Erenumab versus topiramate: migraine-related disability, impact and health-related quality of life
Abstract
Background and purpose
HER-MES was the first head-to-head study of erenumab against topiramate (standard of care). This post hoc analysis of the HER-MES study evaluated the effect of erenumab versus topiramate on patient-reported outcomes at week 24.
Methods
Adult patients with episodic or chronic migraine (n = 777) were randomized (1:1) to monthly subcutaneous erenumab (n = 389) or daily oral topiramate (n = 388). Migraine-related disability, as measured by the Headache Impact Test 6 (HIT-6) and Short Form 36 Health Survey version 2 (SF-36v2), was analysed in the entire study cohort and true completers.
Results
In the erenumab group (vs. topiramate), significant improvements were reported in Headache Impact Test 6 total scores (composite populations, −10.88 vs. −7.72; true completers, −11.92 vs. −10.61) and a higher proportion of patients achieved a ≥5-point reduction from baseline with erenumab (composite populations, 72.2% vs. 53.9%; true completers, 79.64% vs. 71.43%). The adjusted mean change from baseline in the SF-36v2 score was greater with erenumab for both physical component summary (composite population, 5.48 vs. 3.63; true completers, 5.95 vs. 5.23) and mental component summary (composite populations, 1.00 vs. −1.18; true completers, 1.74 vs. −0.33). A higher proportion of patients on erenumab versus topiramate had a ≥5-point improvement in SF-36v2 for the physical component summary (composite populations, 47.7% vs. 37.4%; true completers, 52.1% vs. 48.9%) and mental component summary (composite populations, 25.3% vs. 16.8%; true completers, 27.3% vs. 17.7%).
Conclusions
This post hoc analysis demonstrated that patients treated with erenumab had significant improvements in headache impact and quality of life as measured by patient-reported outcomes versus patients treated with topiramate.
INTRODUCTION
A variety of non-specific prophylactic drugs, including beta-blockers, topiramate, valproate, anti-depressants (mainly amitriptyline) and flunarizine, are currently being used for the treatment of migraine. These therapies have insufficient or limited evidence and may commonly be associated with variable efficacy and/or substantial tolerability issues that often lead to treatment discontinuation in patients with migraine [1-3]. Research shows that the administration of erenumab not only reduces migraine frequency but also improves health-related quality of life (HRQoL), as well as migraine-related disability and impact [4-8]. HER-MES was the first randomized, double-blind, double-dummy, head-to-head, 24-week, phase 4 trial that assessed the tolerability and effectiveness of an anti-calcitonin gene-related peptide receptor (CGRP-R) antibody (erenumab) against an oral standard-of-care migraine prophylactic treatment (topiramate). Topiramate is a non-specific oral migraine preventive medication that was not specifically developed for the treatment of migraine [9-11]. In contrast, erenumab, which acts against the CGRP-R, was specifically developed to target migraine pathophysiology [4-6, 12].
The updated guidelines of the European Headache Federation and the American Headache Society recommend the use of monoclonal antibodies that target CGRP as the first-line therapy for migraine prevention [13, 14]. In Germany, erenumab is now considered a first-line therapy for migraine prevention due to the results of the HER-MES trial. However, failure of prior preventive medication is still required for reimbursement for any CGRP(-R) monoclonal antibody in migraine patients in most European countries [15, 16]. Topiramate is used as the standard of care for migraine in many countries, although recent European Medicines Agency (EMA) recommendations suggest avoiding topiramate in women of fertile age. Topiramate is no longer considered as first-line preventive treatment for migraine in this population [17]. Nonetheless, topiramate was still recommended for patients with migraine in many countries such as Germany at the time of the start of the study.
The results of the HER-MES study in composite populations (i.e., patients on therapy and patients who had stopped medication but continued daily reporting) showed that erenumab provides additional benefit that leads to improved persistence, favourable tolerability and a sustained efficacy profile compared with topiramate [16]. Furthermore, the results of a post hoc analysis of the HER-MES study in patients who tolerated the medication and were able to complete the study treatment (completers) demonstrated significantly superior efficacy of erenumab versus topiramate in achieving a ≥50% reduction in monthly migraine days (MMD), with an early onset of efficacy [18].
International treatment guidelines state that preventive treatments for migraine must restore the patients' ability to function in addition to reducing the duration, frequency and intensity of migraine attacks [19]. Hence, evaluation of patients who reported headache-related functional impairment becomes an important component of migraine management; this can be measured using patient-reported outcomes (PROs), which assess the impact of migraine on how patients feel, function and live their lives [20, 21]. Preventive migraine therapies that show meaningful reductions in MMD and monthly headache days, along with a reduction in disability and improvement in PROs, are an ideal treatment option for patients with migraine [7, 8, 22].
The HER-MES study has previously demonstrated that erenumab significantly improved the quality of life of patients with migraine compared with topiramate. However, the PRO analysis was performed in composite populations [16]. Here, the results are presented of a post hoc analysis of the HER-MES study, which was performed to compare PROs in patients treated with erenumab or topiramate who completed the trial on the study medications, thus mimicking the real-world condition through a randomized, controlled study design.
METHODS
Study design and patient population
HER-MES was a randomized, double-blind, double-dummy, active-controlled, parallel-group, phase 4 trial conducted at 82 study sites in Germany. The details of the study design and eligibility criteria have been published previously [16]. Briefly, the study included a screening period (2 weeks), a baseline period (4 weeks), a double-blind treatment phase (DBTP) (24 weeks) and a safety follow-up period (4 weeks). In the DBTP, patients were randomized (1:1) to either the topiramate (topiramate verum and erenumab placebo) or erenumab group (erenumab verum and topiramate placebo). Novartis provided the investigational medicinal products in a double-dummy setting, that is, the identity of treatments was concealed by the use of study drugs and matching placebo that were identical in packaging, labelling, schedule of administration, appearance, taste and odour [16].
The eligibility criteria for HER-MES have been described in detail previously [16]. Adults aged 18–65 years with a history of episodic migraine or chronic migraine, as defined by the International Classification of Headache Disorders, 3rd edition, for at least 12 months prior to screening were included in the study.
This study assesses PROs in three subgroups of patients including composite populations, completers and true completers. Composite populations included patients on therapy and patients who had stopped medication but continued daily reporting. A patient was defined as a completer who tolerated the medication and was able to complete the study treatment. True completers were defined as patients who tolerated the study medication and completed the DBTP on study medication without multiple imputations (true completers).
Ethics approval
The study was conducted in accordance with the International Council for Harmonization Guideline for Good Clinical Practice, applicable local regulations and guidelines, and the ethical principles laid down in the Declaration of Helsinki. All patients provided written informed consent before participation in the study. The final study protocol, informed patient consent forms and accompanying materials were reviewed and approved by the relevant ethics committees/institutional review boards at each participating site.
Outcomes
The primary (tolerability) and secondary (efficacy) endpoint outcomes in the intention-to-treat analysis of the HER-MES study have been reported previously [16]. This post hoc analysis assessed the changes in the Headache Impact Test 6 (HIT-6) and Short Form 36 Health Survey version 2 (SF-36v2). The analysis was performed on the full analysis set population, which consisted of all randomized patients who received at least one dose of double-blind study medication. The prespecified outcomes were the numerical change in HIT-6 and SF-36v2 scores at week 24 compared with baseline, and the proportions of patients achieving at least a 5-point improvement in HIT-6 and SF36-v2 scores at week 24 compared with baseline.
In addition, this study presents these outcomes for the subgroup of patients who tolerated the study medication and completed the DBTP on study medication without multiple imputations (true completers).
The HIT-6
The HIT-6 is a short-form self-administered questionnaire based on the internet-HIT question pool [23]. The HIT-6 was developed as a global measure of adverse headache impact to assess headache severity in the previous month and the change in a patient's clinical status over a short period of time. Six items assess the frequency of pain severity, headaches limiting daily activity (household, work, school and social), wanting to lie down when a headache is experienced, feeling too tired to work or do daily activities because of a headache, feeling ‘fed up’ or irritated because of a headache, and headaches limiting the ability to concentrate or work on daily activities. Each of the six questions had five response categories with a particular score assigned to the response provided, of which one was picked: ‘never’ (6 points), ‘rarely’ (8 points), ‘sometimes’ (10 points), ‘very often’ (11 points) or ‘always’ (13 points). These points are summed to produce a total HIT-6 score that ranges from 36 to 78. The HIT-6 scores were then categorized into four grades, representing little or no impact (≤49), some impact (50–55), substantial impact (56–59) and severe impact (60–78) due to headache. No recall period was specified for the first three items. For the last three items, the recall period was the past 4 weeks. Patients completed this in their eDiary during their scheduled clinic visit.
The SF-36v2
The SF-36v2 is a widely used and extensively studied instrument to measure HRQoL amongst healthy subjects and patients with acute and chronic conditions. SF-36v2 consists of eight subscales: physical functioning, role-physical, bodily pain, general health, vitality, social functioning, role-emotional and mental health [24, 25]. Two overall summary scores, the physical component (PC) summary and the mental component (MC) summary, can also be computed. In this study, SF-36v2 was used to assess the HRQoL of patients. Given the nature of migraine and the 4-weekly assessment, SF-36v2 (with a 4-week recall period) was used in this study.
Statistical analysis
The post hoc analysis was performed on the full analysis set population, and patients were analysed based on their randomized treatment irrespective of the actual treatment received.
In addition, the PROs of erenumab versus topiramate were evaluated in patients who completed treatment with the study medication (true completers). However, this approach would impair randomization because of the unequal discontinuation rates between erenumab and topiramate. To preserve the randomization, multiple imputation was conducted based on the data of patients who stayed on the study medication during the entire 24 weeks of the treatment phase. For patients who discontinued the study medication, prospective data were imputed as if they had been able to stay on medication. Missing values from patients who discontinued the study were also imputed. PROs were analysed using the linear mixed model for repeated measures. The mixed model for repeated measures included treatment group, baseline value, stratification factor, scheduled visit, and the interaction between treatment group and scheduled visit. For patients who achieved at least a 5-point reduction (clinically meaningful) from baseline to week 24 in HIT-6 scores and SF-36v2 scores, the odds ratio (OR) was calculated using a logistic regression model with categorical variables of treatment and stratification factor (MMD at baseline).
Statistical analyses were performed using SAS (version 9.2). Since erenumab and topiramate were already on the market, no data monitoring committee was required.
RESULTS
Demographics and disease characteristics
In the HER-MES study, 777 patients were randomized to either the erenumab group (n = 389) or the topiramate group (n = 388). One patient in the erenumab group did not receive study medication and was therefore excluded from the analysis. The treatment groups, reasons for treatment or study discontinuation, and patients included in the post hoc analysis were reported previously [18].
Overall, 565 of the 777 patients (72.7%) completed the DBTP and 739 (95.1%) completed the study. The study was prematurely terminated by 16 of 389 patients in the erenumab group and 22 of 388 patients in the topiramate group; 39 of 389 in the erenumab group and 135 of 388 in the topiramate group discontinued the study medication but completed the study. Baseline and disease characteristics across treatment groups were well balanced. The mean dose for patients who remained on study medication until the end of the DBTP was 119 mg/month for erenumab (n = 334) and 92 mg/day for topiramate (n = 231). The mean (standard deviation, SD) age of all patients was 40.7 (12.4) years. At baseline, the mean (SD) MMD was 10.3 (4.0) days in the erenumab group, 10.5 (3.8) days in the topiramate group and 10.4 (3.9) days in all patients. The mean (SD) HIT-6 score at baseline was 63.65 (4.20) in the erenumab group and 63.89 (4.03) in the topiramate group. The mean (SD) SF-36v2 scores at baseline were comparable between the two groups for both the PC summary (erenumab group, 45.26 [7.13]; topiramate group, 44.79 [7.26]) and MC summary (erenumab group, 51.63 [8.43]; topiramate group, 52.07 [8.08]). Baseline PRO scores of completers were also comparable between the treatment groups (Tables 1 and 2).
| Baseline demographics and clinical characteristics | |||
|---|---|---|---|
| Erenumab (N = 388) | Topiramate (N = 388) | All patients (N = 776) | |
| Age, years, mean (SD) | 40.8 (12.4) | 40.7 (12.4) | 40.7 (12.4) |
| Male, n (%) | 57 (14.7) | 53 (13.7) | 110 (14.2) |
| Female, n (%) | 331 (85.3) | 335 (86.3) | 666 (85.8) |
| Race, n (%) | |||
| Caucasian | 383 (98.7) | 387 (99.7) | 770 (99.2) |
| Asian | 1 (0.3) | 0 | 1 (0.1) |
| Unknown | 1 (0.3) | 0 | 1 (0.1) |
| Other | 3 (0.8) | 1 (0.3) | 4 (0.5) |
| Weight (kg) | 73.3 (17.9) | 72.7 (17.5) | 73.0 (17.7) |
| Height (cm) | 169.0 (7.8) | 169.2 (7.4) | 169.1 (7.6) |
| Body mass index, kg/m2 | 25.6 (5.6) | 25.3 (5.6) | 25.5 (5.6) |
| Monthly migraine days, mean (SD) | 10.3 (4.0) | 10.5 (3.8) | 10.4 (3.9) |
| Monthly headache days, mean (SD) | 11.4 (4.2) | 11.5 (4.1) | 11.5 (4.2) |
| Acute headache medication use, n (%) | |||
| Any acute medication | 378 (97.4) | 378 (97.4) | 756 (97.4) |
| Migraine specific | 304 (78.4) | 320 (82.5) | 624 (80.4) |
| Monthly migraine days categories, n (%) | |||
| 4–7 monthly migraine days | 94 (24.2) | 92 (23.7) | 186 (24.0) |
| 8–14 monthly migraine days | 248 (63.9) | 254 (65.5) | 502 (64.7) |
| ≥15 monthly migraine days | 43 (11.1) | 42 (10.8) | 85 (11.0) |
| Patient-reported outcomes at baseline, mean (SD) | |||
|---|---|---|---|
| Erenumab (N = 388) | Topiramate (N = 388) | ||
| HIT-6 numerical | 63.65 (4.20) | 63.89 (4.03) | |
| HIT-6 completer numeric | 63.64 (4.20) | 63.86 (4.08) | |
| SF-36v2 numerical PCS | 45.26 (7.13) | 44.79 (7.26) | |
| SF-36v2 numerical MCS | 51.63 (8.43) | 52.07 (8.08) | |
| SF-36v2 numerical completer PCS | 45.28 (7.08) | 44.78 (7.20) | |
| SF-36v2 numerical completer MCS | 51.45 (8.53) | 52.10 (8.03) | |
- Abbreviations: HIT-6, Headache Impact Test 6; MCS, mental component summary; PCS, physical component summary; SD, standard deviation; SF-36v2, Short Form 36 Health Survey version.
Patient-reported outcomes based on the composite study populations
The HIT-6
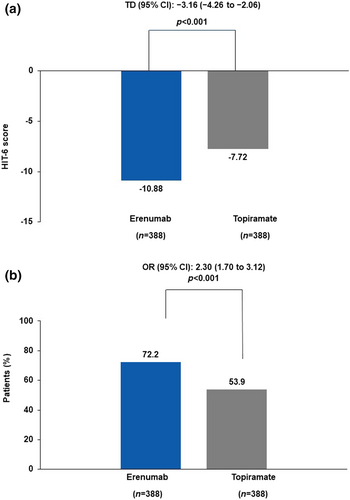
At week 24, the adjusted mean change from baseline in the HIT-6 total numerical score was −10.88 (0.44) for the erenumab group and −7.72 (0.44) for the topiramate group. A statistically significant difference in the adjusted mean was observed between the erenumab and topiramate groups, −3.16 (95% confidence interval [CI] −4.26 to −2.06; p < 0.001; Figure 1a). The proportion of patients with at least a 5-point reduction from baseline in the HIT-6 total numerical score at week 24 was 72.2% (280/388) in the erenumab group compared with 53.9% (209/388) in the topiramate group (OR 2.30; 95% CI 1.70–3.12; p < 0.001; Figure 1b).
The SF-36v2
At week 24, the adjusted mean change from baseline in the SF-36v2 PC numerical scores was 5.48 (0.36) for the erenumab group and 3.63 (0.36) for the topiramate group. The difference in the adjusted mean for erenumab versus topiramate was statistically significant (1.86; 95% CI 0.96–2.75; p < 0.001; Figure 2a). The proportion of patients with at least a 5-point increase from baseline in the monthly average PC domain numerical scores at week 24 was 47.7% (185/388) in the erenumab group and 37.4% (145/388) in the topiramate group (OR 1.77; 95% CI 1.29–2.43; p < 0.001; Figure 2b).
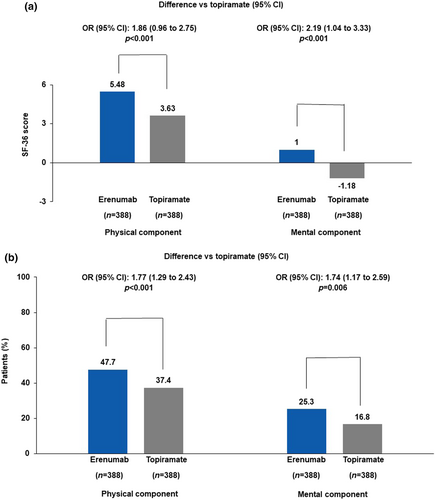
At week 24, the adjusted mean change from baseline in the SF-36v2 MC numerical scores was 1.00 (0.46) for the erenumab group and −1.18 (0.46) for the topiramate group. A difference in the adjusted mean for erenumab versus topiramate was statistically significant (2.19; 95% CI 1.04–3.33; p < 0.001; Figure 2a). The proportion of patients with at least a 5-point increase from baseline in the monthly average MC domain numerical scores at week 24 was 25.3% (98/388) in the erenumab group and 16.8% (65/388) in the topiramate group (OR 1.74; 95% CI 1.17–2.59; p = 0.006; Figure 2b).
Change in PROs in completers of the DBTP
The HIT-6
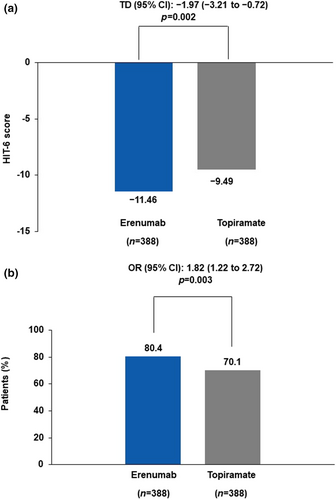
At week 24, the adjusted mean change from baseline in the HIT-6 total numerical score was −11.46 (0.44) for the erenumab group and −9.49 (0.51) for the topiramate group, and the adjusted mean difference was −1.97 (95% CI −3.21 to −0.72; p = 0.002) for the erenumab group versus the topiramate group (Figure 3a). The proportion of patients with at least a 5-point reduction from baseline in HIT-6 total numerical score at week 24 was 80.4% (312/388) in the erenumab group and 70.1% (272/388) in the topiramate group (OR 1.82; 95% CI 1.22–2.72; p = 0.003; Figure 3b).
The SF-36v2
At week 24, the adjusted mean change from baseline in the SF-36v2 PC numerical scores was 5.78 (0.36) for the erenumab group and 4.88 (0.40) for the topiramate group, with adjusted means of 0.90 (95% CI −0.07 to 1.86; p = 0.070) for erenumab versus topiramate (Figure 4a). The proportion of patients with at least a 5-point increase from baseline in the monthly average PC domain numerical scores at week 24 was 53.1% (206/388) in the erenumab group and 51.0% (198/388) in the topiramate group (OR 1.20; 95% CI 0.84–1.71; p = 0.319; Figure 4b).
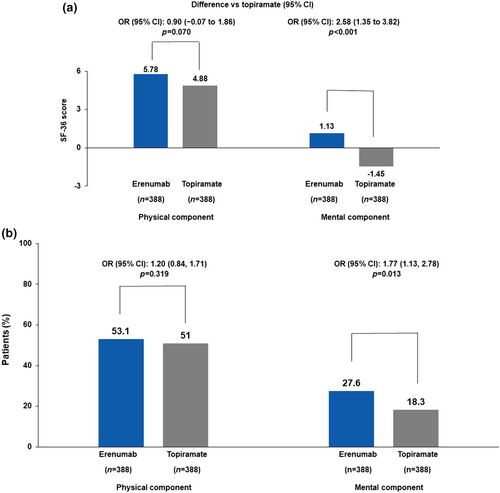
At week 24, the adjusted mean change from baseline in the SF-36v2 MC numerical scores was 1.13 (0.46) for the erenumab group and −1.45 (0.51) for the topiramate group. A significant difference in the adjusted mean was observed for erenumab versus topiramate (2.58; 95% CI 1.35–3.82; p < 0.001; Figure 4a). The proportion of patients with at least a 5-point increase from baseline in the monthly average MC domain numerical scores at week 24 was 27.6% (107/388) in the erenumab group and 18.3% (71/388) in the topiramate group (OR 1.77; 95% CI 1.13–2.78; p = 0.013; Figure 4b).
Patient-reported outcomes based on true completers of the DBTP
The HIT-6
In patients who were true completers, the adjusted mean change from baseline to week 24 in the HIT-6 total score was −11.92 (7.8) for the erenumab group (n = 328) and −10.61 (7.9) for the topiramate group (n = 228). The proportion of patients with at least a 5-point reduction from baseline in the HIT-6 total score at week 24 was 79.6% (266/334) in the erenumab group and 71.4% (165/231) in the topiramate group.
The SF-36v2
At week 24, the adjusted mean change from baseline in the SF-36v2 PC numerical scores was 5.95 (6.8) for the erenumab group (n = 327) and 5.23 (7.8) for the topiramate group (n = 228). The proportion of patients with at least a 5-point increase from baseline in the monthly average PC domain numerical scores at week 24 was 52.1% (174/334) in the erenumab group and 48.9% (113/231) in the topiramate group.
At week 24, the adjusted mean change from baseline in the SF-36v2 MC numerical scores was 1.74 (8.1) for the erenumab group (n = 327) and −0.33 (8.2) for the topiramate group (n = 228). The proportion of patients with at least a 5-point increase from baseline in the monthly average MC domain numerical scores at week 24 was 27.2% (91/334) in the erenumab group and 17.7% (41/231) in the topiramate group.
DISCUSSION
The measurement of HRQoL, as assessed by HIT-6 and SF-36v2 scores, is an important indicator of efficacy and tolerability. The results of the post hoc analysis of PROs in this study showed that the treatment response with erenumab was better than that with topiramate for all analysed subgroups. It is noteworthy that patients treated with erenumab showed numerical superior improvements in all PROs compared to those treated with topiramate. However, the focus of this post hoc analysis was to demonstrate whether the improvement in PROs in patients who have tolerated and completed 24 weeks of treatment with the study medication (completers) is superior in the erenumab group compared to the topiramate group. To perform this analysis, only the PRO values of patients who were on study medication were included. There was an approximately 18% difference between the erenumab (72.2%) and topiramate (53.9%) groups in the proportion of patients with at least a 5-point reduction from baseline in HIT-6 total score at week 24. In the analysis of the completers, there was approximately a 10% difference between the erenumab (80.4%) and topiramate (70.1%) groups in the proportion of patients who achieved at least a 5-point reduction in the HIT-6 score. These results suggest that topiramate is an effective preventive therapy for migraine; however, erenumab is superior to topiramate in migraine prevention as corroborated by a greater improvement in the HRQoL. The results of this post hoc analysis using the multiple imputation approach showed that, other than the change from baseline in the adjusted mean of the SF-36v2 PC scores, all PROs were similar to the results in true completers, whilst having the advantage of preserving the randomization of the study population. In addition, the HER-MES study assessed the tolerability and safety of erenumab compared with topiramate in patients with migraine in Germany [16]. Although the safety and efficacy of topiramate has been demonstrated in pivotal clinical trials [9-11], adherence to and persistence of topiramate amongst patients with migraine is low due to inadequate efficacy and poor tolerability [26-28]. The ATTAIN study, which analysed the pattern of preventive treatment in the real-world setting before the approval of CGRP monoclonal antibodies, revealed that topiramate was the most commonly used preventive treatment (42.7%). Approximately half the patients reported modification of treatment by decreased frequency, treatment discontinuation or skipping doses. The main reasons for treatment modification were side effects and inadequate efficacy. The rate of treatment discontinuation for topiramate was 69% compared with 59% for the overall population, suggesting poor tolerability with topiramate [28]. Similarly, in the HER-MES study, the rate of treatment discontinuation was greater with topiramate than with erenumab (38.9% vs. 10.6%) and was attributed to adverse events [16]. These findings corroborate the previous clinical findings and provide evidence that treatment with erenumab provides greater adherence, persistence and sustained improvement in quality of life.
Limitations
This study has limitations. Although PRO measures for HIT-6 and SF-36 were assessed, including other migraine-specific PROs like Migraine Disability Assessment or Migraine-Specific Quality of Life could have provided a more comprehensive understanding of the disability and quality of life burden associated with migraine. These tools are designed to capture different aspects of the impact of migraine on patients' lives and could complement the HIT-6 and SF-36 measures. For future studies, it might be beneficial to consider a broader range of PROs to gain a more detailed insight into the patient experience.
CONCLUSION
The post hoc analysis of PROs in the HER-MES study showed a favourable and clinically meaningful treatment effect of erenumab versus topiramate on HRQoL. These results are complementary to the primary efficacy results and confirm that erenumab is a promising preventive therapy for migraine in the real world.
AUTHOR CONTRIBUTIONS
Uwe Reuter: Conceptualization; investigation; funding acquisition; writing – original draft; methodology; validation; writing – review and editing; visualization; software; formal analysis; project administration; data curation; supervision; resources. Christian Hentschke: Conceptualization; investigation; funding acquisition; methodology; validation; software; formal analysis; project administration; data curation; resources. Axel Heinze: Conceptualization; investigation; funding acquisition; writing – original draft; methodology; validation; writing – review and editing; software; formal analysis; project administration; data curation; resources. Astrid Gendolla: Conceptualization; investigation; funding acquisition; writing – original draft; methodology; validation; writing – review and editing; software; formal analysis; project administration; data curation; resources. Christian Sieder: Conceptualization; investigation; funding acquisition; writing – original draft; methodology; validation; writing – review and editing; resources; project administration; formal analysis; software; data curation.
ACKNOWLEDGEMENTS
The authors would like to thank the patients and investigators involved in the study, as well as Marc Ehrlich from Novartis Ireland Ltd and Preethi Bheereddy and Rachna Shukla of Novartis Healthcare Pvt Ltd, Hyderabad, India, for providing medical writing support for the manuscript. Open Access funding enabled and organized by Projekt DEAL.
FUNDING INFORMATION
The authors disclosed receipt of the following financial support for the research, authorship and/or publication of this article. This study was funded by Novartis Pharma GmbH, Nuremberg, Germany, which designed the study in collaboration with the investigators and provided the investigational medicinal products in a double-dummy setting, funded data analysis by Winicker Norimed GmbH and medical writing support of the methods and results sections, which was done under the directions of the authors. Novartis had no role in the data collection. Employees of Novartis contributed to data interpretation and writing of the report in their role as authors. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. The funder had a role in the design and conduct of the study; collection, management, analysis and interpretation of the data; and preparation, review or approval of the manuscript. All authors had the final responsibility for the decision to submit the manuscript for publication.
CONFLICT OF INTEREST STATEMENT
Uwe Reuter reports grants, personal fees and other from Novartis; personal fees and other from Amgen during the conduct of the study; personal fees and other from AbbVie; grants, personal fees and other from Allergan; other from Alder; personal fees and other from Eli Lilly; personal fees from Lundbeck; personal fees from Medscape and Perfood; and personal fees and other from Teva Pharmaceuticals, outside the submitted work. Axel Heinze reports personal fees and other from Novartis during the conduct of the study; personal fees and other from AbbVie, personal fees and other from Eli Lilly, personal fees and other from Lundbeck, personal fees and other from Novartis and personal fees and other from Teva Pharmaceuticals, outside the submitted work. Astrid Gendolla reports grants, personal fees and other from Grünenthal, Mundipharma, AbbVie/Allergan, Lilly, Teva, Amgen, Novartis, Hormosan, Stada, Lundbeck, Hexal, Esanum, Perfood, Medscape, Streamed up, Ärztekammer Nordrhein, Ärztekammer Westfalen Lippe and DGS. Christian Sieder and Christian Hentschke are employees of Novartis.
DATA AVAILABILTY STATEMENT
Data are available upon reasonable request. The study data for the analysis described in this report may be made available on request from the authors/investigators or Novartis Pharma GmbH, sponsor of this clinical research.
ETHICS STATEMENT
The study protocol, protocol amendments, the informed consent forms and accompanying materials were reviewed and approved by the Institutional Review Board/Independent Ethics Committee and Health Authorities at each study centre. All participants provided written informed consent before undergoing any study-specific procedures.
CONSENT FOR PUBLICATION
Consent for publication was received.
MEETING PRESENTATION
The data included in this study was partly presented as an e-poster at the 64th American Headache (AHS), Annual Scientific Meeting, 9–12 June 2022.
CLINICAL TRIAL REGISTRATION NUMBER
ClinicalTrials.gov NCT03828539.




