HAPLN3 p.T34A contributes to incomplete penetrance of moyamoya disease in Chinese carrying RNF213 p.R4810K
Jun Xu, Zhengxing Zou, Wanyang Liu and Qian Zhang contributed equally to the manuscript.
Abstract
Background and purpose
The penetrance of the RNF213 p.R4810K, a founder mutation of moyamoya disease (MMD), is estimated to be only 1/150–1/300. However, the factors affecting its penetrance remain unclear. Therefore, our study aims to identify modifier genes associated with the incomplete penetrance of this founder mutation.
Methods
Whole-exome sequencing (WES) was performed on 36 participants, including 22 MMD patients and 14 non-MMD controls with RNF213 p.R4810K mutation. Fisher's exact test was used to assess the presence of genetic variants that differed significantly between MMD patients and non-MMD controls. In order to exclude false-positive results, another 55 carriers were included to perform Fisher's exact test for the selected sites in the WES discovery stage. Subsequently, human brain microvascular endothelial cells were transfected with wild-type and mutant HAPLN3 for tube formation assays and western blotting to explore the impact of candidate genes on angiogenesis.
Results
Analysis of variants from WES data revealed a total of 12 non-synonymous variants. Through bioinformatics analysis, the focus was on the HAPLN3 p.T34A variant with a significant p value of 0.00731 in Fisher's exact test. Validation through Sanger sequencing confirmed the presence of this variant in the WES data. In vitro experiments revealed that silencing HAPLN3 and transfecting HAPLN3 p.T34A significantly enhanced tube formation and increased the relative protein expression of vascular endothelial growth factor in endothelial cells.
Conclusions
These results suggest that HAPLN3 may function as a modifier gene of RNF213 p.R4810K, promoting the development of MMD and contributing to the incomplete penetrance of MMD founder mutations.
INTRODUCTION
Moyamoya disease (MMD, MIM 607151) is a rare cerebrovascular disorder with an unknown aetiology and pathogenesis. It is characterized by progressive stenosis or occlusion of the intracranial internal carotid arteries and their branches, leading to the formation of moyamoya compensatory vessels [1]. MMD significantly increases the risk of stroke and is associated with high mortality and disability rates [2]. However, effective treatments for this disease are currently lacking. Epidemiological studies indicate regional heterogeneity amongst MMD patients, with a particularly high prevalence observed in East Asian populations, mainly in Japan, South Korea and China [3]. In 2011, genome-wide association studies and linkage analyses identified a strong association between the founder variant RING finger protein 213 (RNF213, NM_001256071.3:c.14429G>A; NP_001243000.2:p.R4810K) and MMD in East Asian populations, with an extremely high odds ratio [4, 5]. Subsequent studies investigating RNF213 p.R4810K and its relationship with MMD have also confirmed the significance of this founder variant in the occurrence and progression of MMD [6-8]. The carrier rate of RNF213 p.R4810K amongst Chinese MMD patients is reported to be 23.1%, significantly higher than the 0.9% observed in the general population [9]. However, the penetrance of this variant is estimated to be approximately 1/150–1/300, suggesting that a considerable number of carriers are not affected [10].
The penetrance of a disease genotype refers to the proportion of individuals with a specific genotype who present expected clinical signs of the associated disease. When this proportion reaches 100%, the disease genotype is considered to have complete penetrance; otherwise, it is classified as having incomplete penetrance [11]. Incomplete penetrance is common in diseases and can result from genetic modification, epigenetic, environmental factors etc. [12]. A previous study indicated that familial MMD follows an autosomal dominant mode of inheritance with incomplete penetrance, suggesting that age and genomic imprinting may partially influence penetrance [13, 14]. Additionally, inflammation has been implicated in the transcription of RNF213, providing another plausible explanation for this low penetrance [15, 16]. Some researchers propose that a secondary genetic event is necessary for the development of MMD [17, 18]. Elevated levels of thyroid peroxidase antibody and the presence of a filamin A variant could impact the progression of MMD in RNF213 p.R4810K carriers [19, 20]. Nevertheless, the underlying factors contributing to the incomplete penetrance of RNF213 p.R4810K and its relevance in sporadic cases remain inadequately explored.
Hence, the existence of additional modifier genes that could influence the penetrance of RNF213 p.R4810K was hypothesized. In this study, whole-exome sequencing (WES) and Fisher's exact test were used to identify candidate genes in individuals with the RNF213 p.R4810K mutation. Furthermore, the effect of modifier genes on MMD angiogenesis was explored using in vitro experiments.
METHODS
Study population
In the first stage of the study, 22 MMD patients and 14 non-MMD controls with RNF213 p.R4810K were included. In the WES validation stage, an additional 18 MMD patients and 37 non-MMD controls with RNF213 p.R4810K were included. All patients were recruited from the Department of Neurosurgery at the Chinese PLA General Hospital. The 14 controls, not related to MMD patients, were selected from the physical examination population, whilst the other 37 controls were sourced from the China Metabolic Analytics Project (ChinaMAP). MMD diagnosis was based on the criteria of the Research Committee on the Pathology and Treatment of Spontaneous Occlusion of the Circle of Willis, Health Labor Sciences Research Grant for Research on Measures for Intractable Diseases, Japan [21]. Patients with additional evidence of arteriosclerosis, autoimmune disease, brain neoplasms, meningitis, Down syndrome or other specific aetiologies were excluded.
Peripheral blood samples were collected from all enrolled individuals, and the DNA extraction and single nucleotide polymorphism (SNP) genotyping methods used were in accordance with those described in our previous studies [22].
Whole-exome sequencing (WES) and analysis
Whole-exome sequencing was performed in 36 participants with RNF213 p.R4810K in the WES discovery stage. DNA samples were randomly fragmented using the ultrasonic high-performance sample processing system (Covaris). All exons were captured by hybridization using the SureSelectXT Reagent Kit (Beckman Coulter, USA), library and SureSelectXT Human All Exon Kit V6 probe (Agilent Technologies, CA, USA). The quality of the library was assessed using Qubit and the Agilent 2100 Bioanalyzer (Agilent Technologies), followed by 150 bp double-ended sequencing using the Illumina HiSeq platform (Illumina, CA, USA). Sequence reads were aligned to the human reference genome GRCh37/hg19 using BWA (Burrows-Wheeler Alignment Tool, version 0.7.12). Variant detection was performed with GATK Haplotype Caller, and single-nucleotide variants were filtered using GATK Variant Filtration. SNPs and insertions/deletions (InDels) were annotated using the ANNOVAR software (version 2016Feb01).
Modifying variants identification
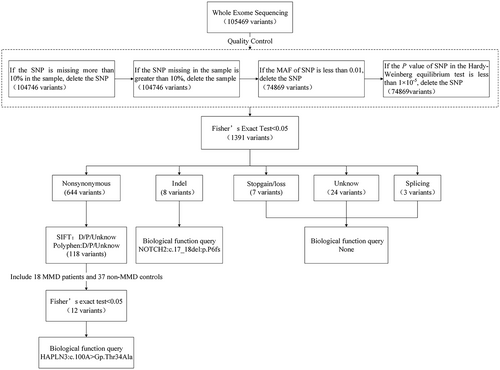
In the WES discovery stage, candidate variants were identified through SNP quality control and Fisher's exact test. Quality control includes four standards: (1) retain a SNP if its missing rate is less than 10% in the samples; (2) retain the sample if the missing rate of SNPs in the sample is less than 10%; (3) the minor allele frequency of SNP is greater than 0.01; (4) the p value of SNP in the Hardy–Weinberg equilibrium test is greater than 1 × 10−5. The main focus was on five types of variants, including non-synonymous, InDel, stopgain/loss, unknown and splicing. Modifying variants were predicted using both the Sorts Intolerant From Tolerant (SIFT) program (http://sift.bii.a-star.edu.sg) and the Polymorphism Phenotyping v2 (PolyPhen-2) program (http://genetics.bwh.harvard.edu/pph2/dokuwiki/start). Sites with SIFT prediction results of ‘D’ (deleterious, score ≤ 0.05) and ‘unknown’ and PolyPhen-2 prediction results of ‘D’ (probability damage, score ≥ 0.957), ‘P’ (possibly damage, 0.453 ≤ score ≤ 0.956) and ‘unknown’ were retained. To exclude false-positive results at these sites, an additional 18 MMD patients and 37 non-MMD controls carrying RNF213 p.R4810K were further analysed in the validation stage, and variants with p values less than 0.05 were queried for their biological function including angiogenesis, immunity and inflammation.
Sanger sequencing
To confirm the variants identified using WES, Sanger sequencing was performed for the selected objects. The primers were designed according to the NCBI database (http://www.ncbi.nlm.nih.gov/); the forward HAPLN3 primer sequence was 5′-GCTCCCCTACCATGGCTATC-3′ and the reverse HAPLN3 primer sequence was 5′- CAGATGAGGAAATGGAGGCAC-3′. After polymerase chain reaction, sequencing was performed using a BigDye Terminator v1.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA, USA) on an ABI GeneAmp 3730xl DNA Sequencer (Applied Biosystems).
Tube formation assay
Human brain microvascular endothelial cells (HBMECs) were purchased from Qingqi (Shanghai) Biotechnology Co. Ltd (http://www.bfbservice.com). HBMECs were inoculated on 96-well plates coated with 50 μL of Matrigel (Sangon, Shanghai, China) at a density of 3 × 105 cells per well and then incubated and photographed for 6 h. Tubes in three randomly selected fields were photographed under 100× magnification (Leica DMi1, Wetzlar, Germany) and analysed using ImageJ software (version 1.8.0, National Institutes of Health, Bethesda, MD, USA).
Western blot
Western blot was used to verify the success of HAPLN3 silencing, overexpression and mutant plasmid transfection, and to detect changes in the protein expression of vascular endothelial growth factor (VEGF). Total proteins were extracted using RIPA lysis buffer (Sangon, Shanghai, China), separated by 12% sodium dodecyl sulfate polyacrylamide gel electrophoresis (Servicebio, Wuhan, China), and the electrophoresed protein lysates were transferred onto polyvinylidene difluoride membranes (GVS, Sanford, FL, USA). Membranes were blocked with 5% skim milk in Tris-buffered saline with 1% Tween 20, and then incubated with primary antibodies overnight at 4°C and with corresponding secondary antibodies (1:5000; Sangon, China). The primary antibodies used were as follows: rabbit anti-human HAPLN3 (1:1000; Absin, China), rabbit anti-human VEGF (1:1000; Absin), rabbit anti-human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (1:3000; Sangon). The protein bands were visualized using the enhanced chemiluminescence reagent kit (WP20005, ThermoFisher, USA) and analysed using ImageJ software.
Bioinformatics and statistical analysis
The STRING database (https://string-db.org/) was used to generate a protein–protein interaction (PPI) network. The protein structure and its mutational site were visualized using the SWISS-MODEL database (https://swissmodel.expasy.org/) and PyMol software (version 2.5, Schrödinger LLC). Quality control and comparison of case–control differences of the variants were analysed using Plink software (version 3; Purcell et al. [23]) and R software 4.3.1 (R Foundation for Statistical Computing, Vienna, Austria). Fisher's exact test was performed to compare the differences between groups. For in vitro cell experiments, one-way analysis of variance (ANOVA) was performed, followed by least significant difference for multiple comparisons (GraphPad 5.02, GraphPad Software Inc., San Diego, CA, USA). A p value <0.05 was considered to indicate a statistically significant difference.
Standard protocol approvals, registrations and patient consents
The ethical issues involved in this study were examined and approved by the Ethics Committee of China Medical University and the Chinese PLA General Hospital. All patients and immediate family members signed written informed consent forms.
RESULTS
Clinical description of study population and sequence analysis
The study subjects in the discovery stage consisted of eight moyamoya patients with the RNF213 p.R4810K AA homozygous mutation and 14 with the GA heterozygous mutations, as well as 14 non-MMD controls with GA heterozygous mutations. The female-to-male ratio was 1.40 (21:15), and the mean age at the onset of symptoms for the patients was 7.41 ± 6.48 years. Amongst the 18 patients in the validation stage, 72.2% were female, with an average age of onset of 18.5 years. The mean baseline age of ChinaMAP controls was 54.02 years, and 64.8% were women. According to the data presented in Table 1, there is no statistically significant difference in phenotype between HAPLN3 p.T34A mutation carriers and non-carriers (p > 0.05). Moreover, the initial symptom of most MMD patients is transient ischaemic attacks. The basic demographic characteristics are shown in Table 1. The percentage of reads correctly aligned to the reference genome (GRCh37/hg19) in this WES ranged from 94.85% to 98.82%, with a total effective yield of 10.46–18.59 GB, of which 5.31–11.35 GB could be aligned to the target region. The average sequencing depth of the target region was 154.37–329.99X, and the coverage of the target region was 98.61%–98.93%. Following quality control, the percentage of bases with quality greater than Q20 in clean reads was 95.72%–97.19%, the range of the Q30 was 88.84%–92.46%, and the average content of GC bases was 48.4%–52.58% (Table S1).
| Sample ID | RNF213 p.R4810K (G>A) | HAPLN3 p.T34A (T>C) | Sex | Age at onset (years) | Initial symptoms | Suzuki stage | PCA involvement | Unilateral/bilateral | Native place |
|---|---|---|---|---|---|---|---|---|---|
| Discovery stage | |||||||||
| MMD-1 | AA | CC | M | 3 | TIA | 2 | No | Bilateral | Anhui |
| MMD-2 | AA | TC | M | 5 | TIA | 3 | No | Bilateral | Jiangxi |
| MMD-3 | AA | CC | M | 27 | CI | 6 | No | Bilateral | Shandong |
| MMD-4 | AA | TT | F | 21 | Dizzy | 6 | Yes | Bilateral | Henan |
| MMD-5 | AA | CC | F | 3 | TIA | 4 | Yes | Bilateral | Hebei |
| MMD-6 | AA | TC | F | 10 | CI | 5 | Yes | Bilateral | Henan |
| MMD-7 | AA | CC | M | 6 | TIA | 6 | Yes | Bilateral | Henan |
| MMD-8 | AA | TC | F | 7 | Dizzy | 4 | Yes | Bilateral | Heilongjiang |
| MMD-9 | GA | TT | F | 4 | TIA | 3 | Yes | Bilateral | Henan |
| MMD-10 | GA | CC | M | 3 | TIA | 4 | Yes | Bilateral | Beijing |
| MMD-11 | GA | TT | F | 5 | Dizzy | 3 | No | Bilateral | Hebei |
| MMD-12 | GA | TC | M | 4 | TIA | 4 | Yes | Bilateral | Beijing |
| MMD-13 | GA | TC | F | 8 | TIA | 6 | No | Bilateral | Shandong |
| MMD-14 | GA | TC | F | 1 | TIA | 3 | Yes | Bilateral | Hebei |
| MMD-15 | GA | TT | F | 3 | TIA | 4 | No | Bilateral | Shandong |
| MMD-16 | GA | CC | M | 4 | TIA | 2 | Yes | Bilateral | Heilongjiang |
| MMD-17 | GA | TT | F | 4 | TIA | 4 | Yes | Bilateral | Jiangsu |
| MMD-18 | GA | CC | M | 5 | TIA | 1 | No | Bilateral | Inner Mongolia |
| MMD-19 | GA | TC | F | 6 | TIA | 4 | Yes | Bilateral | Henan |
| MMD-20 | GA | TT | M | 6 | TIA | – | No | Bilateral | Shanxi |
| MMD-21 | GA | CC | F | 10 | TIA | 4 | No | Bilateral | Hebei |
| MMD-22 | GA | TC | F | 18 | TIA | 3 | Yes | Bilateral | Anhui |
| Con-1 | GA | TC | F | N/A | N/A | N/A | N/A | N/A | Liaoning |
| Con-2 | GA | TT | F | N/A | N/A | N/A | N/A | N/A | Liaoning |
| Con-3 | GA | TT | F | N/A | N/A | N/A | N/A | N/A | Liaoning |
| Con-4 | GA | TC | F | N/A | N/A | N/A | N/A | N/A | Liaoning |
| Con-5 | GA | TT | M | N/A | N/A | N/A | N/A | N/A | Liaoning |
| Con-6 | GA | TT | F | N/A | N/A | N/A | N/A | N/A | Liaoning |
| Con-7 | GA | TC | M | N/A | N/A | N/A | N/A | N/A | Liaoning |
| Con-8 | GA | TC | M | N/A | N/A | N/A | N/A | N/A | Liaoning |
| Con-9 | GA | CC | F | N/A | N/A | N/A | N/A | N/A | Liaoning |
| Con-10 | GA | TT | M | N/A | N/A | N/A | N/A | N/A | Liaoning |
| Con-11 | GA | TC | F | N/A | N/A | N/A | N/A | N/A | Jilin |
| Con-12 | GA | TC | F | N/A | N/A | N/A | N/A | N/A | Jilin |
| Con-13 | GA | TT | M | N/A | N/A | N/A | N/A | N/A | Jilin |
| Con-14 | GA | TC | M | N/A | N/A | N/A | N/A | N/A | Jilin |
| Validation stage | |||||||||
| MMD-23 | GA | TC | F | 48 | TIA | 4 | No | Bilateral | Hebei |
| MMD-24 | GA | TC | F | 4 | CI | 5 | Yes | Bilateral | Guangdong |
| MMD-25 | GA | TT | M | 3 | TIA | – | Yes | – | Inner Mongolia |
| MMD-26 | GA | TT | F | 36 | TIA | 5 | Yes | Bilateral | Heilongjiang |
| MMD-27 | GA | TC | F | 6 | TIA | 5 | Yes | Bilateral | Shandong |
| MMD-28 | GA | CC | F | 4 | TIA | 4 | No | Bilateral | Shandong |
| MMD-29 | GA | TC | F | 49 | Dizzy | 5 | Yes | Bilateral | Shandong |
| MMD-30 | GA | CC | F | 31 | Dizzy | 5 | Yes | Bilateral | Henan |
| MMD-31 | GA | TT | M | 40 | TIA | 6 | No | Bilateral | Hebei |
| MMD-32 | GA | CC | M | 43 | TIA | 4 | Yes | Bilateral | Shandong |
| MMD-33 | GA | TC | F | 2 | Epilepsy | 5 | Yes | Bilateral | Xinjiang |
| MMD-34 | GA | CC | F | 5 | Dizzy | 3 | Yes | Bilateral | Henan |
| MMD-35 | GA | CC | M | 6 | TIA | 4 | No | Bilateral | Liaoning |
| MMD-36 | GA | CC | M | 4 | Headache | 3 | No | Bilateral | Jiangsu |
| MMD-37 | GA | TT | F | 5 | Headache | 6 | Yes | Bilateral | Shaanxi |
| MMD-38 | GA | TC | F | 7 | TIA | 4 | No | Bilateral | Hebei |
| MMD-39 | GA | TC | F | 6 | Cerebral ischaemia | 4 | No | Bilateral | Anhui |
| MMD-40 | GA | TC | F | 34 | CI | 6 | Yes | Bilateral | Hebei |
- Abbreviations: CI, cerebral infarction; Con, control; F, female; M, male; MMD, moyamoya disease; N/A, not applicable; PCA, posterior cerebral artery; TIA, transient ischaemic attack; −, missing value.
Variant identification for modifier founder mutation
A detailed screening flowchart of the variants is illustrated in Figure 1. 1391 variants were screened out after Fisher's exact test in the discovery stage (Table S2). Finally, 12 pathogenic or damaging non-synonymous variants and one InDel variant potentially associated with the MMD founder mutation were identified. Eight of the 12 non-synonymous mutations were in CCDC168; linkage disequilibrium analysis of these eight variants showed two haplotypes: CTAGGGTA, with a frequency of 0.546, and TCGTCCGG, with a frequency of 0.425. These variants were closely linked and highly correlated in the block region, meaning that, when an individual carried one of the SNPs, he was likely to carry the others as well (Figure S1). Table 2 describes the distribution in case–control and details of these sites.
| Discovery stage | Validation stage | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | dbSNP ID | Chr | Position | Ref/alt | Amino acid change/mRNA transcript | Polyphen | SIFT | Case_yes | Case_no | Con_yes | Con_no | p value | Case_yes | Case_no | Con_yes | Con_no | p value |
| CCDC168 | rs9518825 | 13 | 103388370 | C/T | p.G4893S NM_013362.4 | – | – | 25 | 19 | 6 | 22 | 0.0037 | 43 | 37 | 31 | 71 | 0.0023 |
| ZNF225 | rs76921846 | 19 | 44635869 | G/C | p.G368R NM_001039752.4 | D | D | 0 | 44 | 4 | 24 | 0.0199 | 0 | 80 | 10 | 92 | 0.0027 |
| CCDC168 | rs7322112 | 13 | 103391634 | C/T | p.V3805I NM_001146197.3 | – | – | 20 | 24 | 22 | 6 | 0.0071 | 38 | 42 | 71 | 31 | 0.0037 |
| CCDC168 | rs1449707 | 13 | 103394003 | G/A | p.T3015I NM_001146197.3 | – | – | 20 | 24 | 22 | 6 | 0.0071 | 38 | 42 | 71 | 31 | 0.0037 |
| CCDC168 | rs9585986 | 13 | 103394613 | T/G | p.T2812P NM_001146197.3 | – | – | 20 | 24 | 22 | 6 | 0.0071 | 38 | 42 | 71 | 31 | 0.0037 |
| CCDC168 | rs6491708 | 13 | 103396716 | C/G | p.E2111Q NM_001146197.3 | – | – | 20 | 24 | 22 | 6 | 0.0071 | 38 | 42 | 71 | 31 | 0.0037 |
| CCDC168 | rs9582626 | 13 | 103397583 | C/G | p.A1822P NM_001146197.3 | – | – | 20 | 24 | 22 | 6 | 0.0071 | 38 | 42 | 71 | 31 | 0.0037 |
| CCDC168 | rs9300759 | 13 | 103397937 | T/G | p.N1704H NM_001146197.3 | – | – | 24 | 20 | 6 | 22 | 0.0071 | 42 | 38 | 31 | 71 | 0.0037 |
| SLC22A10 | rs199812058 | 11 | 63072226 | C/A | p.T488N NM_001146197.3 | D | D | 0 | 44 | 4 | 24 | 0.0199 | 0 | 80 | 9 | 93 | 0.0051 |
| CCDC168 | rs12583104 | 13 | 103400729 | G/A | p.P773L NM_001146197.3 | – | – | 19 | 25 | 22 | 6 | 0.0134 | 39 | 41 | 71 | 31 | 0.0059 |
| HAPLN3 | rs8024779 | 15 | 89436244 | T/C | p.T34A NM_001307952.2 | – | D | 24 | 20 | 9 | 19 | 0.0316 | 46 | 34 | 38 | 64 | 0.0073 |
| OR56B1 | rs7397032 | 11 | 5758062 | T/C | p.C106R NM_001005180.3 | D | D | 37 | 7 | 17 | 11 | 0.0486 | 66 | 14 | 65 | 37 | 0.0075 |
- Abbreviations: alt, alternative allele; Case_yes, the number of Alt in the case; Case_no, the number of Ref in the case; Chr, chromosome; Con_yes, the number of Alt in the control; Con_no, the number of Ref in the control; dbSNP, Single Nucleotide Polymorphism Database; SIFT, Sorts Intolerant From Tolerant; Polyphen, D, probably damaging; Ref, reference allele; SIFT, D, deleterious; −, unknown.
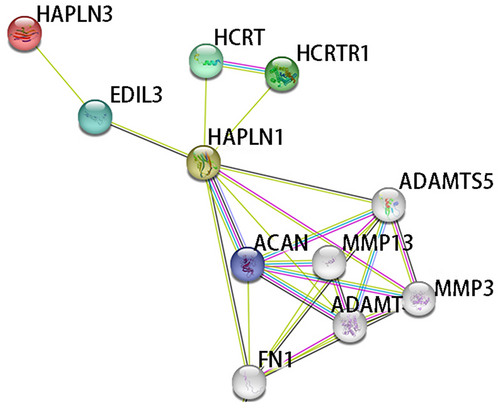
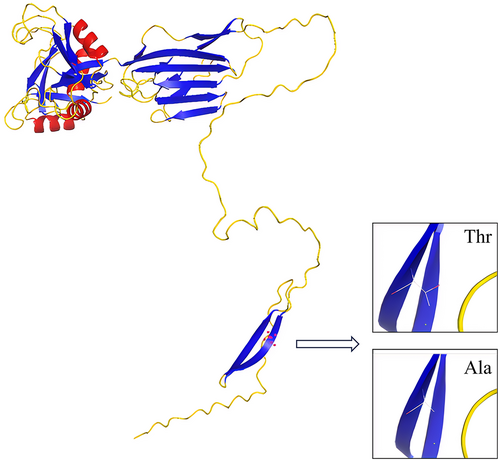
According to the published paper related to MMD, the relevant biological database of candidate genes and PPI analysis, HAPLN3 was determined as the only gene for further study. HAPLN3 belongs to the hyaluronan and proteoglycan binding link protein gene family, which is abundantly expressed in most tissues. The protein encoded by this gene may function in hyaluronic acid (HA) binding and cell adhesion, and it also plays an important role in the construction and stabilization of hyaluronan-dependent extracellular matrix. The three-dimensional structure diagram of the HAPLN3 protein is shown in Figure 2, and the mutation (Thr > Ala) is located in the protein beta-sheet. It can be seen that HAPLN3 is related to its homologous protein family gene HAPLN1 through epidermal growth factor-like repeats and discoidin I-like domains 3 (EDIL3) by the PPI analysis (Figure 3). EDIL3 mediate angiogenesis that may be important in vascular wall remodelling and development. HAPLN1 is also closely related to matrix metalloproteinase (MMP) family proteins. Several studies have shown that MMP plays an important role in the occurrence and development of MMD.
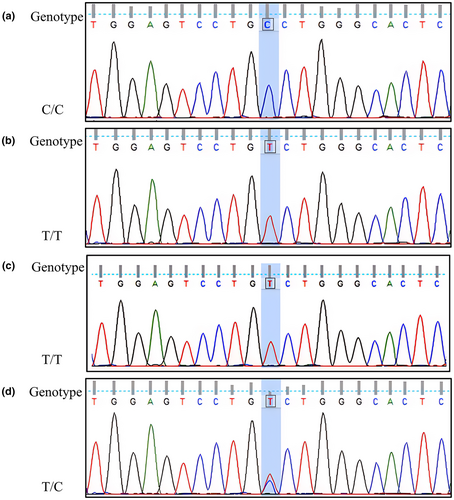
In addition, Sanger sequencing was performed on the study samples to further verify the accuracy of WES at this site located in exon 2. Two cases and two controls with representative results for display were selected. The results of Sanger sequencing were consistent with WES (Figure 4).
Effects of variant-affected HAPLN3 on the angiogenesis of HBMECs
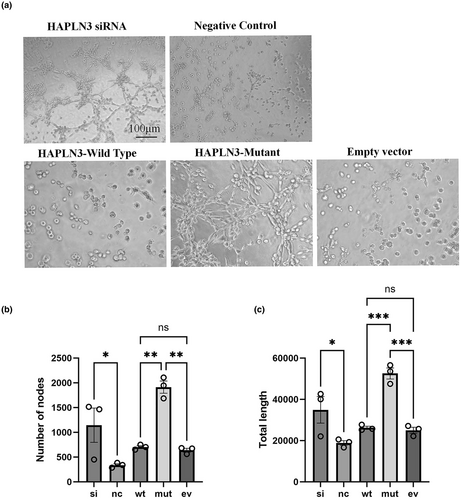
The experimental groups included the HAPLN3 silencing group, the negative control group, the HAPLN3 wild-type plasmid transfection group, the HAPLN3 mutant plasmid transfection group and the empty vector group. The branch nodes and total capillary length in these five groups of tube formation experiments were quantitatively compared. As shown in Figure 5a, silencing of HAPLN3 enhanced the tube formation ability compared with the negative control group. There was no significant difference between the HAPLN3 wild-type plasmid transfection group and the empty vector group, but the tube formation ability of the HAPLN3 mutant plasmid transfection group was increased compared with the empty vector group. Figure 5b,c also shows that the number of branch nodes and total capillary length of the HAPLN3 silencing group were increased and statistically significant compared with the negative control group, and the quantitative indicators of the HAPLN3 mutant plasmid transfection group were also increased relative to the other two groups. These results show that silencing of HAPLN3 and transfection of the mutant variant enhanced the tube-forming ability of endothelial cells, which may be related to angiogenesis during the formation of MMD.
Effects of variant-affected HAPLN3 on VEGF
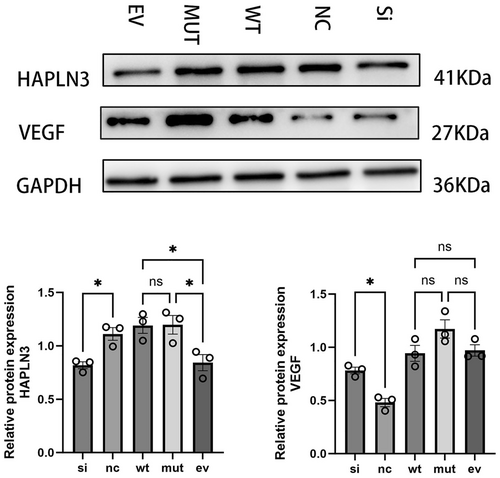
To further confirm the effect of HAPLN3 on angiogenesis, western blot was used to determine the expression level of VEGF in each treatment group. VEGF is a heparin-binding growth factor specific to vascular endothelial cells, which can promote the formation of new blood vessels and the growth of vascular endothelial cells. It is currently the factor with the highest specificity and the strongest activity in promoting angiogenesis. As shown in Figure 6, after silencing HAPLN3, the protein expression levels of HAPLN3 were significantly reduced, whilst the opposite effect was observed with the overexpression of HAPLN3, suggesting successful transfection. Silencing HAPLN3 or transfecting the HAPLN3 mutant plasmid can significantly increase the protein expression of VEGF compared to the negative control group or empty vector group. Furthermore, no significant difference in protein expression was found between the HAPLN3 wild-type plasmid transfection group and the empty vector group. The results above indicate that the loss or change of HAPLN3 may further affect the binding of HA in blood vessels and the stability of the extracellular matrix, thereby influencing the compensatory proliferation of blood vessels, leading to the occurrence of MMD. This is consistent with the result of WES in our previous population, which showed that the frequency of HAPLN3 p.T34A mutation in MMD patients with RNF213 p.R4810K is higher than in non-MMD controls.
DISCUSSION
The RNF213 p.R4810K mutation is a well-known genetic risk factor for MMD [24] and is primarily identified in East Asian populations with MMD, especially in Japan, Korea and China, but is rare in Caucasians and Southeast Asians [25, 26]. However, limited studies have explored the factors contributing to the incomplete penetrance of this locus. In this study, WES analysis on MMD patients and non-MMD controls with RNF213 p.R4810K mutation was conducted, focusing on angiogenesis-related target genes. Through bioinformatics analysis and Sanger sequencing, HAPLN3 p.T34A was identified as the most promising candidate variant associated with MMD. The mutant allele frequency amongst the total cases was 57.50%, significantly higher than the 38.39% observed in the controls, suggesting that this variant may be a detrimental modifier of the RNF213 p.R4810K mutation in MMD. This finding was further supported by in vitro experiments, where the tubulogenic capacity and VEGF protein levels were increased in the HAPLN3 silencing or point mutation group compared to the control group.
Previous studies have indicated that MMD is a polygenic disorder influenced not only by RNF213 but also by other genes like ANO1 (anoctamin 1) [27], DIAPH1 (diaphanous-related formin 1) [28], PHACTR1 (phosphatase and actin regulator 1) [29], CBL (CBL proto-oncogene) [30], and others. In addition to gene interactions in polygenic diseases, there may be additional modifier genes impacting disease manifestation [31]. Hence, it was hypothesized that the incomplete penetrance of the founder mutation in MMD could be related to modifications of other genes. By conducting Fisher's exact test on case–control subjects with RNF213 p.R4810K in the WES discovery phase and subsequently enlarging the sample size in the second stage, a total of 12 target variants with statistical significance in both stages were identified. Finally, the focus was on HAPLN3 p.T34A with a p value of 0.00731.
HAPLN3, located in the chromosomal region 15q26.1, is a member of the hyaluronan and proteoglycan binding link protein gene family and is widely expressed in various tissues. Gene set enrichment analysis results indicated that the mitogen-activated protein kinase, VEGF and Notch signal pathways were significantly enriched in the HAPLN3 high expression phenotype [32]. Studies have also shown that genes associated with MMD are typically enriched in these pathways [33, 34]. The protein encoded by HAPLN3 is involved in HA binding and cell adhesion, and plays an important role in the construction and stabilization of the hyaluronan-dependent extracellular matrix [35]. Structural modelling of HAPLN3 reveals that the HAPLN3 p.Thr34Ala mutation occurs within the β fold, potentially affecting HA binding, consistent with the prediction result of SIFT that is deleterious. HA is a unique polysaccharide with a simple chemical structure but remarkable properties, serving as a significant constituent of the extracellular matrix associated with processes like angiogenesis, cell motility and inflammation [36, 37]. In vessels, HA, along with other extracellular matrix molecules, forms a luminal mesh known as the endothelial glycocalyx, crucial for regulating endothelial functions and maintaining vascular integrity [38, 39]. Moreover, HA not only acts as a biomarker for identifying brain disorders such as atherosclerosis, stroke and vascular dementia [40-42], but also plays an important role in preserving the blood–brain barrier (BBB). Disruption of the BBB integrity can lead to barrier permeability damage [43, 44], reinforcing the progression of MMD [45, 46]. In vitro studies have suggested that HA can serve as a bioactive molecule, influencing functional vascular smooth muscle regeneration and endothelial cell proliferation through interactions with CD44 and the receptor for HA-mediated motility [47, 48]. A recent study has revealed that MMD patients carrying the RNF213 p.R4810K mutation exhibit reduced levels of HA in the matrix surrounding endothelial cells. This reduction makes the endothelium susceptible to shear stress, prompting endothelial progenitor cells to infiltrate the vascular intima and generate HA, eventually leading to compensatory HA accumulation and the development of pathological changes of intimal thickening and vascular stenosis [49].
The PPI analysis reveals a connection between HAPLN3 and its homologous gene, HAPLN1, mediated by EDIL3. EDIL3 encodes a protein that functions as an integrin ligand, implicated in angiogenesis in pathological conditions and potentially targeted for the treatment of pathological angiogenesis [50, 51]. Additionally, the involvement of HAPLN1 influences the proliferation, migration and phenotypic transition of vascular smooth muscle cells [52]. Importantly, the study indicates a significant association between HAPLN1 and members of the MMP family. A previous study has highlighted the pivotal role of MMP family proteins including MMP2, MMP3 and MMP9 in the development of MMD, with distinct expression patterns in the plasma or serum of MMD patients compared to healthy controls [53-55]. Consequently, HAPLN3 was identified, closely related to HA binding, as the target modifier gene in this study.
Three in vitro models were established through transfection to induce silencing, overexpression and point mutation, with successful construction confirmed via western blotting. HBMECs are a crucial component of the BBB and have been confirmed to actively participate in the pathophysiological processes of various neurological diseases [56, 57]. Angiogenesis, a pivotal event in processes like embryonic development, injury repair and tissue regeneration, serves as a crucial physiological mechanism underlying vascular-related diseases [58], and the in vitro angiogenesis model was developed using Matrigel matrix gel to assess the impact of HAPLN3 intervention on HBMEC angiogenesis [59]. Both the silencing of the HAPLN3 group and transfection with the HAPLN3 mutant plasmid exhibited enhanced tube formation ability compared to other groups. Angiogenesis, a multifaceted process intricately linked to endothelial cell activation and proliferation, is primarily orchestrated by VEGF, known as a potent stimulator of angiogenesis. Studies have demonstrated that VEGF promotes angiogenesis in both in vivo and in vitro settings, playing an important role in pathological angiogenesis [60, 61]. Notably, increased VEGF expression levels have been reported in the dural tissue and plasma of MMD patients [62, 63]. This suggests a potential functional impairment in HAPLN3, possibly contributing to the MMD development in conjunction with the RNF213 p.R4810K mutation.
Our study has several limitations, primarily due to the relatively small sample size and limited biological replicates available in the WES discovery stage and in vitro experiments, which may reduce the statistical power of the results. It is proposed that future studies with larger sample sizes replicate our findings to confirm their reliability and generalizability. The possibility of population stratification should also be considered, although our study faces certain challenges due to the low frequency of RNF213 p.R4810K carriers in the control group. Additionally, the RNF213 p.R4810K mutation in this study represents a founder mutation, tracing back to a common ancestor nearly 10,000 years ago, which ensures the homogeneity of the study population [4]. This homogeneity may mitigate the impact of population stratification on our research. Moving forward, principal component analysis and family-based designs will be employed to further minimize the effects of population stratification. The specific mechanism by which HAPLN3 affects the founder mutation of MMD remains unclear and requires further elucidation. Moreover, there is a lack of in vivo experiments and studies to validate the clinical phenotype of RNF213 p.R4810K carriers for HAPLN3.
In summary, our findings suggested that the HAPLN3 p.T34A mutation site significantly increased the risk amongst founder mutation carriers. In vitro experiments on HAPLN3 in endothelial cells showed that HAPLN3 knockout and point mutation intervention promoted angiogenesis and increased relative protein expression of VEGF. Ultimately, the HAPLN3 p.T34A led to the functional loss of HAPLN3, potentially acting as a modifying gene for RNF213 p.R4810K and contributing to the incomplete penetrance of the MMD founder mutation.
AUTHOR CONTRIBUTIONS
Jun Xu: Writing – original draft; software; investigation. Zhengxing Zou: Data curation. Wanyang Liu: Methodology; software. Qian Zhang: Data curation. Juan Shen: Methodology. Fangbin Hao: Data curation. Gan Chen: Methodology. Dan Yu: Data curation. Yunzhu Li: Methodology. Zhengshan Zhang: Data curation. Yuchen Qin: Methodology. Rimiao Yang: Data curation. Yue Wang: Writing – review and editing. Lian Duan: Writing – review and editing.
ACKNOWLEDGEMENTS
All individuals are thanked for their participation in the study and our sincere gratitude is expressed to the ChinaMAP. The comprehensive resources and datasets made available through ChinaMAP have been instrumental in our research. The funders had no role in study design, data collection and analysis, interpretation of data, writing of the report and decision to submit the paper for publication.
FUNDING INFORMATION
This research is supported by the National Natural Science Foundation of China (82173610); China Postdoctoral Science Foundation (2024T171043, 2023MD744266, GZC20233120); Department of Science and Technology of Liaoning Province (2023JH2/20200025, 2023-MSLH-371).
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
With the permission of the corresponding authors and their institutions, combined with relevant documents, all data used for this analysis will be shared, following ethics approval, with other investigators for the reasonable purpose of replicating the procedures and results, if requested.




