Microstructure of the cerebellum and its afferent pathways underpins dystonia in myoclonus dystonia
Emmanuel Roze and Yulia Worbe contributed equally to this work.
Abstract
Background and Purpose
Myoclonus dystonia due to a pathogenic variant in SGCE (MYC/DYT-SGCE) is a rare condition involving a motor phenotype associating myoclonus and dystonia. Dysfunction within the networks relying on the cortex, cerebellum, and basal ganglia was presumed to underpin the clinical manifestations. However, the microarchitectural abnormalities within these structures and related pathways are unknown. Here, we investigated the microarchitectural brain abnormalities related to the motor phenotype in MYC/DYT-SGCE.
Methods
We used neurite orientation dispersion and density imaging, a multicompartment tissue model of diffusion neuroimaging, to compare microarchitectural neurite organization in MYC/DYT-SGCE patients and healthy volunteers (HVs). Neurite density index (NDI), orientation dispersion index (ODI), and isotropic volume fraction (ISOVF) were derived and correlated with the severity of motor symptoms. Fractional anisotropy (FA) and mean diffusivity (MD) derived from the diffusion tensor approach were also analyzed. In addition, we studied the pathways that correlated with motor symptom severity using tractography analysis.
Results
Eighteen MYC/DYT-SGCE patients and 24 HVs were analyzed. MYC/DYT-SGCE patients showed an increase of ODI and a decrease of FA within their motor cerebellum. More severe dystonia was associated with lower ODI and NDI and higher FA within motor cerebellar cortex, as well as with lower NDI and higher ISOVF and MD within the corticopontocerebellar and spinocerebellar pathways. No association was found between myoclonus severity and diffusion parameters.
Conclusions
In MYC/DYT-SGCE, we found microstructural reorganization of the motor cerebellum. Structural change in the cerebellar afferent pathways that relay inputs from the spinal cord and the cerebral cortex were specifically associated with the severity of dystonia.
INTRODUCTION
Myoclonus dystonia due to a pathogenic variant in SGCE (MYC/DYT-SGCE) is a rare disorder, characterized by childhood onset of subcortical myoclonus and dystonia [1]. As a result, the exact pathophysiology of different motor symptoms of MYC/DYT-SGCE remains poorly understood [2].
As in other dystonic syndromes, the dystonia and myoclonus in MYC/DYT-SGCE have been associated with dysfunctions involving primarily the cerebellum [2-4], the basal ganglia [5, 6], and the sensorimotor cortex [7-9] and thus are considered to be a result of abnormalities within the motor system [1]. However, it is unclear which structures and pathways of the corticobasal ganglia–cerebellar motor network preferentially contribute to the expression of symptoms in MYC/DYT-SGCE.
Diffusion-weighted imaging (DWI) is an effective in vivo tool to investigate properties and potential abnormalities of white matter tracts and gray matter microstructure [10]. Previous imaging study of the motor pathways in MYC/DYT-SGCE showed increased white matter volume and fractional anisotropy (FA) with a decreased mean diffusivity (MD) in the subthalamic area of the brainstem and red nucleus and decreased MD in the cortical sensorimotor areas [11], suggesting abnormalities in pathways connecting the cerebellum with the thalamus. However, such alteration in FA and MD might be the consequence of different developmental processes such as dysmyelination [12] or deafferentation phenomena [13]. Classic DWI approaches are limited in providing specific information with regard to the microarchitectural abnormalities of gray and white matter [10], which might be a key pathophysiological feature in this disorder. Moreover, the location of the abnormalities within the motor network tracts has not yet been properly investigated in MYC/DYT-SGCE.
We hypothesized that microstructural abnormalities in the motor network are implicated in MYC/DYT-SGCE pathogenesis. Recently, a study using the CRISPR/Cas9 editing of a human cortical glutamatergic cell line with an SGCE heterozygous mutation showed higher dendritic complexity and longer axon initial segments in these neurons [9]. We hypothesize that similar neurite reorganization may occur in cells within other regions of the motor network, particularly within the cerebellum, impacting dendrites in gray matter. Additionally, we hypothesized that axonal projections in white matter might also be altered in this disorder. To test this hypothesis, we used neurite orientation dispersion and density imaging (NODDI), which is an emerging accurate diffusion magnetic resonance imaging (MRI) method to probe microstructural complexity of dendrites and axons [10, 14]. This model is more successful compared to classical diffusion tensor imaging (DTI) approaches in characterizing microstructural changes, particularly in the white matter, as was shown by histological analyses of spinal cord lesions in multiple sclerosis [15]. NODDI separates the signal arising from different tissue compartments and estimates the density and fanning of neurites, as well as the partial volume contamination from cerebrospinal fluid [10]. In the NODDI model, each voxel is assumed to be a combination of three compartments: intracellular (modeled as restricted anisotropic non-Gaussian diffusion), extracellular (modeled as hindered anisotropic Gaussian diffusion), and cerebrospinal fluid (modeled as isotropic Gaussian diffusion). The common measures used are (i) the neurite density index (NDI), which reflects the fraction of the signal represented by the intra-axonal compartment; (ii) the neurite orientation dispersion index (ODI), which indicates the fanning of neurites when increasing; and (iii) the isotropic volume fraction (ISOVF), which reflects extracellular free water [14]. Thus, NODDI provides a qualitative indication of the brain tissue microarchitecture and allows making inferences regarding the histopathology and mechanisms involved in pathophysiology [15]. NODDI has been employed in various physiological and pathological conditions such as ageing, Parkinson disease, stroke, and amyotrophic lateral sclerosis [16].
In this study, we applied NODDI analysis alongside classic diffusion tensor approach analysis to examine microarchitectural abnormalities and their potential association with the motor phenotype in MYC/DYT-SGCE. Furthermore, we employed tractography analysis to precisely pinpoint and localize these potential abnormalities within white matter pathways.
METHODS
Subjects
All participants signed informed consent. All the procedures contributing to this work comply with the ethical standards of the relevant national committee on human experimentation (CPP/AU-1360) and with the Helsinki Declaration. Patients' inclusion criteria were (i) age between 15 and 60 years old, (ii) no botulinum toxin injections for at least 3 months prior to participation, (iii) stability of the pharmacological treatment for at least 1 month prior to the study and no medication interruption during the study, and (iv) symptom severity enabling the patient to remain lying still on the back for at least 30 min. Patients and healthy volunteers (HVs) were matched for gender, age, handedness, and educational level. Disease severity was assessed using the Burke–Fahn–Marsden Dystonia Rating Scale (BFM) [17] for dystonia and Unified Myoclonus Rating Scale (UMRS) for myoclonus at rest (section 2) and with action (section 4) [18]. Clinical evaluations were based on video recordings and assessed post hoc by two experts in movement disorders (C.Ta. and E.M.M.), who performed the ratings on a consensus basis; they rated together at the same time the different items on the clinical scales, blinded to the results of the neuroimaging analysis.
Imaging acquisition
All scans were acquired on a 3-T MRI scanner (Prisma, Siemens, Erlangen, Germany) with a 64-channel head coil.
A multishell DTI acquisition was performed (repetition time = 3.5 s, echo time = 75 ms, multiband of three, isotropic voxel size = 1.75 mm, 60 directions with b = 2000 s/mm2, 32 directions with b = 1000 s/mm2, and eight directions with b = 300 s/mm2, one b = 0 image was acquired after every 10 diffusion-weighted directions).
Diffusion-weighted MRI preprocessing
Data preprocessing was performed using the FMRIB Software Library (FSL; https://fsl.fmrib.ox.ac.uk/fsl/fslwiki) and included correction for motion and eddy currents, nonlinear registration, and alignment to the Montreal Neurological Institute space. The EDDY Quality Control tools calculated the average signal to noise ratio (SNR) across all voxels within the brain mask to give for each subject a summary measure of the overall quality of the data [19]. Subjects exceeding 1.5 SD for the SNR or the absolute motion were removed. SNR, as the primary quality measure, addresses preprocessing effects and determines data usability, highlighting both successful corrections and failures, even amid subject movement [19].
Computational methods for DWI
Preprocessed DWI data were independently modeled using the NODDI and the classical DTI approaches. NODDI models were fitted using the AMICO (Accelerated Microstructure Imaging via Convex Optimization) toolbox to generate NDI, ODI, and ISOVF maps. DTI representation was fitted using DTIFIT, implemented in FSL with default parameters, to generate FA and MD maps.
Individual subjects' maps derived from NODDI and DTI approaches were smoothed (full width at half maximum = 5 mm) and masked with subjects' brain templates (i.e., union of their gray and white matter) derived from their anatomical imaging (acquisition parameters and preprocessing in supplementary materials: Data S1).
Fiber tractography
To specify the pathways concerned by the white matter clusters derived from both DWI analysis approaches, we performed a probabilistic tractography analysis in the group of patients. We investigated the main inflow and outflow pathways of the cerebellum and basal ganglia, namely, the cerebellothalamic (CTP), corticopontocerebellar (CPCP), spinocerebellar (SCP), motor corticostriatal (mCSP), and motor thalamocortical (mTCP) pathways. We used “bedpostx” [20] and “probtrackx2” [21] implemented in FSL 6.0 with the default parameters at each step of the procedure (parameters provided in supplementary materials: Data S1) to reconstruct the fibers of the CTP, CPCP, SCP, mCSP, and mTCP. These tractography analyses were performed following the physiological directions of these pathways of interest and for each side separately (accounting for decussation when appropriate) and guided using waypoint, termination, and exclusion masks according to the neuroanatomical knowledge (see supplementary materials: Data S1 for settings and masks definition). The seed and termination masks used were a merged mask of supplementary motor area and primary motor cortex, thalamus, putamen, and spinal cord. As the cerebellar lobule VI was the most represented in our results, considering group comparisons and regression analyses, this region was chosen as the cerebellar seed/target. For each subject, individual density maps were normalized on their individual total number of valid generated tracts (i.e., the “waytotal”) [22]. Then, group tracts were created by averaging binarized (threshold of 0.2) normalized tract density maps across subjects.
Statistical analysis
Demographic and clinical data analysis
Statistical analysis was performed using the Statistical Package for Science version 21 (SPSS, Chicago, IL, USA). Comparability between groups was tested using independent t-tests and Fisher exact tests.
Neuroimaging data analysis
Voxelwise statistical analysis of NODDI (NDI, ODI, and ISOVF maps) and classical DTI metrics (FA and MD maps) data was performed using the general linear model approach of SPM12. We used independent-sample t-tests to compare MYC/DYT-SGCE and controls. For patients, we implemented UMRS and BFM scales as covariate of interest to identify clusters associated with these variables. The peak statistical threshold was set to p < 0.001 with a cluster level threshold of p < 0.05 corrected with false discovery rate method for all these analyses. To ensure the absence of any spurious effects of medication in our findings, we conducted additional regression analyses for each identified cluster (i.e., in group comparisons and regression analyses), examining the association between the DWI metric in this cluster and medications (see supplementary materials: Data S1).
Nvoxelscluster∩pathway represents the count of voxels that belong to both the cluster and the specific pathway, and Nvoxelscluster indicates the total voxel count within that cluster.
RESULTS
Participants
Twenty-one MYC/DYT-SGCE patients and 25 HVs were enrolled in the study. Three patients and one HV were identified as outliers due to excessive movement and/or disease severity and were removed from the analysis. Consequently, the final analysis was performed on 18 MYC/DYT-SGCE patients and 24 HVs. As presented in Table 1, groups were comparable for age, sex, handedness, and level of education (all p > 0.05).
| Characteristic | MYC/DYT-SGCE, n = 18 | HVs, n = 2 | t | p |
|---|---|---|---|---|
| Agea | 29.33 ± 2.80 | 29.88 ± 2.17 | −0.16 | 0.88* |
| Handedness, R:Lb | 15:3 | 19:5 | 1.00** | |
| Sex, F:Mb | 9:9 | 14:10 | 0.76** | |
| Years of educationa | 13.56 ± 0.35 | 13.08 ± 0.38 | 0.89 | 0.38* |
| BFMa | 10.11 ± 1.59 | |||
| UMRS 2a | 4.22 ± 1.01 | |||
| UMRS 4a | 20.44 ± 2.72 | |||
| Medicationc | 7 | 0 | <0.001** |
- Abbreviations: BFM, Burke–Fahn–Marsden Dystonia Rating Scale; F, female; HV, healthy volunteer; L, left; M, male; MYC/DYT-SGCE, myoclonus dystonia due to a pathogenic variant in SGCE; R, right; UMRS, Unified Myoclonus Rating Scale (section 2 = at rest; section 4 = with action).
- a Reported as mean ± SEM.
- b Reported as the number of subjects.
- c Zonisamide (n = 3), benzodiazepine (n = 2), anticholinergics (n = 2), selective serotonin reuptake inhibitors (n = 1), tetrabenazine (n = 1), botulinum toxin injections (n = 1).
- * Independent t-test.
- ** Fischer exact test.
NODDI analysis results
The overall quality of the imaging dataset (SNR) was comparable across groups (mean ± SEM: MYC/DYT-SGCE: 20.82 ± 0.74, HVs: 21.32 ± 0.74; t40 = −0.47, p = 0.64).
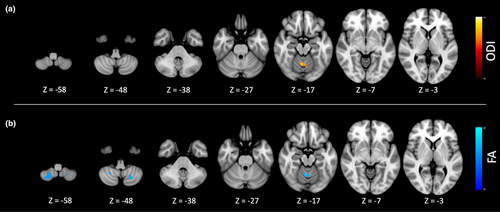
As presented in Table 2 and Figure 1a, group comparison showed an increase in ODI index in patients with MYC/DYT-SGCE within a cluster encompassing the gray matter of the bilateral motor cerebellum and the associated vermis.
| C | Peak voxel, x, y, z (MNI) | K E | p corr a | Location | |
|---|---|---|---|---|---|
| Group comparison | |||||
| ODI, MYC/DYT-SGCE > HVs | 1 | 3, −60, −20 | 196 | 0.04 |
Gray matter Cerebellum IV, V, VI (L + R) Vermis IV, V, VI (L + R) |
| FA, MYC/DYT-SGCE < HVs | 1 | 6, −57, −18 | 188 | 0.02 |
Gray matter Vermis IV, V, VI Cerebellum IV, V, VI (R) Cerebellum IV, V (L) |
| 2 | −22, −66, −46 | 170 | 0.02 | Cerebellum VIIb, VIII (L) | |
| 3 | 21, −58, −56 | 373 | 0.001 | Cerebellum VIII (R) | |
| BFM | |||||
| NDI, negative | 1 | 12, −50, −45 | 450 | 0.02 |
Gray matter Cerebellum VIII, IX (R) |
| 1 | −4, −18, −20 | 1030 | <0.001 |
White matter b Brainstemc CPCP: 81%; SCP: 31%; CTP: 17% |
|
| 2 | −20, −51, −39 | 357 | 0.04 |
Mid Cerebellar Ped (L) CPCP: 48%; SCP: 35%; CTP: 16% |
|
| 3 | 2, −38, −51 | 466 | 0.02 |
Medulla oblongata CPCP: 1%; SCP: 68%; CTP: 9% |
|
| ODI, negative | 1 | 50, −26, −4 | 188 | 0.02 |
Gray matter Temporal Sup (R) Temporal Mid (R) |
| 2 | 22, −51, 6 | 226 | 0.01 |
Calcarine (R) Precuneus (R) Lingual (R) |
|
| 3 | 6, −69, −27 | 261 | 0.01 |
Vermis VI, VII, VIII Cerebellum VI, VIII (R) |
|
| ISOVF, positive | 1 | 14, −21, −27 | 345 | 0.001 |
White matter b Brainstem CPCP: 95%; SCP: 14%; CTP: 15% |
| FA, positive | 1 | 22, −52, 6 | 239 | 0.01 |
Gray matter Calcarine (R) Precuneus (R) |
| 2 | 6, −70, −26 | 301 | 0.005 |
Cerebellum VI (R) Precuneus (R) Lingual (R) Vermis VI, VII |
|
| MD, positive | 1 | −28, 3, −36 | 784 | <0.001 |
Gray matter Temp Inf (L) Parahippocam. (L) Fusiform (L) |
| 2 | 12, −30, −34 | 1248 | <0.001 |
White matter b Brainstemc CPCP: 61%; SCP: 32%; CTP: 2% |
|
- Abbreviations: C, Cluster number; BFM, Burke–Fahn–Marsden Dystonia Rating Scale; CPCP, corticopontocerebellar pathway; CTP, cerebellothalamic pathway; FA, fractional anisotropy; HV, healthy volunteer; ISOVF, isotropic volume fraction; KE, number of voxels; L, left; MD, mean diffusivity; Mid, middle; MNI, Montreal Neurological Institute space; MYC/DYT-SGCE, myoclonus dystonia related to SGCE pathogenic variant; NDI, neurite density index; ODI, orientation dispersion index; Parahippocam., parahippocampic cortex; Ped, pedunculus; R, right; SCP, spinocerebellar pathway; Sup, superior; Temp Inf, temporal inferior.
- a Corrected for multiple testing with the false discovery rate method.
- b For each white matter cluster, the proportion of voxels belonging to the different pathways of interest is indicated (voxels could belong to multiple pathways).
- c Extended from mesencephalon to the lower pons.
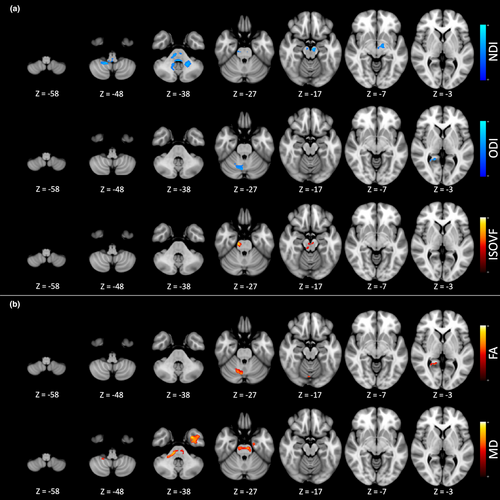
Regression analysis (Figure 2a) exhibited an association between BFM and NODDI parameters mainly in the motor cerebellum and in the brainstem. A higher BFM score was associated with (i) a lower NDI signal in the white matter of the left middle cerebellar pedunculus, the brainstem, and the gray matter of the right motor cerebellum; (ii) a lower ODI signal in the gray matter of the right motor cerebellum and corresponding vermis, the right temporal lobe, and the right occipital lobe; and (iii) a higher ISOVF signal in the brainstem. There was no association of the UMRS parts 2 and 4 with NODDI parameters. No association was found between the NODDI metric in these clusters and the medications (see supplementary results: Data S1).
FA and MD diffusion tensor analysis results
As depicted in Table 2 and Figure 1b, group comparisons revealed a reduction in the FA index among patients with MYC/DYT-SGCE within the gray matter regions of the bilateral motor cerebellum and the associated vermis.
Regression analysis (Figure 2b) demonstrated associations between the BFM score and diffusion tensor parameters, primarily in the cerebellum and brainstem. Specifically, a higher BFM score was associated with (i) a higher FA signal in the gray matter of the right motor cerebellum and of the right occipital cortex and (ii) a higher MD in the white matter of the brainstem and of the left temporal cortex. No association was observed between UMRS parts 2 and 4 and FA or MD indexes. No association was found between the DTI metric in these clusters and the medications (see supplementary results: Data S1).
Tractography analysis results
The masks of the pathways of interest are presented in Figure S1. As presented in Table 2 and Figure 3, white matter clusters associated with BFM severity were overlayed with these pathways' masks. Importantly, voxels could potentially belong to multiple pathways [23].
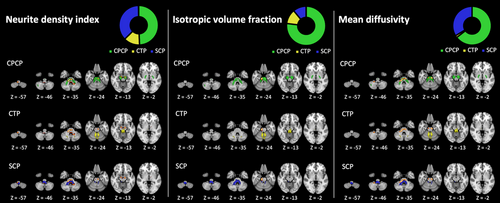
For the NDI, the voxels of the brainstem and left middle cerebellar pedunculus clusters mainly belonged to the CPCP and SCP. For the ISOVF index, the brainstem cluster had 95% of its voxels belonging to the CPCP. Regarding the MD index, the brainstem cluster had 61% of its voxels belonging to the CPCP and 32% to the SCP.
DISCUSSION
Using two DWI approaches to investigate the brain microarchitecture in MYC/DYT-SGCE, we showed that, compared to controls, patients exhibited an increase in ODI and decreased FA within the motor cerebellar cortex. A greater severity of dystonia was related to lower ODI and NDI and higher FA within the motor cerebellar cortex and to lower NDI and higher ISOVF and MD within the cerebellar afferent pathways, specifically the CPCP and SCP. Altogether, these findings suggest that the severity of dystonia in MYC/DYT-SGCE was primarily related to microstructural abnormalities in the cerebellum and its afferent pathways, which relay inputs from the spinal cord and the cerebral cortex.
Study limitations
Our study presents several limitations. First, we have not identified any relation between the DWI metrics and the severity of myoclonus. Head movement during acquisitions could affect our findings. However, the overall quality of the imaging dataset as measured with the SNR was similar between the groups, and our findings with regard to dystonia in MYC/DYT-SGCE pointed to specific involvement of motor cerebellum [24] and its afferent pathways. Thus, it is unlikely that a systemic bias such as excessive movements would significantly affect our results. An alternative explanation might be that the structural abnormalities we found do not explain the entire neural activity underpinning myoclonus. Furthermore, the coupling between neuronal activity and neuronal microstructure, as assessed with DWI, may have certain limitations. Further studies using additional imaging modalities such as functional MRI are warranted to investigate the anatomical correlates of myoclonus.
Second, no structural alterations in the motor cortex were detected, but these have been previously identified using cell biology techniques [9]. It is possible that these alterations are of a subtle nature, making their detection challenging when using DWI techniques. Future neuroimaging studies, utilizing a higher magnetic field strength, such as 7 T, may potentially improve the model's sensitivity.
Third, although the NODDI model was developed for studying both gray matter and white matter [14], it has been more robustly validated for the study of white matter [15]. Therefore, its interpretation for the study of gray matter should be approached with greater caution.
Lastly, the overall mild severity of the disease might also constrain the capacity to identify subtle structural changes. However, we had to include patients who were able to remain still in the MRI machine for the entire acquisition procedure.
Microstructure of the cerebellar cortex and correlation with severity of dystonia
Compared to HVs, patients exhibited an increase in the ODI metric and a decrease in the FA metric in the motor cerebellar cortex, reflecting likely morphological changes to neurites [25]. ODI represents the alignment and coherence of the neurites in a voxel, and an increased ODI indicates a higher dispersion of neurites [26]. FA characterizes the directionality of water diffusion [27], and despite the need for caution in interpreting FA in gray matter [28], a decrease in FA during brain development may indicate an increase in dendritic elongation and branching orthogonal to cortical columns [29]. In summary, abnormalities observed in ODI and FA metrics could reflect a higher degree of complexity of the gray matter dendritic trees [30]. A recent study found increased branching and longer branches in cortical glutamatergic cell dendrites in subjects with SGCE mutation [9]. The results of the present study suggest that other cellular lines such as cerebellar neurons can also be affected by these dendritic changes.
A first interpretation of these findings may be that structural abnormalities reflect an alteration in the functioning of the cerebellar cortex. In neurodevelopmental disorders, changes in neurite morphology have been associated with diminished synaptic efficiency and may result from alterations in neuronal homeostasis [9, 31]. Within the cerebellum, the highest expression of SGCE is found in the Purkinje cells and neurons of the dentate nucleus [32]. Thus, the microstructural change we found in the cerebellar cortex might reflect reorganization of the Purkinje cell neurites. The Purkinje cells send γ-aminobutyric acid (GABA) inhibitory projections to the dentate nucleus [33]. The SGCE protein may have a role in GABAergic inhibitory synapse homeostasis [34, 35]. This suggests that cerebellar GABAergic deficit may play a key role in the pathophysiology of MYC/DYT-SGCE,4 which aligns with the observed increase in metabolism within the cerebellar motor cortex and deep nuclei in MYC/DYT-SGCE, suggesting disinhibition of these structures [3].
However, a more severe dystonia correlated with a lower density of neurites in the motor cerebellar cortex, which might suggest a compensatory nature of these changes. Despite the specific mutation in SGCE, the dystonia severity in MYC/DYT-SGCE varies [1], suggesting that cerebellar plasticity might influence symptom severity.
Overall, in MYC/DYT-SGCE, the GABAergic deficiency may alter the firing rate and regularity of cerebellar neurons, thereby affecting cerebellar microcircuit functioning and leading to aberrant cerebellar output signals [2]. Intrinsic plasticity within cerebellar microcircuitry may be critical in developmental disorders and supports the concept of “functional cerebellar reserve” [36]. For instance, granule cells have been implicated in the neuroplastic recovery process following cerebellar damage during neurodevelopment [37] and may be involved in the restoration of cerebellar hyperactivity in MYC/DYT-SGCE [2, 38]. Further longitudinal studies are warranted to fully address this question.
Aside from the motor cerebellar cortex, severity of dystonia was associated with a decrease in ODI and an increase in FA in the right occipital lobe, a decrease in ODI the right temporal lobe, and an increase of MD in the left temporal lobe. Structural abnormalities of these cortical areas have occasionally been found in focal dystonia [39, 40]. In MYC/DYT-SGCE, reduced regional cerebral blood flow in the temporal lobe by single photon emission computed tomography [41] and structural abnormalities in occipital cortex related to sensory information processing have been described [42].
Severity of dystonia and afferent and efferent cerebellar pathways
Severity of dystonia in MYC/DYT-SGCE was also correlated with microstructure of white matter, as reflected by NDI, ISOVF, and MD indexes, of the CPCP and the SCP. In the brain, the SGCE protein is expressed within the cortex, cerebellum, and spinal cord [9, 43], and these structures were found to be involved in dystonia pathogenesis [44, 45].
Interestingly, we have not found a strong association between the severity of dystonia and abnormalities in the CTP. It does not rule out the potential contribution of the cerebellar efferent pathway's contribution to symptoms but suggests it may be less significant in MYC/DYT-SGCE dystonia.
More severe dystonia was associated with a decrease in NDI and increase in ISOVF and MD within the cerebellar afferent pathways. The NDI metric reflects the space within axons, and ISOVF corresponds to the volume fraction of unhindered water (cerebrospinal fluid), whereas MD reflects the mean rate of water diffusion within tissues [14, 15]. Taken together, this suggests that overall lower density of axons in these pathways may underlie a higher severity of dystonia in MYC/DYT-SGCE. When referring to the histological validation of the NODDI model, chronic multiple sclerosis lesions affected by axonal loss were associated with a tendency toward decreased NDI and FA along with increased ISOVF and MD [15]. In patients with multiple sclerosis or cerebral palsy, these abnormal NODDI changes were interpreted as reflecting axonal loss, potentially arising from Wallerian degeneration [46-48]. However, in MYC/DYT-SGCE, earlier observations have indicated an increase in FA and a decrease in MD within the brainstem compared to controls, suggesting a contrary enhancement of directional axonal growth. The authors proposed that these changes might reflect axonal growth linked to plasticity secondary to the hyperkinetic state [11]. The lower severity of dystonia associated with increased axonal density rather suggests that plasticity mechanism acts more like a compensatory response, akin to the plasticity observed with motor skill training [49]. Cerebellar function may be compensated by extracerebellar areas, a phenomenon known as “structural cerebellar reserve” [36].
Nevertheless, the correlational nature of the present analysis prevents us from making definitive inferences about the pathological or compensatory nature of the observed associations. Furthermore, the interpretation of DTI in the white matter can be challenging, because it is jointly influenced by demyelination, neuroaxonal loss, and even changes in the glial component [15].
Finally, we did not observe a group difference in these pathways, compared to a previous report [11], that might stem from some methodological differences, including a more restrictive whole-brain analysis and the inclusion of younger patients.
Also, microarchitecture of the basal ganglia and their connecting pathways did not exhibit any difference between groups or relation with disease severity. Previous reports pointed to the striatopallidothalamocortical network's role in MYC/DYT-SGCE motor symptoms [1], as supported by intraoperative pallidal recordings and symptom improvement with deep brain stimulation [1, 6]. However, it has been well established that bidirectional direct connections exist between cerebellum and basal ganglia [2]. A recent mouse model of acute knockdown of SGCE produced dystonia and jerking movements when applied in the cerebellum but not in the basal ganglia [2]. This supports a model of dystonia where basal ganglia abnormalities may stem from abnormal cerebellar signals [50]. Our findings further support that dysfunction of the cerebellum and cerebellar afferent pathways might be primarily involved in the MYC/DYT-SGCE dystonic phenotype.
AUTHOR CONTRIBUTIONS
Clément Tarrano: Writing – original draft; investigation; writing – review and editing; formal analysis; methodology. Giuseppe Zito: Writing – review and editing; investigation. Cécile Galléa: Conceptualization; writing – review and editing; supervision; writing – original draft; investigation. Cécile Delorme: Investigation; writing – review and editing; resources. Eavan M. McGovern: Investigation; writing – review and editing; formal analysis. Cyril Atkinson-Clement: Writing – review and editing; formal analysis; investigation. Vanessa Brochard: Writing – review and editing; investigation; data curation; project administration. Stéphane Thobois: Writing – review and editing; resources. Christine Tranchant: Writing – review and editing; resources. David Grabli: Writing – review and editing; resources. Bertrand Degos: Writing – review and editing; resources. Jean Christophe Corvol: Writing – review and editing; resources. Jean-Michel Pedespan: Writing – review and editing; resources. Pierre Krystkowiak: Writing – review and editing; resources. Jean-Luc Houeto: Writing – review and editing; resources. Adrian Degardin: Writing – review and editing; resources. Luc Defebvre: Writing – review and editing; resources. Mélanie Didier: Writing – review and editing; investigation. Romain Valabrègue: Software; formal analysis; writing – review and editing. Emmanuelle Apartis: Writing – review and editing; resources. Marie Vidailhet: Funding acquisition; writing – review and editing; resources; supervision; project administration; investigation. Emmanuel Roze: Conceptualization; writing – original draft; project administration; supervision; writing – review and editing; funding acquisition; investigation; resources. Yulia Worbe: Conceptualization; investigation; writing – original draft; methodology; supervision; project administration; writing – review and editing; funding acquisition.
ACKNOWLEDGMENTS
We thank Drs. Lucie Guyant-Marechal, Elisabeth Sadot, Philippe Damier, Marion Simonetta-Moreau, and Damien Ricard for their help with the inclusion of subjects.
FUNDING INFORMATION
This study received financial support from the Dystonia Medical Research Foundation (Chicago, IL, USA); from the “Association des Malades atteints de Dystonie” (AMADYS) through the “Fonds de Dotation Brou de Laurière”, from the “Investissements d'Avenir” programme funded by the “Agence Nationale de la Recherche” (ANR-10-IAIHU-06) and from the European Union's Horizon 2020 research and innovation program under EJP RD COFUND-EJP No 825575–EurDyscover; and from the Fondation pour la Recherche Médicale.
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest concerning the present research. Regarding financial disclosures for the preceding 12 months, C.Ta. has received a PhD grant from the “Fondation pour la Recherche Médicale.” C.D. has received a research grant from the FIA; travel funding from Merz Pharma, Abbvie, Boston Scientific, and Medtronic; and honoraria from Elivie, Merz Pharma, and Medtronic. J.C.C. has served on advisory boards for Biogen, Denali, Idorsia, Prevail Therapeutic, Servier, Theranexus, and UCB and has received grants from Sanofi and the Michael J. Fox Foundation for other projects. S.T. has received grants from France Parkinson and PHRC and honorarium from Abbvie, Boston, and Merz. E.M.M. has received speaking honoraria from AbbVie and the Dutch MDS symposium and travel support from Elivie; has served on an advisory board for AbbVie; and has received research grants from the STAR MD and the RCSI Richard Steeven's Scholarship. D.G. has received grants from AP-HP (DRC-PHRC) and France Parkinson; has served on scientific advisory boards for AbbVie and Zambon; received research funding from Air Liquide and Orkyn; has received speech honoraria from Medtronic, AbbVie, Merz, Orkyn, Aguettant, and EverPharma; and has received travel funding from AbbVie and Merz. E.R. has received honoraria for speaking from Orkyn, Aguettant, and Elivie and for participating on an advisory board from Merz Pharma. He has received research support from Merz Pharma, Orkyn, Aguettant, Elivie, Ipsen, Everpharma, Fondation Desmarest, AMADYS, ADCY5.org, Fonds de dotation Patrick Brou de Laurière, Agence Nationale de la Recherche, Societé Française de Médecine Esthétique, and Dystonia Medical Research Foundation. None of the other authors has any disclosure relevant to this work.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




