meta-analyses, which concluded that there is a substantially increased prevalence of migraine in patients with epilepsy and vice versa [1-3].
To date, more than 30 studies have explored the comorbidity between epilepsy and migraine [1-4], with most studies having focus on the prevalence of migraine in hospital-based epilepsy cohorts [2-4]. Although the hospital setting helps ensure accurate epilepsy diagnosis, it is well documented that clinic-based studies may overestimate the prevalence of comorbidities, known as Berkson's bias [16]. This may be the case, for example, if patients with more comorbidities, including migraine, are more likely to be referred to hospital or receive follow-up than patients with fewer comorbidities. Estimation of comorbidity is therefore best done in population samples.
A 2015 meta-analysis of 10 population-based studies on the association between epilepsy and migraine [1] concluded with a 52% higher prevalence of migraine in people with than in people without epilepsy (prevalence odds ratio [PR] = 1.52, 95% CI = 1.29–1.79) and a 79% higher prevalence of epilepsy in people with than without migraine (PR = 1.79, 95% CI = 1.43–2.25). We were unable to identify population-based studies published after this study. It is noteworthy that in six of the 10 studies included in the meta-analysis, epilepsy was diagnosed solely based on self-report, using a single questionnaire item, and two further studies relied on nonvalidated diagnostic codes. When the same meta-analysis performed a sensitivity analysis and included solely studies in which the diagnoses were determined by a physician's assessment, no association was found (PR = 0.93, 95% CI = 0.61–1.41) [1]. This was mirrored in our study, where we only found a positive association in the second sensitivity analysis, when we included participants who self-reported current or previous epilepsy but had no epilepsy diagnosis in hospital records from 1987 to 2019.
Unfortunately, we do not have information on the validity of self-reported epilepsy in HUNT. This group may include persons who were once suspected of having epilepsy but then received other diagnoses, such as functional seizures and transient global amnesia, both of which have a high rate of comorbid migraine [17, 18]. Diagnostic misunderstandings may also have occurred in persons with migraine treated with antiseizure medications or undergoing electroencephalographic examinations. This group may also include patients with epilepsy that resolved before 1987, including children with self-limited focal epilepsies of childhood, which have been associated with an increased rate of migraine [19]. Finally, it is possible that some were diagnosed and followed up completely outside of specialized health care, but we believe this to be a small group.
We hypothesize that the overall comorbidity of epilepsy and migraine reported in previous studies may in large part be due to methodological flaws, in particular selection bias in clinic-based studies and the use of unvalidated diagnostic instruments in population-based studies. This does not alter the finding that epilepsy and migraine are linked in certain genetic conditions and that research into these disorders has provided important insights into overlapping pathophysiological mechanisms. These disorders may be underrepresented in population-based studies because of their disease burden. For example, variants in the genes CACNA1A, ATP1A2, SCN1A, and PRRT2, which are involved in ion channel function, are known to increase the risk of both epilepsy and hemiplegic migraine [20]. However, these rare disorders are unlikely to drive an overall comorbidity between epilepsy and migraine.
Four previous small population-based studies found no association between epilepsy and migraine, in line with our results. First, the comorbidity of migraine and epilepsy was investigated in a door-to-door survey of nearly all residents in the Norwegian municipality of Vågå (1656 participants) [21], which found a similar prevalence of epilepsy in people with (3.6%) and without (3.9%) migraine. Second, a long-term follow-up study of a population-based cohort of 220 patients with childhood epilepsy in Finland matched against 99 controls randomly selected from the general population found no association between epilepsy and migraine [22]. Third, an Icelandic study of 324 epilepsy cases identified in a national surveillance program and matched with 647 randomly selected controls from the general population found an association between epilepsy and migraine with aura, but no association for migraine overall, that is, when including migraine without aura [1, 23]. In all three studies, the diagnosis of migraine and epilepsy was made by a physician. Finally, in a large cohort study of 276,921 patients seen by Dutch general practitioners, no significant association between epilepsy and migraine was found (OR = 1.41, 95% CI = 0.73–2.72) [24]. It should be noted that the diagnoses were based on unvalidated International Classification of Primary Care codes, and the migraine rate was substantially lower (1%) than the expected population prevalence (14%) [25], which calls into question the representativeness of the sample.
Although we found no overall increased prevalence of migraine in people with epilepsy, we observed an increased prevalence of high-frequency migraine that was offset by a decrease in low-frequency migraine. This may suggest that migraine tends to be more severe in people who also suffer from epilepsy. Several possible mechanisms can be hypothesized. A comorbid disorder such as epilepsy may increase allostatic load, leading to higher migraine attack frequency [26]. In addition, several antiseizure medications may have headache as a common side effect and could potentially exacerbate pre-existing migraine [27]. These aspects could not be investigated further in our study as no detailed data on the use of individual antiseizure medications were available. Furthermore, the classification into high- and low-frequency headache groups could lead to information bias, as epilepsy patients are more likely to use health services and may have a greater awareness of neurological symptoms, which could lead to an overrepresentation of migraine symptoms in these patients. Further research should aim to shed light on the relationship between high-frequency migraine and epilepsy.
The strengths of our study include the large and unselected population sample combined with the use of validated diagnoses for both epilepsy and migraine. In particular, a careful review of medical records was performed to exclude cases of misdiagnosed or nondocumented epilepsy, including acute symptomatic seizures, functional seizures, convulsive syncope, and hyperventilation syndrome, as well as various unclassified paroxysmal events, resulting in a rigorously validated cohort of patients with confirmed epilepsy.
There are several limitations that should be considered. First, because participation in HUNT required the ability to complete a questionnaire, individuals with cognitive deficits, such as those with developmental disability, are likely to be underrepresented. Also, participants with epilepsies that resolved before 1987 may have been missed. However, the prevalence of 0.57% validated epilepsy among HUNT2 and HUNT3 participants is comparable to the expected population prevalence [9, 28], suggesting that individuals with epilepsy are not substantially underrepresented overall. Second, the HUNT questionnaire was designed primarily to diagnose migraine, and we could not differentiate between migraine subtypes, such as with or without aura, or study other headache disorders. In contrast to the epilepsy diagnoses, which were individually reviewed and validated, the migraine diagnoses were based on a diagnostic tool that has been validated and used in several previous studies. The validation studies show some misclassification between the headache groups, which may contribute to attenuating the observed comorbidity with epilepsy. Third, treatment with antiseizure medications might bidirectionally confound the comorbidity. In epilepsy, these drugs can alleviate a coexisting migraine, whereas when used for migraine, they can suppress the occurrence of seizures. Both effects may attenuate the observed comorbidity between the two disorders. Lastly, as we did not have information on the age at onset of migraine, we were unable to investigate the temporal relationship between the two diagnoses.
In conclusion, we found no association between epilepsy and migraine in this large population-based study. This is in contrast with most previous studies, which may have been limited by recruitment bias or the use of nonvalid diagnoses. Our results are consistent with previous, smaller, population-based studies that used validated diagnoses, and suggest that the association between epilepsy and migraine might be weaker than previously suggested [1-4]. Patients with migraine who also have epilepsy tend to have more frequent migraine. The reasons for this should be the subject of future research.
AUTHOR CONTRIBUTIONS
Helene Engstrand: Investigation; writing – original draft; methodology; writing – review and editing; visualization; formal analysis; validation; project administration; data curation; conceptualization. Eline Revdal: Investigation; writing – review and editing; data curation; formal analysis; conceptualization. Maria Bengtson Argren: Writing – review and editing; conceptualization. Knut Hagen: Resources; writing – review and editing; conceptualization. John-Anker Zwart: Funding acquisition; writing – review and editing; supervision; conceptualization. Eylert Brodtkorb: Writing – review and editing; conceptualization. Bendik Slagsvold Winsvold: Conceptualization; methodology; funding acquisition; data curation; formal analysis; investigation; writing – review and editing; writing – original draft; project administration; supervision; validation.
ACKNOWLEDGEMENTS
The Trøndelag Health Study (HUNT) is a collaboration between the HUNT Research Centre (Faculty of Medicine and Health Sciences, Norwegian University of Science and Technology), Trøndelag County Council, Central Norway Regional Health Authority, and the Norwegian Institute of Public Health.
FUNDING INFORMATION
This work was funded by the Norwegian Research Council (#328615).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




