Association of serum uric acid to serum creatinine ratio with 1-year stroke outcomes in patients with acute ischemic stroke: A multicenter observational cohort study
Dandan Zhang and Zhongzhong Liu contributed equally to this work.
Abstract
Background and purpose
Considering the reliance of serum uric acid (SUA) levels on renal clearance function, its role in stroke outcomes remains controversial. This study investigated the association of renal function–normalized SUA (SUA to serum creatinine ratio, SUA/SCr), a novel renal function index, with the 1-year outcomes in patients with acute ischemic stroke (AIS).
Methods
This is a prospective, multicenter observational study. Renal function–normalized SUA levels were determined by calculating the ratio of SUA to SCr. One-year outcomes included stroke recurrence, all-cause mortality, and poor prognosis. Multivariable Cox regression analyses and restriction cubic splines for curve fitting were used to evaluate SUA/SCr's association with 1-year stroke outcomes.
Results
Among 2294 enrolled patients, after adjustment for potential confounders, multivariable Cox regression analyses showed that each one-unit increase in SUA/SCr corresponded to a 19% decrease in 1-year stroke recurrence in patients with AIS. SUA/SCr was analyzed as a continuous variable and categorized into quartiles (Q1–Q4). Compared with the Q1 reference group, Q2, Q3, and Q4 showed significantly lower 1-year stroke recurrence risks. The trend test indicated significant differences in the 1-year stroke recurrence trend from Q1 to Q4. In these patients, SUA/SCr did not show a significant association with poor prognosis or all-cause mortality. Curve fitting revealed SUA/SCr had a negative but nonlinear association with 1-year stroke recurrence.
Conclusions
In patients with AIS, low SUA/SCr may be an independent risk factor for 1-year stroke recurrence. Changes in SUA/SCr had no significant impact on 1-year poor prognosis and all-cause mortality.
INTRODUCTION
The latest Chinese stroke surveillance report (January 2019 to December 2020) showed that >86.9% of patients with stroke had ischemic stroke [1]. The 1-year disability, mortality, and recurrence rates were 14.0%, 8.5%, and 5.6%, respectively. Acute ischemic stroke (AIS) is the main cause of stroke patients, posing a major threat to public health [1]. Therefore, actively identifying and managing risk factors that affect stroke outcomes are of great significance.
Serum uric acid (SUA), the ultimate outcome of purine metabolism, also acts as an important endogenous antioxidant in the blood [2, 3]. Previous studies have produced conflicting results regarding the relationship between SUA levels at admission and acute stroke outcomes, with findings varying from positive outcomes [4-9] to insignificant association [10-12] and negative outcomes [13-15]. In addition, some studies have found a U-shaped association between excessively low or high SUA levels and worse stroke outcomes [16].
These controversial conclusions may be because the level of endogenous SUA concentration is highly dependent on renal clearance. Approximately two thirds of SUA is excreted through the kidneys [2]. When evaluating the association of SUA levels with stroke outcomes, it is necessary to consider the impact of renal excretion on SUA levels. Therefore, renal function–normalized SUA (SUA to serum creatinine ratio, SUA/SCr) is deemed to provide a more precise assessment of endogenous SUA concentrations. SUA/SCr is related to nonalcoholic fatty liver disease, all-cause mortality, metabolic syndrome, and impaired islet function [17-21]. In addition, Gong et al. [22] conducted a large-scale study of 8169 patients with AIS based on the Third China National Stroke Registry and found that SUA/SCr was associated with stroke prognosis. Few studies have separately explored the relationship between SUA/SCr and poor prognosis or stroke recurrence [23-25]. However, the association of SUA/SCr with 1-year outcomes, including stroke recurrence, all-cause mortality, and poor prognosis, has rarely been explored.
Therefore, in this study, we utilized the Xi'an multicenter Stroke Registry Research Platform to explore the association of the SUA/SCr with recurrence of stroke, all-cause mortality, and poor prognosis within 1 year in patients with AIS in Xi'an, China. Effective interventions targeting these factors are expected to minimize the rate of poor stroke outcomes.
METHODS
Study population
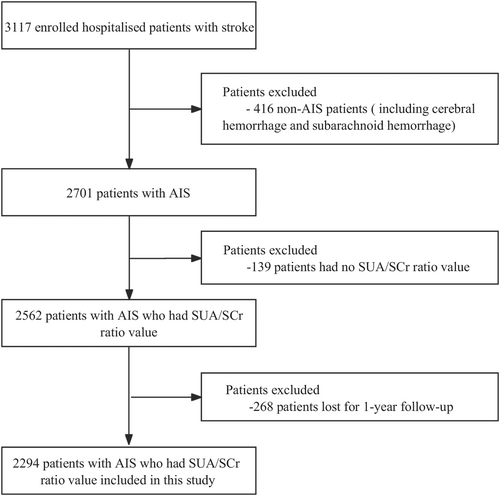
Individuals suffering from stroke who were registered at four hospitals ranked as tertiary level and grade A in Xi'an, China, between January and December 2015 were recruited for this research. The inclusion criteria were as follows: a clinical diagnosis of AIS, with imaging findings in line with the diagnostic criteria outlined by the American Heart Association/American Stroke Association and confirmed through brain computed tomography or cranial magnetic resonance imaging [26]. The patients were between the ages of 18 and 97 years and had experienced symptom onset within 7 days. The exclusion criteria were as follows: patients with cerebral or subarachnoid hemorrhage (n = 416), patients with loss of SUA/SCr value (n = 139), and patients without a 1-year follow-up (n = 268). Initially, a comprehensive medical examination was performed on 3117 stroke patients. Subsequent evaluations were carried out at 1, 3, 6, and 12 months after symptom onset. Among these patients, 2294 with AIS were finally included. Figure 1 illustrates the thorough screening process and research flowchart. This study was conducted in accordance with the Declaration of Helsinki's principles. Consent for the study was granted by the institutional review board of Xi'an No. 1 Hospital and the ethics panels of each of the involved hospitals (approval no. 2014 [5]; registration number: ChiCTR-EOC-17012190). All the patients provided written and oral informed consent.
Baseline data collection
Utilizing the Xi'an Stroke Registry Research Platform, our team carried out the cohort research with observations from multiple centers. Relevant clinical information was obtained, including baseline characteristics, health behaviors, anamnesis, admission assessment, and laboratory tests. Within 24 h after admission, fasting blood samples were collected, stored, and processed in accordance with the requirements of uniform regulations in every hospital. The various measuring instruments, reagents, and methods used in the study were standardized. The definition criteria were the same as those in our previous article [27]. All the participating hospitals followed uniform diagnostic guidelines.
Clinical outcome assessments
The endpoint events for this study were defined as follows. At the 1-year follow-up, a new episode of acute stroke (including subarachnoid hemorrhage, cerebral hemorrhage, or cerebral infarction) was categorized as a 1-year stroke recurrence. Poor prognosis was identified as a modified Rankin Scale (mRS) score in the range of 3–6 at the 1-year follow-up (score spans from 0 [indicating absence of symptoms] to 6 [indicating mortality]). All-cause mortality outcome events were gathered during the 1-year follow-up postenrollment. A committee consisting of four or five stroke experts from each hospital established the endpoint events.
Grouping and data comparison
SUA/SCr was studied as both a categorical variable (quartiles Q1–Q4) and a continuous variable. The SUA/SCr was categorized from low to high, according to its level (Q1: <3.137, Q2: 3.137–3.921, Q3: 3.922–4.789, Q4: ≥4.790).
Follow-up
All patients received routine follow-up at 1, 3, 6, and 12 months after being diagnosed with AIS. Trained study coordinators conducted follow-ups with all enrolled patients via phone conversations or direct interviews, guaranteeing the follow-up time deviation was within a 5-day limit. Patients who declined further involvement in this research or were uncontactable by phone after three tries each day for five continuous workdays were deemed lost to follow-up.
Statistical methods
Data analyses were carried out using the statistical software package R (v3.3.2) and Free Statistics software (v1.7). Data that were in accordance with a normal distribution were identified as mean ± SD, and the comparisons among groups were based on analysis of variance. Medians (interquartile ranges) are used to represent nonnormally distributed variables. Multiple group comparisons were carried out using the Kruskal–Wallis rank-sum test. Multivariable Cox regression analysis was used to evaluate the association between SUA/SCr and stroke recurrence and all-cause mortality. Logistic regression analysis was used to evaluate the association between SUA/SCr and poor prognosis. The principles of covariate screening include the following aspects: (i) according to clinical experience and relevant variables reported in previous authoritative journals, (ii) whether a variable causes the SUA/SCr estimate of 1-year stroke outcomes to change by >10%, and (iii) the variable should be included in the regression model as a potential confounding factor if univariate regression analysis indicates a significant correlation between the variable and 1-year stroke outcomes. The cDAG (causal directed acyclic graph) figure generation was done with online software (https://www.dagitty.net/). The 5th, 35th, 65th, and 95th percentiles of SUA/SCr distribution were used as knots and the median of the third quartile of SUA/SCr as the reference point. Restricted cubic spline was used for curve fitting to assess the relationship between SUA/SCr and stroke recurrence and all-cause death within 1 year. The curve fittings were adjusted for all covariates mentioned in the study. Stacked bar charts were utilized to assess the distribution of mRS scores within SUA/SCr quartiles after 1 year. Stratified analyses were performed in subgroups of age (<65 and ≥65 years), sex (male and female), estimated glomerular filtration rate (eGFR; <60 and ≥60 mL/min/1.73 m2), history of hypertension, diabetes mellitus, atrial fibrillation, and prior stroke, and presence or absence of pneumonia. The interaction effect was assessed by including interaction terms between SUA/SCr and each indicator listed above in the model, testing with the likelihood ratio test. A two-tailed p-value of <0.05 signified statistical significance.
RESULTS
Baseline characteristics
Comparison of clinical characteristics between patients lost to follow-up and those not lost to follow-up revealed that the main feature variables in both groups were similar, indicating that the patients selected for analysis were representative of the entire patient cohort (Table S1). Of the 2294 participants involved in the research, the average age was 64.5 ± 12.2 years, with 1434 being males. The SUA/SCr quartile (Q1–Q4) groups were compared based on their baseline demographic, biochemical, and clinical characteristics (Table 1). Compared with the other quartiles, participants in Q1 of SUA/SCr tended to be older; had no alcohol consumption; had a greater prevalence of pneumonia and diabetes mellitus; had higher levels of fasting venous plasma glucose (FPG), aspartate aminotransferase, homocysteine, blood urea nitrogen, and serum creatinine; and had lower body mass index, triglycerides, eGFR, and serum uric acid (p < 0.05). There was no significant difference in other variables among SUA/SCr quartile groups (p > 0.05).
| Variables | Overall, n = 2294 | SUA/SCr quartiles | p | |||
|---|---|---|---|---|---|---|
| Q1, n = 574 | Q2, n = 573 | Q3, n = 573 | Q4, n = 574 | |||
| Demographic information | ||||||
| Age, years | 64.5 ± 12.2 | 66.0 ± 11.5 | 65.8 ± 11.8 | 63.5 ± 12.6 | 62.6 ± 12.7 | <0.001 |
| Sex, n (%) | 0.233 | |||||
| Male | 1434 (62.5) | 357 (62.2) | 367 (64) | 370 (64.6) | 340 (59.2) | |
| Female | 860 (37.5) | 217 (37.8) | 206 (36) | 203 (35.4) | 234 (40.8) | |
| Medical insurance type, n (%) | 0.065 | |||||
| Urban employees' medical insurance | 1099 (47.9) | 253 (44.1) | 308 (53.7) | 265 (46.3) | 273 (47.6) | |
| New type rural cooperative medical system | 907 (39.5) | 250 (43.5) | 198 (34.6) | 232 (40.5) | 227 (39.5) | |
| Commercial insurance | 9 (0.4) | 4 (0.7) | 1 (0.2) | 3 (0.5) | 1 (0.2) | |
| Out-of-pocket medical | 279 (12.2) | 67 (11.7) | 66 (11.5) | 73 (12.7) | 73 (12.7) | |
| Educational level, n (%) | 0.558 | |||||
| Elementary or below | 1075 (46.9) | 265 (46.2) | 262 (45.7) | 284 (49.6) | 264 (46) | |
| Middle school | 460 (20.0) | 125 (21.8) | 107 (18.7) | 108 (18.8) | 120 (20.9) | |
| High school or above | 759 (33.1) | 184 (32.0) | 204 (35.6) | 181 (31.6) | 190 (33.1) | |
| Lifestyle | ||||||
| Smoking, n (%) | 0.754 | |||||
| Never smoking | 1285 (56.0) | 334 (58.2) | 324 (56.5) | 304 (53.1) | 323 (56.3) | |
| Smoking cessation | 452 (19.7) | 108 (18.8) | 114 (19.9) | 118 (20.6) | 112 (19.5) | |
| Current smoking | 557 (24.3) | 132 (23) | 135 (23.6) | 151 (26.3) | 139 (24.2) | |
| Alcohol consumption, n (%) | 0.041 | |||||
| No | 1744 (76.0) | 458 (79.8) | 441 (77) | 421 (73.5) | 424 (73.9) | |
| Yes | 550 (24.0) | 116 (20.2) | 132 (23) | 152 (26.5) | 150 (26.1) | |
| Medical history, n (%) | ||||||
| Hypertension | 0.656 | |||||
| No | 670 (29.2) | 166 (28.9) | 177 (30.9) | 169 (29.5) | 158 (27.5) | |
| Yes | 1624 (70.8) | 408 (71.1) | 396 (69.1) | 404 (70.5) | 416 (72.5) | |
| Diabetes mellitus | 0.016 | |||||
| No | 1754 (76.5) | 415 (72.3) | 436 (76.1) | 460 (80.3) | 443 (77.2) | |
| Yes | 540 (23.5) | 159 (27.7) | 137 (23.9) | 113 (19.7) | 131 (22.8) | |
| Atrial fibrillation | 0.663 | |||||
| No | 2126 (92.7) | 526 (91.6) | 530 (92.5) | 534 (93.2) | 536 (93.4) | |
| Yes | 168 (7.3) | 48 (8.4) | 43 (7.5) | 39 (6.8) | 38 (6.6) | |
| Prior stroke | 0.156 | |||||
| No | 1631 (71.1) | 391 (68.1) | 401 (70) | 421 (73.5) | 418 (72.8) | |
| Yes | 663 (28.9) | 183 (31.9) | 172 (30) | 152 (26.5) | 156 (27.2) | |
| Pneumonia | 0.002 | |||||
| No | 2172 (94.7) | 526 (91.6) | 552 (96.3) | 546 (95.3) | 548 (95.5) | |
| Yes | 122 (5.3) | 48 (8.4) | 21 (3.7) | 27 (4.7) | 26 (4.5) | |
| Examination on admission | ||||||
| SBP on admission, mmHg | 145.9 ± 21.9 | 146.1 ± 22.7 | 146.2 ± 21.9 | 145.4 ± 21.1 | 146.0 ± 22.0 | 0.934 |
| DBP on admission, mmHg | 85.7 ± 12.5 | 86.2 ± 13.1 | 85.3 ± 11.9 | 85.2 ± 12.1 | 86.2 ± 13.0 | 0.324 |
| HR, beats/min | 74.9 ± 10.6 | 75.4 ± 11.4 | 74.1 ± 9.9 | 75.0 ± 10.9 | 75.0 ± 10.1 | 0.163 |
| Admission NIHSS score, IQR | 4.0 (2.0–6.0) | 4.0 (2.0–7.0) | 4.0 (1.0–6.0) | 4.0 (2.0–6.0) | 4.0 (2.0–6.0) | 0.06 |
| BMI, kg/m2 | 23.9 ± 3.4 | 23.7 ± 3.7 | 23.7 ± 3.0 | 23.8 ± 3.4 | 24.3 ± 3.5 | 0.002 |
| Laboratory-related tests | ||||||
| Total cholesterol, mmol/L | 4.4 ± 1.1 | 4.3 ± 1.0 | 4.4 ± 1.1 | 4.4 ± 1.0 | 4.5 ± 1.2 | 0.239 |
| Triglycerides, mmol/L | 1.7 ± 1.4 | 1.5 ± 1.2 | 1.6 ± 1.2 | 1.7 ± 1.3 | 1.9 ± 1.7 | <0.001 |
| HDL cholesterol, mmol/L | 1.1 ± 0.3 | 1.1 ± 0.3 | 1.1 ± 0.3 | 1.1 ± 0.3 | 1.1 ± 0.3 | 0.635 |
| LDL cholesterol, mmol/L | 2.6 ± 0.8 | 2.6 ± 0.8 | 2.6 ± 0.8 | 2.6 ± 0.8 | 2.7 ± 0.9 | 0.183 |
| Glycated hemoglobin, % | 6.4 ± 1.6 | 6.5 ± 1.8 | 6.5 ± 1.7 | 6.3 ± 1.4 | 6.3 ± 1.4 | 0.124 |
| FPG, mmol/L | 6.0 ± 2.4 | 6.4 ± 2.8 | 5.9 ± 2.3 | 5.8 ± 2.1 | 5.9 ± 2.1 | <0.001 |
| Alanine aminotransferase, U/L | 24.0 ± 20.8 | 23.1 ± 23.5 | 22.9 ± 22.0 | 24.3 ± 20.7 | 25.6 ± 16.4 | 0.102 |
| Aspartate aminotransferase, U/L | 24.9 ± 17.3 | 26.3 ± 21.6 | 23.4 ± 18.0 | 24.3 ± 14.8 | 25.7 ± 13.4 | 0.021 |
| Alkaline phosphatase, U/L | 79.8 ± 33.1 | 80.5 ± 29.5 | 77.5 ± 24.1 | 82.4 ± 44.2 | 78.7 ± 31.2 | 0.069 |
| Homocysteine, μmol/L | 21.9 ± 14.5 | 23.4 ± 14.2 | 22.8 ± 15.9 | 21.3 ± 13.9 | 20.1 ± 13.9 | 0.007 |
| eGFR, mL/min/1.73 m2 | 76.3 ± 15.2 | 71.7 ± 18.7 | 77.6 ± 13.5 | 79.3 ± 11.9 | 76.6 ± 14.7 | <0.001 |
| Serum creatinine, μmol/L | 75.7 ± 35.8 | 91.8 ± 61.8 | 76.8 ± 19.7 | 71.0 ± 15.3 | 63.3 ± 16.3 | <0.001 |
| Blood urea nitrogen, mmol/L | 5.1 ± 1.9 | 5.5 ± 2.3 | 5.1 ± 2.0 | 5.1 ± 1.6 | 4.8 ± 1.6 | <0.001 |
| Serum uric acid, μmol/L | 288.0 ± 97.1 | 207.6 ± 88.0 | 272.2 ± 69.9 | 308.0 ± 67.1 | 364.2 ± 87.7 | <0.001 |
| White blood cells, ×109/L | 7.0 ± 2.6 | 7.2 ± 3.0 | 6.9 ± 2.5 | 6.9 ± 2.3 | 7.0 ± 2.4 | 0.111 |
| Platelets, ×109/L | 189.7 ± 60.6 | 185.9 ± 63.8 | 192.4 ± 63.0 | 190.2 ± 56.7 | 190.2 ± 58.5 | 0.333 |
- Note: Data presented are mean ± SD, median (Q1–Q3), or n (%). Q1: <3.137, Q2: 3.137–3.921, Q3: 3.922–4.789, Q4: ≥4.790.
- Abbreviations: AIS, acute ischemic stroke; BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; FPG, fasting venous plasma glucose; HLD, high-density lipoprotein; HR, heart rate; IQR, interquartile range; LDL, low-density lipoprotein; NIHSS, National Institutes of Health Stroke Scale; Q, quartile; SBP, systolic blood pressure; SCr, serum creatinine; SUA, serum uric acid.
Association of SUA/SCr with stroke outcomes in patients with AIS
Using SUA/SCr as a continuous variable, after adjusting for confounding factors (confounding factor screening results are provided in Tables S2 and S3 and Figure S1), multivariable Cox regression analyses showed that for each unit increase in SUA/SCr, the risk of stroke recurrence decreased by 19% at 1 year (hazard ratio [HR] = 0.81, 95% confidence interval [CI] = 0.71–0.93, p = 0.002; Table 2). The SUA/SCr was divided into quartiles (Q1–Q4), and Q1 was used as the reference. After adjusting for confounders, multivariable Cox regression analyses showed that the risk of 1-year stroke recurrence was lower in the Q2, Q3, and Q4 groups than in the Q1 group (Q2: HR = 0.48, 95% CI = 0.29–0.81, p = 0.005; Q3: HR = 0.62, 95% CI = 0.39–0.99, p = 0.046; Q4: HR = 0.44, 95% CI = 0.26–0.76, p = 0.003; Table 2). In univariable and multivariable Cox regression analyses, the trend of 1-year stroke recurrence in Q1 to Q4 groups was significantly different (p < 0.001 and p = 0.003 for trend tests). However, there was no significant association between SUA/SCr and the risk of 1-year all-cause mortality or poor prognosis. Following the analysis of SUA/SCr levels in patients with AIS at 3 months, the results revealed that similar to 1-year stroke outcomes, SUA/SCr level was significantly associated with reduced risk of 3-month stroke recurrence, but not with 3-month all-cause mortality and 3-month poor prognosis (Table S4).
| Outcomes | Overall, n | Event, n (%) | Crude model HR/OR (95% CI) | p | Adjusted model HR/OR (95% CI) | p |
|---|---|---|---|---|---|---|
| Stroke recurrence | ||||||
| SUA/SCr, per 1 unit increase | 2294 | 121 (5.3) | 0.75 (0.66–0.86) | <0.001 | 0.81 (0.71–0.93) | 0.002 |
| SUA/SCr quartiles | ||||||
| Q1 | 574 | 53 (9.2) | Ref. | Ref. | ||
| Q2 | 573 | 22 (3.8) | 0.4 (0.24–0.66) | <0.001 | 0.48 (0.29–0.81) | 0.005 |
| Q3 | 573 | 28 (4.9) | 0.52 (0.33–0.82) | 0.005 | 0.62 (0.39–0.99) | 0.046 |
| Q4 | 574 | 18 (3.1) | 0.33 (0.19–0.56) | <0.001 | 0.44 (0.26–0.76) | 0.003 |
| Trend test | <0.001 | 0.003 | ||||
| Poor prognosis | ||||||
| SUA/SCr, per 1 unit increase | 2294 | 453 (19.7) | 0.88 (0.82–0.95) | 0.001 | 0.97 (0.89–1.05) | 0.435 |
| SUA/SCr quartiles | ||||||
| Q1 | 574 | 147 (25.6) | Ref. | Ref. | ||
| Q2 | 573 | 103 (18) | 0.64 (0.48–0.85) | 0.002 | 0.79 (0.56–1.11) | 0.175 |
| Q3 | 573 | 104 (18.2) | 0.64 (0.49–0.86) | 0.002 | 0.89 (0.63–1.27) | 0.521 |
| Q4 | 574 | 99 (17.2) | 0.61 (0.45–0.81) | 0.001 | 0.88 (0.62–1.26) | 0.498 |
| Trend test | 0.001 | 0.631 | ||||
| All-cause mortality | ||||||
| SUA/SCr, per 1 unit increase | 2294 | 176 (7.7) | 0.87 (0.79–0.97) | 0.014 | 0.96 (0.86–1.06) | 0.398 |
| SUA/SCr quartiles | ||||||
| Q1 | 574 | 63 (11) | Ref. | Ref. | ||
| Q2 | 573 | 37 (6.5) | 0.57 (0.38–0.86) | 0.007 | 0.79 (0.51–1.21) | 0.282 |
| Q3 | 573 | 39 (6.8) | 0.61 (0.41–0.91) | 0.015 | 0.81 (0.53–1.21) | 0.301 |
| Q4 | 574 | 37 (6.4) | 0.57 (0.38–0.86) | 0.007 | 0.84 (0.55–1.29) | 0.431 |
| Trend test | 0.008 | 0.391 |
- Note: Crude model does not adjust. Adjusted model adjusts for age, sex, smoking, alcohol consumption, prior stroke, National Institutes of Health Stroke Scale score at admission, pneumonia, body mass index, alkaline phosphatase, white blood cell count, hypertension, diabetes mellitus, and atrial fibrillation. Q1: <3.137, Q2: 3.137–3.921, Q3: 3.922–4.789, Q4: ≥4.790.
- Abbreviations: AIS, acute ischemic stroke; CI, confidence interval; HR, hazard ratio; OR, odds ratio; Q, quartile; Ref., reference; SCr, serum creatinine; SUA, serum uric acid.
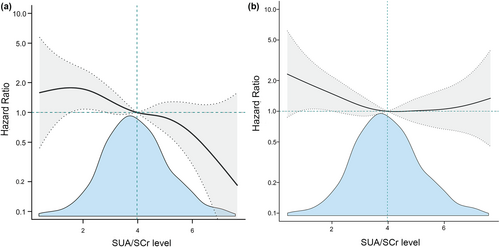
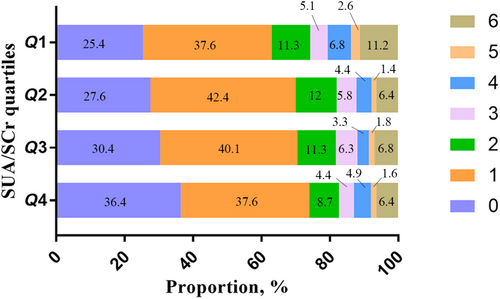
Following accounting for potential confounding factors, fitted smooth curve analysis demonstrated a negative but nonlinear association between SUA/SCr and 1-year stroke recurrence (Figure 2a) as well as a large upward-opening U-shaped association between SUA/SCr and 1-year mortality (Figure 2b). We also analyzed the distribution of 1-year mRS scores in patients with AIS. The Q1 group had the highest proportion of patients with poor prognosis, whereas the Q4 group had the lowest (Figure 3 and Table S5). In addition, we analyzed the single SUA association with 1-year stroke outcomes, and the results showed that there was no significant association between SUA and 1-year stroke outcomes (Table S6). Receiver operating characteristic curve analysis of SUA/SCr and SUA alone for predicting 1-year stroke recurrence showed that the area under the curve of SUA/SCr was 0.615 (95% CI = 0.564–0.667), and the area under the curve of SUA was 0.554 (95% CI = 0.501–0.608). There was a significant difference between the two groups (p = 0.015; Figure S2).
Subgroup analyses
Stratified and interaction analyses were conducted to determine whether the association of SUA/SCr with recurrence of stroke within 1 year remained consistent in all subgroups (Table 3). The interaction between sex, age, eGFR, and the incidence of prior stroke, diabetes mellitus, hypertension, atrial fibrillation, and pneumonia was not found to be significant. However, the stratified analysis found that low baseline SUA/SCr significantly increased the hazard of recurrence of stroke within 1 year in patients aged ≥65 years (HR = 0.83, 95% CI = 0.7–0.97, p = 0.022), males (HR = 0.78, 95% CI = 0.65–0.93, p = 0.005), those with eGFR ≥ 60 (HR = 0.84, 95% CI = 0.72–0.99, p = 0.042), those with hypertension (HR = 0.84, 95% CI = 0.73–0.97, p = 0.017), those without diabetes mellitus (HR = 0.84, 95% CI = 0.72–0.99, p = 0.036), those without atrial fibrillation (HR = 0.82, 95% CI = 0.71–0.94, p = 0.006), those without prior stroke (HR = 0.78, 95% CI = 0.66–0.92, p = 0.004), and those without pneumonia (HR = 0.77, 95% CI = 0.66–0.91, p = 0.001).
| Subgroup | Overall, n | Event, n (%) | Crude model HR (95% CI) | p | Adjusted model HR (95% CI) | p | p for interaction |
|---|---|---|---|---|---|---|---|
| Age, years | |||||||
| <65 | 1126 | 38 (3.4) | 0.74 (0.59–0.93) | 0.011 | 0.81 (0.64–1.03) | 0.088 | 0.617 |
| ≥65 | 1168 | 83 (7.1) | 0.78 (0.66–0.92) | 0.003 | 0.83 (0.7–0.97) | 0.022 | |
| Sex | |||||||
| Male | 1434 | 79 (5.5) | 0.76 (0.64–0.89) | 0.001 | 0.78 (0.65–0.93) | 0.005 | 0.828 |
| Female | 860 | 42 (4.9) | 0.75 (0.6–0.93) | 0.008 | 0.84 (0.69–1.03) | 0.087 | |
| eGFR, mL/min/1.73 m2 | |||||||
| <60 | 286 | 27 (9.4) | 0.81 (0.64–1.01) | 0.067 | 0.78 (0.59–1.02) | 0.066 | 0.667 |
| ≥60 | 2008 | 94 (4.7) | 0.77 (0.66–0.9) | 0.001 | 0.84 (0.72–0.99) | 0.042 | |
| Hypertension | |||||||
| No | 670 | 27 (4) | 0.72 (0.54–0.96) | 0.027 | 0.79 (0.58–1.08) | 0.134 | 0.875 |
| Yes | 1624 | 94 (5.8) | 0.76 (0.66–0.89) | <0.001 | 0.84 (0.73–0.97) | 0.017 | |
| Diabetes mellitus | |||||||
| No | 1754 | 90 (5.1) | 0.77 (0.66–0.9) | 0.001 | 0.84 (0.72–0.99) | 0.036 | 0.441 |
| Yes | 540 | 31 (5.7) | 0.71 (0.55–0.91) | 0.007 | 0.8 (0.63–1.02) | 0.069 | |
| Atrial fibrillation | |||||||
| No | 2126 | 98 (4.6) | 0.76 (0.66–0.88) | <0.001 | 0.82 (0.71–0.94) | 0.006 | 0.732 |
| Yes | 168 | 23 (13.7) | 0.71 (0.5–1) | 0.050 | 0.82 (0.58–1.16) | 0.267 | |
| Prior stroke | |||||||
| No | 1631 | 67 (4.1) | 0.72 (0.6–0.86) | <0.001 | 0.78 (0.66–0.92) | 0.004 | 0.14 |
| Yes | 663 | 54 (8.1) | 0.82 (0.67–1) | 0.052 | 0.89 (0.73–1.1) | 0.279 | |
| Pneumonia | |||||||
| No | 2172 | 90 (4.1) | 0.74 (0.63–0.86) | <0.001 | 0.77 (0.66–0.91) | 0.001 | 0.088 |
| Yes | 122 | 31 (25.4) | 0.95 (0.77–1.17) | 0.622 | 0.93 (0.73–1.17) | 0.517 | |
- Note: Crude model does not adjust. Adjusted model adjusts for sex, smoking, alcohol consumption, prior stroke, National Institutes of Health Stroke Scale score at admission, body mass index, alkaline phosphatase, and white blood cell count.
- Abbreviations: CI, confidence interval; HR, hazard ratio; SCr, serum creatinine; SUA, serum uric acid.
DISCUSSION
The primary discovery of this multicenter study was that in patients with AIS, a low SUA/SCr may be an independent risk factor for 1-year stroke recurrence. Nevertheless, the SUA/SCr did not show a significant association with poor prognosis and all-cause mortality within 1 year. Notably, there was a negative and nonlinear association of the SUA/SCr with 1-year stroke recurrence.
Previous studies have explored the relationship between SUA and stroke outcomes, yielding inconsistent results. These inconsistent conclusions may be because renal excretion of endogenous SUA is typically dependent on kidney function. Patients with renal insufficiency are at a greater risk of elevated uric acid levels [2]. Renal insufficiency may be one of the main confounding factors in studies on the association of SUA with stroke outcomes. Therefore, renal function–normalized uric acid (SUA/SCr) levels more accurately reflect endogenous SUA levels [18, 19, 22].
Gong et al. [22] found an association of lower SUA/SCr levels with 3-month and 1-year poor prognosis (mRS score = 3–6) in patients with AIS. They observed that mRS scores of 3–6 were the highest in the group with the lowest SUA/SCr levels. Similarly, in our research, Q1 exhibited the highest percentage of patients with mRS scores of 3–6 in comparison to the other groups (Figure 3). However, there was no significant association of SUA/SCr with poor prognosis within 3 months and 1 year in patients with AIS. We also found that low SUA/SCr may be an independent risk factor for stroke recurrence within 3 months and 1 year. Both studies suggested that high SUA/SCr may be protective in stroke patients. The discrepant conclusions between the two studies may be attributed to distinctions in research design, methodology, and research populations. Whereas Gong et al. [22] used data from the Third China National Stroke Registry, we utilized data from the Xi'an Stroke Registry. Their research was based on national average data, whereas our study was based on regional data; therefore, our findings have a certain significance for regional disease prevention and control. Sun et al. [23] conducted a single-center study and reported a positive correlation between SUA/SCr and stroke recurrence. This is inconsistent with our findings from this multicenter study, as we found that low SUA/SCr increased the risk of stroke recurrence. Variances in research outcomes can be ascribed to discrepancies in patient populations, number of patients, and research approaches. Sun et al. [23] conducted a retrospective analysis of 428 patients with ischemic stroke, aged between 18 and 49 years. Our research involved a prospective analysis of 2294 patients with AIS (mean age = 64.5 ± 12.2 years) to evaluate the causality relationship between SUA/SCr and stroke recurrence. In addition, Xu et al. [24] found that lower SUA/SCr could be used as a predictor of adverse functional prognosis after mechanical thrombectomy in AIS. Liu et al. [25] found that SUA/SCr was negatively associated with the risk of early neurological deterioration in branch atheromatous disease–stroke patients. Their study also suggested that high SUA/SCr may have a protective effect in stroke patients.
Previous studies have mainly focused on the association between SUA and stroke outcomes, and there are few studies on the association between SUA/SCr and stroke outcomes. A single-center study by Zhu et al. showed a positive nonlinear association of SUA levels with the 3-year recurrence in ischemic stroke patients [14]. This result is inconsistent with the findings of our research. We found a negative nonlinear association of the SUA/SCr with 1-year stroke recurrence in patients with AIS in our multicenter study. Previous studies have found that every 60 μmol/L increase in SUA is associated with a 7%–11% increased hazard of kidney disease and a 14% increased risk of renal function deterioration [28, 29]. Therefore, the SUA/SCr can more accurately reflect endogenous SUA levels. Mapoure et al. studied a population of 480 patients with AIS and found that in patients with AIS, hyperuricemia was an independent predictor of 3-month mortality [30]. However, this result is inconsistent with our findings in a cohort of 2294 patients with AIS, where we found no association of SUA/SCr levels with 1-year mortality. In addition to discrepancies in study populations, regions, and methodologies, significant differences in mortality rates may be one of the reasons for these different results (28.3% vs. 7.7% in our study).
Subgroup analyses revealed no significant interactions in patients with diabetes mellitus or a history of stroke. Nevertheless, the stratified analysis revealed a significant decrease in the hazard of 1-year stroke recurrence as the SUA/SCr increased in patients without diabetes mellitus or prior stroke. Prior studies have found a positive association of the SUA/SCr with FPG [18]. In patients with AIS, diabetes mellitus is an independent risk factor for stroke recurrence [31]. These results support the findings of the present research. The association of SUA/SCr with stroke recurrence in patients with diabetes was less significant. Xu et al. [32] found that prior stroke increased the risk of recurrent stroke. This finding is consistent with our findings. The association of the SUA/SCr with the hazard of recurrence of stroke was less significant in patients with a prior stroke. In conclusion, for patients without diabetes mellitus or prior stroke, increased focus should be given to the consequences of SUA/SCr and recurrent stroke.
The underlying mechanism linking renal function–normalized SUA (SUA/SCr) with stroke recurrence is not fully understood. First, SUA exerts a direct antioxidant effect. When AIS occurs, several related events are triggered in a sequence termed the ischemic cascade, where the primary driving force seems to be the production of peroxynitrite and free radicals [33]. SUA exhibits strong antioxidant activity at physiological concentrations [34]. It serves as a potent free radical scavenger, specifically preventing peroxynitrite formation, and plays the role of a potent peroxynitrite scavenger. In addition, it scavenges free radicals produced by the decomposition of peroxynitrite [3, 35]. Second, SUA promotes neuroprotection in the brain by inducing neuronal glutathione [36]. Third, SUA may act through astroglia to protect neurons from glutamate-induced toxicity [37]. These mechanisms suggest that SUA exerts neuroprotective effects. A lower SUA/SCr at stroke onset indicates reduced neuroprotection. However, more studies are essential to verify these discoveries.
The novelty of this study is to explore and find that low SUA/SCr may be an independent risk factor for 1-year stroke recurrence in patients with AIS through a multicenter prospective study. However, our study has some limitations. First, dynamic changes in the SUA/SCr were not recorded. The stroke outcomes may have been affected by changes in SUA/SCr during the follow-up period. Second, owing to the lack of data on patients undergoing mechanical thrombectomy and/or intravenous thrombolysis, we could not analyze patients in this category. Third, due to the design of the observational study, there may be some unmeasured or residual confounding effects. Finally, the selected hospitals were all tertiary hospitals in Xi'an, and smaller community hospitals were not included. This may have led to selection bias in the inclusion of patients. Therefore, larger studies involving different regional populations are expected to validate and expand our results.
Conclusions
In patients with AIS, low SUA/SCr may be an independent risk factor for 1-year stroke recurrence, and the changes in the SUA/SCr had no significant impact on all-cause death and poor prognosis within 1 year in these patients. Our research could offer valuable insights for clinicians in the use of the SUA/SCr as a preferred predictor of stroke recurrence.
AUTHOR CONTRIBUTIONS
Dandan Zhang: Writing – original draft; writing – review and editing; methodology; software; formal analysis. Zhongzhong Liu: Writing – original draft; writing – review and editing; methodology; software; formal analysis. Weiyan Guo: Investigation. Qingli Lu: Investigation. Zhen Lei: Investigation. Pei Liu: Investigation. Tong Liu: Investigation. Linna Peng: Investigation. Qiaoqiao Chang: Investigation. Mi Zhang: Investigation. Xuemei Lin: Investigation. Fang Wang: Investigation. Songdi Wu: Data curation; project administration; supervision; validation; conceptualization.
ACKNOWLEDGMENTS
We are grateful to all the imaging and laboratory technicians, medical staff, and nurses from the participating hospitals.
CONFLICT OF INTEREST STATEMENT
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
ETHICS STATEMENT
This study was conducted in accordance with the Declaration of Helsinki's principles. Consent for the study was granted by the institutional review board of Xi'an No. 1 Hospital and the ethics panels of each of the involved hospitals (approval no. 2014 [5]; registration number: ChiCTR-EOC-17012190).
CONSENT
All the patients provided written and oral informed consent.
Open Research
DATA AVAILABILITY STATEMENT
Data are available to researchers on request for purposes of reproducing the results or replicating the procedure by directly contacting the corresponding author.




