Education for fatigue management in people with multiple sclerosis: Systematic review and meta-analysis
Abstract
Background and Purpose
Fatigue is a common and disabling symptom in multiple sclerosis (MS). Educational interventions have shown potential to reduce fatigue. The aim was to systematically review the current best evidence on patient education programmes for MS-related fatigue.
Methods
This was a systematic review and meta-analysis following Cochrane methodology. A systematic search was conducted in eight databases (September 2023). Moreover, reference lists and trial registers were searched and experts in the field were contacted. Randomized controlled trials were included evaluating patient education programmes for people with MS with the primary aim of reducing fatigue.
Results
In total, 1176 studies were identified and assessed by two independent reviewers; 15 studies (1473 participants) were included. All interventions provided information and education about different aspects of MS-related fatigue with different forms of application, some with components of psychological interventions. Amongst those, the most frequently applied were cognitive behavioural therapy (n = 5) and energy-conservation-based approaches (n = 4). Studies differed considerably concerning mode of intervention delivery, number of participants and length of follow-up. Interventions reduced fatigue severity (eight studies, n = 878, standardized mean difference −0.28; 95% confidence interval −0.53 to −0.03; low certainty) and fatigue impact (nine studies, n = 824, standardized mean difference −0.21; 95% confidence interval −0.42 to 0.00; moderate certainty) directly after the intervention. Mixed results were found for long-term effects on fatigue, for secondary endpoints (depressive symptoms, quality of life, coping) and for subgroup analyses.
Conclusion
Educational interventions for people with MS-related fatigue may be effective in reducing fatigue in the short term. More research is needed on long-term effects and the importance of specific intervention components, delivery and context.
BACKGROUND
Multiple sclerosis (MS) is an incompletely understood disease of the central nervous system that usually manifests in young adulthood [1]. Disease progression is largely unpredictable, and patients may experience a wide range of symptoms. Fatigue is a symptom related to various chronic and neurological illnesses, for example cancer, rheumatoid arthritis, stroke, traumatic brain injury and MS [2]. In MS, fatigue is one of the most common symptoms affecting 75%–95% of patients at least once in their life [3, 4]. Current definitions include a subjective lack of physical/mental energy or both, which is perceived by the individual or the caregiver to interfere with usual or desired activities, an overwhelming sense of tiredness, lack of energy or feelings of exhaustion. The feeling of physical tiredness and lack of energy is distinct from sadness or weakness [5]. Fatigue is considered to be the most disabling symptom by many people with MS, more important than mobility restrictions or pain [6]. Therefore, fatigue is a common reason for unemployment [7] and often a cause for social withdrawal [8]. Nevertheless, fatigue may affect patients very differently; and its causes are yet poorly understood [5].
According to current understanding, there are primary and secondary mechanisms that lead to fatigue [9]. Whilst primary fatigue is a direct result of physical changes within the body through the disease itself, secondary fatigue is due to other factors that can cause and worsen fatigue. Amongst the primary mechanisms are axonal loss, reorganization and increased brain activity recruitment, as well as immunological and neuroendocrine factors. Factors related to secondary fatigue include sleeping problems, depression, stress, side effects of pharmacological therapies and reduced physical activity [10, 11]. Moreover, it has been postulated that fatigue is perpetuated by certain cognitions and beliefs, such as negative thinking and symptom focusing, a reduced level of physical activity, and a low level of self-efficacy [11-14].
Currently there are no convincing pharmacological treatments available for MS-related fatigue [11-14]. However, there have been a number of studies assessing the effects of non-pharmacological interventions, such as physical exercise or cognitive behavioural therapy (CBT) [13, 15-19]. In the last few years, various patient education programmes for MS-related fatigue have been developed and evaluated. Patient education programmes aim to teach patients about fatigue and about ways of managing and coping with fatigue in daily life. For this review, ‘education programme’ is defined as any intervention in which patients receive information or education about fatigue and about strategies to deal with fatigue. Programmes may be delivered to patients in groups or as individual sessions either face-to-face, online, via telephone or a combination of the three. Patients may receive information on the origins of fatigue, lifestyle changes and strategies for managing fatigue, stress and negative thinking. In addition, patients may receive homework exercises to integrate newly acquired strategies into their daily lives. Studies may compare education programmes to a comparator intervention, to usual or optimized standard care.
Since fatigue is believed to be a multidimensional symptom composed of primary and secondary factors, education programmes are mostly designed as multidimensional approaches targeting, for example, patients' lifestyle, sleeping behaviour, coping mechanisms, negative thinking about MS, and symptom attribution and disease knowledge [20]. Generally, the programmes aim to gradually change patients' beliefs about MS and fatigue, and behaviour in relation to fatigue, by providing relevant information and strategies [21, 22]. Different concepts are used. For example, whilst CBT aims to identify and replace unhelpful thoughts and behaviour by more helpful alternatives, the concept of energy conservation aims to teach patients strategies to optimize activities of daily living to maximize available energy. Homework is used to encourage patients to integrate the newly learned strategies into their daily lives. An own previous review [23] indicated a favourable effect of CBT approaches.
Fatigue is an important symptom in people with MS and often a reason for disability even in the absence of significant physical disability. Since there is no convincing pharmacological treatment for fatigue, and patient education programmes have been proposed as a promising non-pharmacological approach, it seems important to synthesize the current evidence assessing whether these programmes are effective. Importantly, it seems crucial to determine which types of patient education programmes promise to be most successful, considering for example (a) content; (b) programme duration; and (c) programme format.
Recently, there have been a number of systematic reviews and meta-analyses of non-pharmacological interventions for MS-related fatigue, some focusing on specific educational programmes [21, 23] and others focusing on non-pharmacological interventions, including educational programmes [13, 15, 17-19].
The primary objective of this review was to determine the effectiveness of patient education programmes on MS-related fatigue compared to (optimized) usual care or alternative approaches. Additionally, the aim was to assess whether the effects of patient education programmes on fatigue differed according to different intervention approaches or type of comparator intervention. Furthermore, the aim was to assess the effect on secondary outcomes, that is, depressive symptoms, quality of life (QoL) and coping/self-efficacy.
METHODS
This systematic review and meta-analysis that followed Cochrane methodology was reported following the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) [24] guidelines, and was registered in PROSPERO (CRD42019146246), along with its published protocol [25].
The aim was to include all individual and cluster-randomized controlled trials (RCTs), including studies using a cross-over design, that evaluated the effect of patient education programmes on MS-related fatigue. Studies using other study designs were not considered since randomly allocated control groups are needed to control for changes in fatigue not mediated by the intervention (e.g., changes in temperature, therapist attention or natural course). No restrictions concerning language or publication status were used.
Studies, irrespective of setting, evaluating adults with a neurologist-confirmed MS diagnosis of any type (e.g., relapsing–remitting, secondary or primary progressive), according to common diagnostic criteria (e.g., [26]) were included. In studies also including other patient groups apart from people without MS, results for the subgroup of MS patients were used. If these data were not available, studies were included only if at least 80% of participants were people with MS. Participants were also required to have confirmed fatigue at baseline, assessed with any validated fatigue scale (e.g., Fatigue Scale of Motor and Cognitive Functions [27] or Fatigue Scale [28].
Any intervention was defined as ‘education programme’ in which patients received information or education about strategies to deal with fatigue, including those that contain components of psychological interventions. Studies were included that evaluated patient education programmes addressing fatigue in individual or group interventions administered face-to-face, online or via telephone. As this review focuses on assessing the potential benefit of educational interventions for fatigue in MS, studies evaluating effects of drugs, physical exercise or other types of interventions (such as, for example, cooling studies, bee sting therapy, yoga) were not included. Studies were included irrespective of duration and length. The descriptions of the respective comparator interventions were checked, including information on training of the healthcare provider delivering the control treatment, presence of a manual, possible therapist supervision, treatment fidelity monitoring, and other potentially important aspects. As usual care, regular neurological follow-up visits were defined. Optimized usual care aimed to level differences in control group patients, for example by providing basic information on MS and MS-related fatigue. Comparator interventions were designed specifically to control for time and attention of healthcare professionals, but did not focus on reducing fatigue. Studies using active comparator groups were included; however, studies were excluded if the comparator intervention clearly focused on fatigue reduction.
Outcomes
The primary outcomes were (a) fatigue severity, (b) fatigue impact and (c) (serious) adverse events, as reported in primary studies. ‘Fatigue severity’ is defined as the subjectively perceived level of fatigue that a person feels in daily life, whilst ‘fatigue impact’ describes the influence and consequences of perceived fatigue on a person's daily life. Different validated instruments measuring fatigue severity as well as fatigue impact are available in MS-related fatigue. Fatigue scales often include aspects of both fatigue severity and fatigue impact, resulting in a challenge assessing solely one dimension. Only validated scales which assess fatigue severity, for example Fatigue Severity Scale (FSS) [29], or fatigue impact, for example Fatigue Impact Scale (FIS) [30], or a combination of both were included.
As seconday outcome measures, (a) depressive symptoms, (b) QoL and (c) coping/self-efficacy were assessed when obtained with any validated scale.
Furthermore, disease-related endpoints were assessed, such as activities of daily living, medication, relapses, physical impairment (Expanded Disability Status Scale [31]) and caregiver outcomes.
Search methods
On 30 September 2023, an information specialist at the Department of Oncology, Laboratory of Clinical Research Methodology, Istituto di Ricerche Farmacologiche Mario Negri IRCCS, Milan, Italy, ran a bespoke search for the following databases: Cochrane Central Register of Controlled Trials in the Cochrane Library; MEDLINE (PubMed) (from inception to 30 September 2023); Embase (Embase.com) (from 1974 to 30 September 2023); Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCOhost) (from 1981 to 30 September 2023); PsycINFO (from inception until 30 September 2023); Latin American and Caribbean Health Science Information Database (LILACS) (Bireme) (from 1982 to 30 September 2023); ClinicalTrials.gov (www.clinicaltrials.gov); World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch). The detailed search methods are reported in File S1.
Handling of different study designs
For cross-over studies, only first period data up to the first point of cross-over were used to rule out carry-over effects [32]. For continuous outcomes, only the total number of participants was divided and means and standard deviations were left unchanged [32]. In the case of studies comparing one active intervention with more than one control intervention, the group with the less active control group approach was used (e.g., usual care instead of provision of general information materials).
Measures of treatment effect
For all continuous outcome data, mean differences with 95% confidence intervals (CIs) were used. For pooling data from studies using different measurement scales for the same outcome, standardized mean differences (SMDs) were calculated. All statistical analyses were performed using Revman Web [33].
Handling of missing data
In the case of missing data, study authors were contacted and missing data, if available, were included in analyses. The answers obtained and the dates of our contacts were recorded.
Assessment of clinical heterogeneity
For the assessment of clinical heterogeneity, extracted data for between-study variability with respect to participants, interventions and outcome measurements were examined. RevMan Web was used to assess statistical heterogeneity using I2 and χ2 statistics, and visual inspections of forest plots were used to assess heterogeneity of data. Due to the overall heterogeneity amongst studies, random-effects models were used for all meta-analyses.
Assessment of risk of bias in included studies
To assess the risk of bias of included studies, the guidance from the Cochrane Handbook for Systematic Reviews of Interventions [32] was followed.
Two authors (SK, AG) independently assessed and scored the included studies' methodological quality to identify any potential sources of bias. Criteria for appraisal of studies were internal validity and low risk of bias through selection bias, performance bias, attrition bias and detection bias. Study validity was determined by categorizing individual studies into low, high and unclear risk of bias. As recommended by the Cochrane Handbook for Systematic Reviews of Interventions [32], a two-part tool, addressing six domains (sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other issues) was used, the first part describing what has been reported in the study and the second part assessing the related risk of bias (low, high or unclear). Domains of sequence generation, allocation concealment (avoidance of selection bias) and selective outcome reporting (avoidance of reporting bias) were addressed using a single entry for each study. Furthermore, blinding of participants, staff and outcome assessors (avoidance of performance bias and detection bias) was assessed. Assessment of risk of bias across studies followed the instructions provided in the Cochrane Handbook for Systematic Reviews of Interventions [32] (chapter 8.7), that is, low risk of bias across studies was defined if the majority of studies providing information for a comparison stemmed from studies at low risk of bias, unclear risk of bias across studies if the majority of studies were rated as low or unclear risk of bias, and high risk of bias across studies if one or more studies with high risk of bias importantly influenced the summary results [32].
Data synthesis
For the primary outcomes (fatigue severity and impact), meta-analyses were performed because studies were considered to be sufficiently clinically homogeneous, in terms of participants, interventions and outcomes. Meta-analyses were performed using the generic inverse variance method in RevMan Web [33]. For the secondary outcomes (depressive symptoms, QoL and coping/self-efficacy), meta-analyses were performed if sufficient data were available. Studies were only included in meta-analyses if means and standard deviations of outcomes for both intervention and control groups were reported. If this was not the case, results were reported narratively.
Summary of findings and assessment of the certainty of the evidence
For the primary outcomes, the primary endpoints fatigue severity and fatigue impact at two time points (i.e., directly after the intervention and at the 2–4-month follow-up), as well as the secondary endpoints depressive symptoms at two time points (i.e., directly after the intervention and at the 2–4-month follow-up) and QoL (directly after the intervention) were included in a ‘Summary of Findings’ table (Table 1). Further follow-up data were reported quantitatively when possible and qualitatively in the case of insufficient data available for meta-analysis. Outcome data were reported as points on particular scales (e.g., FSS and Modified Fatigue Impact Scale [29, 30]).
| Outcomes | Risk with patient education compared to risk with usual care/sham intervention | No. of participants (studies) | Certainty of the evidence (GRADE) | Comments |
|---|---|---|---|---|
|
Fatigue severity, directly post-intervention (presented in points on FSS: higher is worse) Scale from 1 to 7 |
SMD 0.28 points lower (0.53 lower to 0.03 lower) | 878 (8 RCTs) | ⨁⨁◯◯ Lowa, b | Based on weighted mean SD of 0.9986 calculated from three studies [44, 46, 47] using the FSS; SMD −0.28 (−0.53, −0.03) |
|
Fatigue severity, 2–4 months follow-up (presented in points on FSS: higher is worse) Scale from 1 to 7 |
SMD 0.18 points lower (0.4 lower to 0.04 higher) | 660 (6 RCTs) | ⨁⨁⨁◯ Moderatec | Based on weighted mean SD of 1.092 calculated from two studies [46, 47] using the FSS; SMD −0.18 (−0.40, 0.04) |
|
Fatigue impact, directly post-intervention (presented in points on MFIS: higher is worse) Scale from 0 to 84 |
SMD 0.21 points lower (0.42 lower to 0) | 824 (9 RCTs) | ⨁⨁⨁◯ Moderated | Based on weighted mean SD of 15.25 calculated from six studies [40, 41, 45-47, 49] using the MFIS; SMD −0.21 (−0.42, 0.00) |
|
Fatigue impact, 2–4 months follow-up (presented in points on MFIS: higher is worse) Scale from 0 to 84 |
SMD 0.15 points lower (0.33 lower to 0.03 higher) | 484 (5 RCTs) | ⨁⨁⨁◯ Moderatee | Based on weighted mean SD of 16.893 calculated from four studies [41, 45, 47, 49] using the MFIS; SMD −0.15 (−0.33, 0.03) |
|
Depression, directly after intervention (presented in points on HADS-D: higher is worse) Scale from 0 to 21 |
SMD 0.2 points lower (0.4 lower to 0) | 841 (7 RCTs) | ⨁⨁⨁◯ Moderatef | Based on weighted mean SD of 3.773 calculated from three studies [35,37, 38] using the HADS-D; SMD −0.20 (−0.40, 0) |
|
Depression, 2–4 months follow-up (presented in points on HADS-D: higher is worse) Scale from 0 to 21 |
SMD 0.13 points lower (0.29 lower to 0.03 higher) | 600 (5 RCTs) | ⨁⨁⨁◯ Moderateg | Based on weighted mean SD of 3.791 calculated from two studies [35,37] using the HADS-D; SMD −0.13 (−0.29, 0.03) |
| Quality of life, directly after intervention | SMD 0.23 higher (0.03 lower to 0.5 higher) | 213 (2 RCTs) | ⨁◯◯◯ Very lowh, i | Of 11 studies assessing quality of life, only two studies [36, 43] could be included in the meta-analysis using different scales, SMD 0.23 (−0.03 to 0.50). It was not possible to calculate a WMSD to calculate absolute effects |
- Abbreviations: CI, confidence interval; FSS, Fatigue Severity Scale; HADS-D, Hospital Anxiety and Depression Scale—Depression subscale; MFIS, Modified Fatigue Impact Scale; RCT, cluster-randomized controlled trial; SMD, standardized mean difference; WMSD, weighted mean standard difference.
- a Downgraded one level for risk of bias: high risk (two studies) and unclear risk (four studies) of performance bias; high risk of attrition bias (one study).
- b Downgraded by one level for inconsistency: I2 = 65%.
- c Downgraded one level for risk of bias: high risk (one study) and unclear risk (three studies) of performance bias; high risk of attrition bias (one study).
- d Downgraded one level for risk of bias: high risk (three studies) and unclear risk (three studies) of performance bias; high risk of attrition bias (two studies).
- e Downgraded one level for risk of bias: high risk (one study) and unclear risk (one study) of performance bias; high risk of attrition bias (one study).
- f Downgraded one level for risk of bias: high risk (two studies) and unclear risk (one study) of performance bias; high risk of attrition bias (two studies).
- g Downgraded one level for risk of bias: high risk (one study) and unclear risk (one study) of performance bias; high risk of attrition bias (two studies).
- h Downgraded one level for risk of bias: high risk (two studies) of performance bias; high risk of attrition bias (one study).
- i Downgraded two levels for imprecision: number of participants less than 400 and CI included the null effect.
The certainty of the evidence was assessed with reference to overall risk of bias of included studies, directness of the evidence, consistency of results and precision of estimates, displaying the results in the Summary of Findings table (Table 1) using GRADEpro software, according to the methods outlined in the Cochrane Handbook of Systematic Reviews [32]. The certainty of the evidence for each relevant outcome was categorized as high, moderate, low or very low [30].
Subgroup investigations
To investigate if certain study characteristics influenced efficacy of interventions, five subgroup analyses were performed: group versus single-patient approaches; CBT versus non-CBT approaches; face-to-face versus online versus telephone approaches; short- versus long-term approaches; type of comparator, that is, usual care versus sham intervention.
RESULTS
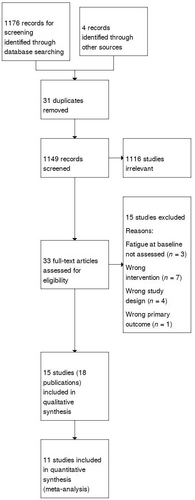
Our search identified 1176 records (1149 after the removal of duplicates). During an initial screening, 1116 citations were excluded. Of the 33 full-text articles retained for further screening, 15 were excluded because they did not focus on patient education for fatigue (n = 7), did not have the correct study design (n = 4), did not assess fatigue at baseline (n = 3) or did not have the prespecified primary outcome (n = 1). Eighteen publications (referring to 15 RCTs see Figure 1) [17, 34-47] were included, of which 11 studies were suitable for meta-analyses. Table S1 reports the characteristics of the included studies, and Table S2 reports the excluded studies and reasons for exclusion.
Interventions
In all programmes, participants were informed and educated about different aspects of MS-related fatigue. The interventions and approaches varied between programmes, with some studies employing components of psychological interventions. A CBT approach was used in five studies [17, 35-38]. CBT aims to identify and change unhelpful thinking to alleviate symptoms, in this case MS-related fatigue. Moreover, four interventions were based on an energy conservation approach [39, 44, 46, 47]. Energy conservation teaches techniques to identify and modify participants' activities through systematic analysis of work, home and leisure activities, to reduce fatigue. Kos et al. [40] used a behaviour-change multidisciplinary fatigue management programme educating participants about energy saving methods, medical treatment options, psychosocial support and physiotherapeutic approaches. Hugos et al. evaluated the ‘Fatigue: Take Control’ programme in two studies [41, 42] with adaptations between the two studies. Ehde et al. [45] used a self-management approach which provided education and promotes skill-building to help balance the physical, emotional and social aspects of living with a chronic illness. For additional information on the content of the respective programmes, see File S2 (TIDieR framework). All programmes used an integrated homework part.
Delivery of programmes
Ten programmes were delivered in a group setting, partly via telephone [35, 36, 39-42, 46-49]. Two studies employed a single-participant face-to-face approach [17, 45], and three studies used an online approach [37, 38, 43]. Duration of interventions ranged from 4 to 16 weeks; sessions were delivered once/twice a week. Follow-up duration ranged from 10 [47] to 52 [46] weeks.
Participants
Participant numbers ranged from 23 [47] to 275 [37]. All participants had a diagnosis of MS and substantial fatigue at baseline measured with different fatigue scales (i.e., FSS, FIS, Fatigue Scale, Fatigue Scale of Motor and Cognitive Functions). In the study by Ghahari et al. [43], nearly 80% of participants were diagnosed with MS, whilst smaller groups of patients with Parkinson's disease and the post-polio syndrome were also included.
Control groups
Five studies [42, 43, 45, 46, 50] used an information-only intervention as comparator, whilst two studies [34, 49] provided control patients with relaxation training and one study [47] used a peer support group as control. A wait-list-control approach was employed in four studies [38, 39, 41, 44]. Three other programmes used a usual care group as control [36, 38, 44].
Methods of data collection
In all studies, primary and secondary outcomes were assessed using patient-reported outcome measures (scales and questionnaires), most of which had been validated in the target language of the respective population.
Risk of bias in included studies
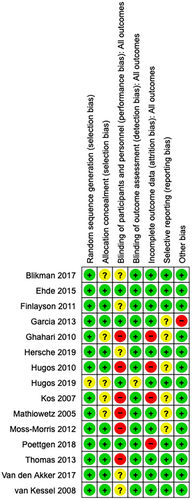
Overall, only one study was of high methodological quality [45]. All other studies had at least one unclear or high risk of bias (Figure 2).
Primary outcome measures
Fatigue severity
The meta-analysis of fatigue severity directly after the intervention including eight studies with 878 patients favoured the intervention with an SMD of −0.28 (95% CI −0.53 to −0.03), I2 = 65% [35-38, 44, 46, 47, 50]. Therefore, this meta-analysis suggested that patient education programmes may slightly reduce fatigue severity in patients with MS directly after the intervention (low certainty evidence, see Figure 3a). Additionally, but not included in the meta-analysis, Kos et al. [40] did not find significant changes for fatigue severity directly after the intervention compared to placebo.
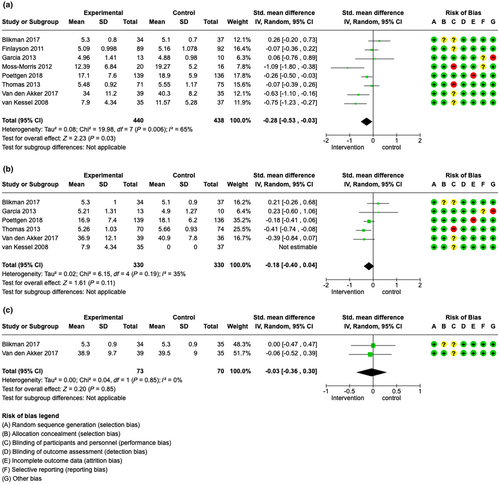
At the 2–4-month follow-up, data of six studies [35-37, 44, 46, 50] (660 patients overall) were available and favoured the intervention with an SMD of −0.18 (95% CI −0.40 to 0.04), I2 = 35%. Education programmes may therefore slightly reduce fatigue severity in patients with MS after 2–4 months (moderate certainty evidence, see Figure 3b).
Insufficient data were available for meta-analysis at 6–8-months’ follow-up. van Kessel et al. [35] and colleagues reported a reduction of fatigue severity (intervention group, IG 10.37 (SD 6.37); control group, CG 12.49 (SD 5.24)).
At long-term follow-up, data of two studies [46, 50] were available for meta-analysis. Here, studies with data of 143 patients favoured the intervention with an SMD of −0.03 (95% CI −0.36 to 0.30), I2 = 0% (see Figure 3c). Interestingly, Kos et al. [40] reported a reduction of fatigue in the intervention group compared to the control group after 6 months, whilst no change in fatigue severity was documented directly after the intervention.
Fatigue impact
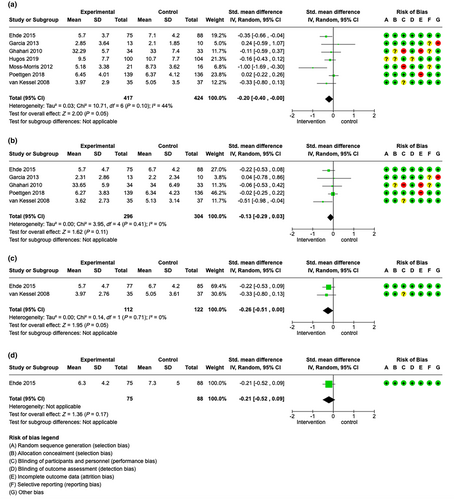
Nine studies (824 patients) were included that evaluated fatigue impact directly after the intervention in the meta-analysis [38, 40, 42-47, 49], favouring the intervention with an SMD of −0.21 (95% CI −0.42 to 0.00), I2 = 48%. This meta-analysis suggested that patient education programmes may slightly reduce fatigue impact directly after the intervention (moderate certainty evidence, see Figure 4a). Moreover, the following data were available but not included in the meta-analyses: Mathiowetz et al. [39] reported a significant reduction in fatigue impact levels directly after the intervention (overall FIS reduction p = 0.0014); Kos et al. [40] reported no significant change in fatigue impact levels directly after the intervention.
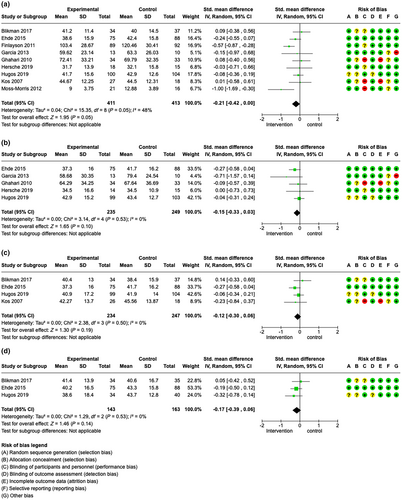
At 2–4 months’ follow-up, the meta-analysis of five studies (484 patients) [41, 43, 45, 47] favoured the intervention with an SMD of −0.15 (95% CI −0.33 to 0.03), I2 = 0%, suggesting that education programmes may slightly reduce fatigue impact in patients with MS (moderate certainty evidence, see Figure 4b).
At 6–8-months’ follow-up, meta-analysis including four studies and 481 patients [40, 42, 45, 46] favoured the intervention with an SMD of −0.12 (95% CI –0.30 to 0.06), I2 = 0% (see Figure 4c). Notably, Kos et al. [40], whose study had not shown a difference directly after the intervention, also reported a significant reduction of fatigue impact scores after 6 months.
Finally, at long-term follow-up, the meta-analysis comprising three studies (306 patients) [42, 45, 46] favoured the intervention with an SMD of −0.17 (95% CI −0.39 to 0.06), I2 = 0% (see Figure 4d).
Adverse events
Six studies [36, 37, 44-46, 50] assessed adverse events, with only two studies detecting adverse events (increase in fatigue and anxiety [37], MS relapses during the programme and non-MS related surgeries [50]); the other studies did not report the occurrence of any adverse events.
Secondary outcome measures
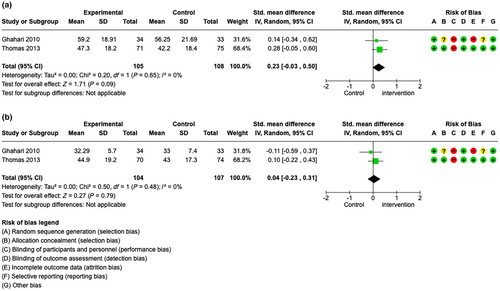
The results of the secondary outcome measures are reported in File S3. In brief, some evidence was found that fatigue education programmes may also slightly reduce depressive symptoms up to 12 months after the intervention (Figure 5a–d). For QoL, meta-analyses of two studies (Figure 6a,b) and individual studies found mixed results. For coping/self-efficacy, no meta-analysis was possible. Individual studies showed mixed results.
Subgroup analyses
Subgroup analyses were performed with the aim of detecting differences between groups using different approaches and different settings, as well as different intervention modalities, on both fatigue severity and fatigue impact.
Group versus single-patient approaches
For fatigue severity, directly post-intervention, data from six studies were available for comparison, which favoured the single-intervention approach (Figures S1–S5, 1.1). At the 2–4-month follow-up, data from four studies were available, showing a slightly pronounced effect of single-patient interventions on fatigue severity (Figures S1–S5, 1.2). At later time points, no data were available for comparison.
For fatigue impact, directly after the intervention data from nine studies were available for comparison, favouring the single-patient interventions (Figure S1b, 1.3). At the 2–4-month follow-up, the single-patient studies also showed a greater effect than the group-intervention-based studies (Figure S1b, 1.4). Interestingly, the long-term follow-up favoured the group interventions (Figure S1b, 1.5).
Cognitive behavioural therapy versus non-CBT-based interventions
Comparing CBT-based approaches with other approaches, the CBT-based interventions showed a greater effect on both fatigue severity and fatigue impact directly after the intervention (for fatigue severity, see Figure S2a, 2.1; for fatigue impact, see Figure S2b, 2.3). At follow-up, comparable data were only available for fatigue severity after 2–4 months (Figure S2a, 2.2). Here, the effect of CBT-based approaches was also more pronounced than that of the non-CBT interventions. For later time points, no comparable data were available.
Face-to-face versus online versus telephone approaches
Here, the mode of delivery of the programmes to assess the potential impact on fatigue was compared. The greatest effect on both fatigue impact and fatigue severity was shown by the programmes using an online intervention when assessed directly after the intervention (Figure S3a, 3.1 and S3b, 3.3). However, at the 2–4-month follow-up (Figure S3a, 3.2), this remained only for fatigue severity whilst fatigue impact was reduced to the greatest extent in the telephone group (Figure S3b, 3.4). Interestingly, the personal contact groups were consistently associated with the smallest changes in difference in fatigue severity and fatigue impact over all time points (insufficient data were available for the 6- and 12-month follow-ups) (Figure S3a,b).
Short-term versus long-term approaches
Directly after the intervention, long-term approaches achieved greater reductions of fatigue compared to those with a medium or short duration (defined as >6 weeks, 6 weeks and <6 weeks' duration of intervention) (Figure S4a,b). This effect, however, did not remain for the 2–4-month follow-up of either fatigue severity or fatigue impact, nor for the longer follow-up time points where better or similar results were achieved by the studies with a shorter intervention duration (Figure S4a,b).
Usual care versus sham intervention
At all available time points, the group of studies that used a sham intervention showed smaller differences in both fatigue severity and fatigue impact reduction than the group of studies that used a usual care group (Figure S5a,b).
Sensitivity analyses
Results from the sensitivity analyses excluding studies with increased risk of bias due to incomplete data reporting are given in File S4.
DISCUSSION
This systematic review assessed the effects of patient education programmes for reducing fatigue in people with MS. The 15 identified studies used a variety of approaches in terms of both intervention programmes and study methodology. Programmes varied in terms of duration, session length and session number per week, mode of delivery (face-to-face, telephone, online) and number of participants (single sessions, group sessions). Furthermore, the content of the programmes varied; whilst some approaches were partially overlapping and using the same programme basis or theory, some were distinct. Most commonly, energy conservation strategies and CBT approaches in various combinations were used. Overall, the included studies showed a slight effect on fatigue severity and fatigue impact, as well as on depressive symptoms, directly after the intervention and after 3 months. Some studies reported follow-up data of up to 12 months after the intervention, with fewer participants and therefore lower confidence in study results. Our review results showed no clear indication of adverse events.
Subgroup analyses showed greater benefit of programmes using a CBT-based approach in comparison to programmes not explicitly using CBT techniques, directly after the intervention and after 3 months. This finding is in line with a previous review [23]. Further, the programmes with a single-participant approach might reduce fatigue more effectively compared to group approaches. A possible explanation for this finding is that the individual participant may benefit more from personalization of the programme and more pronounced tailoring. Comparing the mode of delivery, programmes using an online or telephone approach showed better results in reducing fatigue compared to face-to-face sessions. A possible reason for this could be the reduced effort to travel to the respective sessions, which may cause stress and therefore worsen fatigue. Additionally, online interventions may better allow participants to work in their own time, and to take pauses at will. Also, the duration of the intervention seemed to somehow impact the reduction of fatigue. Longer duration of an intervention (>6 weeks duration) showed a greater reduction of fatigue levels. This may be explained by an increased amount of therapist time, attention and practice of newly acquired fatigue reduction strategies. This result, however, was only present directly after the interventions; later follow-up time points did not show any difference compared to control groups. Moreover, the reduction of fatigue was more pronounced when compared to a usual care group compared to an active control group, probably indicating an ameliorating effect of the active control groups on fatigue. The certainty of evidence ranged from very low to moderate, mainly due to performance and attrition bias, as well as to inconsistency.
The impact of different psychological interventions on MS-related fatigue has been investigated in several previous reviews in which, to a certain degree, similar results were found. Wendebourg et al. [23] found a greater impact on fatigue reduction of CBT interventions over other approaches. Furthermore, interventions delivered to individuals seemed to be more efficacious than group interventions. The positive effect of combined CBT plus energy conservation compared to energy conservation therapy alone was also described by Harrison et al. [19]. Asano et al. [21] identified four characteristics of patient education programmes that were associated with a significant reduction of fatigue (at least 6 weeks of programme, 1 session per week, no less than 50 min duration per session, and a trained facilitator). The same characteristics were described in a recent review by Askari et al. [18]. Additionally, the use of a printed guiding handout was considered as beneficial for a significant fatigue reduction. In a mixed population review, Hersche et al. [49] also found an advantage of longer duration interventions. Whilst in our meta-analysis a benefit of the longer duration interventions was also found, a relatively long duration per individual session might also bear the risk of increasing fatigue; however, this question was not examined in the present review.
Limitations
Several limitations to our study must be acknowledged. Although the studies were deemed as sufficiently homogeneous to perform meta-analyses, there was a certain level of heterogeneity amongst both the studied patient groups and the programmes, and not all studies reported sufficient data to be included in the meta-analyses, which limits the generalizability of our results.
Moreover, some studies were of low methodological quality, which further reduces the confidence in the review results' certainty. Due to a small number of studies available for pre-planned subgroup analyses, there is frequently low certainty about the relevance of specific intervention characteristics and components.
In this review, patients seemed to benefit more from interventions with a longer duration; therefore, interventions should ideally be at least 6 weeks or longer. This raises the question whether a prolonged effect might be achieved by the implementation of ‘booster sessions’, that is, sessions with a repetitive character after the end of the programme designed to refresh the gained knowledge. However, this question has not yet been systematically investigated and may present an interesting characteristic to be studied further. Long-term studies assessing the effect of education programmes up to 12 months are scarce; thus, again, further research is needed.
No ongoing studies were identified and therefore the review results will probably not be challenged by new study results soon. However, the results were limited by the fact that all included studies were conducted in high-income countries with comparatively high-resource availability. Thus, the transferability of our findings beyond these populations is unclear.
CONCLUSION
The current evidence shows a small beneficial effect of various education programmes on fatigue severity and impact. Whilst the included programmes varied substantially in content, duration and mode of delivery, they seem to present a feasible and safe approach to reduce fatigue in patients with MS. Since most evaluated programmes showed some benefit on fatigue, adaptations of programmes to fit into different models of clinical MS patient care also seem feasible.
AUTHOR CONTRIBUTIONS
Maria Janina Wendebourg: Investigation; writing – original draft; visualization; writing – review and editing; formal analysis; data curation; project administration; software. Jana Poettgen: Conceptualization; writing – review and editing. Marcia Finlayson: Writing – review and editing. Marien Gonzalez-Lorenzo: Methodology; writing – review and editing. Christoph Heesen: Conceptualization; writing – review and editing. Sascha Köpke: Conceptualization; investigation; writing – original draft; writing – review and editing; methodology; visualization; data curation; supervision; formal analysis; validation; software. Andrea Giordano: Conceptualization; writing – original draft; writing – review and editing; methodology; data curation; formal analysis; software.
ACKNOWLEDGEMENTS
Andrea Fittipaldo (Department of Oncology, Laboratory of Clinical Research Methodology, Istituto di Ricerche Farmacologiche Mario Negri IRCCS, Milan, Italy), information specialist, is thanked for her support in developing and running the search strategy. Open Access funding enabled and organized by Projekt DEAL.
FUNDING INFORMATION
The authors received no funding for this study.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
All data used in this review are either published in the included studies or available from the respective primary studies' authors.




