Patient-reported daily functioning after SARS-CoV-2 vaccinations in autoimmune neuromuscular diseases
Abstract
Background and purpose
There are concerns for safety regarding SARS-CoV-2 vaccines for patients with autoimmune neuromuscular disease. We compared daily functioning using disease-specific patient-reported outcome measures (PROMs) before and after SARS-CoV-2 vaccinations.
Methods
In this substudy of a prospective observational cohort study (Target-to-B!), patients with myasthenia gravis (MG), chronic inflammatory demyelinating polyneuropathy (CIDP), multifocal motor neuropathy (MMN), and idiopathic inflammatory myopathy (IIM) vaccinated against SARS-CoV-2 were included. Surveys of daily functioning (Myasthenia Gravis Activities of Daily Living, Inflammatory Rasch-Built Overall Disability Scale, Multifocal Motor Neuropathy Rasch-Built Overall Disability Scale, and Health Assessment Questionnaire–Disability Index) were sent before first vaccination and every 60 days thereafter for up to 12 months. Regression models were constructed to assess differences in PROM scores related to vaccination, compared to scores unrelated to vaccination. We also assessed the proportion of patients with deterioration of at least the minimal clinically important difference (MCID) between before first vaccination and 60 days thereafter.
Results
We included 325 patients (median age = 59 years, interquartile range = 47–67, 156 [48%] female sex), of whom 137 (42%) had MG, 79 (24%) had CIDP, 43 (13%) had MMN, and 66 (20%) had IIM. PROM scores related to vaccination did not differ from scores unrelated to vaccination. In paired PROMs, MCID for deterioration was observed in three of 49 (6%) MG patients, of whom none reported a treatment change. In CIDP, MCID for deterioration was observed in eight of 29 patients (28%), of whom two of eight (25%) reported a treatment change.
Conclusions
SARS-CoV-2 vaccination had no effect on daily functioning in patients with autoimmune neuromuscular diseases, confirming its safety in these patients.
INTRODUCTION
Since the start of the SARS-CoV-2 pandemic in early 2020, vaccination proved to be essential in reducing transmission and mortality from the virus, especially in vulnerable groups [1]. Patients with autoimmune neuromuscular disease (NMD) may be vulnerable because of the disease itself, for example, because of respiratory involvement in myasthenia gravis (MG), or because of the use of immunosuppressants [2]. Yet, among these patients fear of deterioration after vaccination is common, which may lead to vaccine hesitancy [3]. Previously, we observed that self-reported increases in disease activity, measured on a Likert scale, were more frequent in patients with autoimmune NMD compared to other immune-mediated inflammatory diseases (IMIDs), although we could not demonstrate a temporal association between self-reported increases and vaccination [4]. Other prospective studies focusing on MG or inflammatory neuropathies have shown no change in disease activity after SARS-CoV-2 vaccination [5-7]. However, no systematically collected disease-specific data on changes in patient-reported outcome measures (PROMs) before and after SARS-CoV-2 vaccination are available to better investigate a potential association between vaccination and relapse of an autoimmune NMD.
We aimed to assess changes in patient daily functioning using PROMs after SARS-CoV-2 vaccination in the most prevalent autoimmune NMDs.
METHODS
Study design
This is a substudy of a prospective observational multicenter cohort study (Target-to-B! Immunity Against SARS-CoV-2). The Target-to-B! (T2B!) study was approved by the medical ethical committee (NL74974.018.20 and EudraCT 2021-001102-30) and registered in the Dutch Trial Register (trial ID NL8900). All participants provided signed informed consent. Here, we report on a secondary objective of the study for a selection of specific IMIDs (autoimmune NMDs), namely, changes in disease activity after SARS-CoV-2 vaccination. A detailed description of the T2B! study protocol including eligibility criteria and data on other outcomes from some of the patients in this cohort have been published before [4, 8-14].
Setting and participants
For the T2B! cohort, patients with IMIDs were actively recruited in the Netherlands, before and during the national SARS-CoV-2 vaccination campaign, from 2 February 2021 to 1 August 2021. Participants were vaccinated in the national vaccination campaign or at the coordinating study site, with one or two doses of any of the four different vaccines available in the Netherlands during 2021: BNT162b2 (Pfizer/BioNTech), mRNA-1273 (Moderna), ChAdOx1 nCoV-19 (Oxford/AstraZeneca), or Ad.26.COV2.S (Janssen) as primary immunization and mRNA-1273 or BNT162b2 as additional vaccination (i.e., a third and/or booster vaccination, no bivalent vaccines). In this cohort, primary immunizations ranged from 6 February 2021 to 3 December 2021, and additional vaccinations ranged from 8 September 2021 to 6 July 2022. For this substudy, we selected patients with an autoimmune NMD, either MG, chronic inflammatory demyelinating polyneuropathy (CIDP), multifocal motor neuropathy (MMN), or an idiopathic inflammatory myopathy (IIM; excluding inclusion body myositis), who received at least one vaccination as part of primary immunization, and who completed at least two surveys during the study.
Clinical data collection
Clinical data were collected by sending online surveys to participants and by the investigators using a standardized electronic case record form (eCRF). A baseline survey was sent at enrollment and registered demographics and disease-specific PROMs for each of the autoimmune NMD. As PROMs, for MG the Myasthenia Gravis Activities of Daily Living (MG-ADL) was used, for CIDP the Inflammatory Rasch-Built Overall Disability Scale (I-RODS), for MMN the Multifocal Motor Neuropathy Rasch-Built Overall Disability Scale (MMN RODS), and for IIM the Health Assessment Questionnaire–Disability Index (HAQ-DI) [15-18]. All these scales were designed to capture daily functioning in relation to disease activity. Vaccination data were registered by sending additional surveys. Follow-up surveys containing disease activity PROMs were sent every 60 days after the start of primary immunization for up to 413 days. Changes in medication for the underlying condition were registered by the investigators using a standardized eCRF.
Outcome definitions
The primary outcome for this substudy was the difference in PROM scores from surveys related to vaccination (defined as surveys completed within 8–60 days after any SARS-CoV-2 vaccination) compared to surveys unrelated to vaccination (defined as surveys without prior vaccination, or vaccination >60 days prior to the survey) for each autoimmune NMD over the entire follow-up period. The 8-day cutoff was chosen to avoid short-term adverse events influencing the PROMs, as these are common and are a different phenomenon from increased disease activity, but usually resolve within 1 week after SARS-CoV-2 vaccination [11]. The 60-day cutoff was chosen because a new onset of autoimmune diseases was reported to occur within this time frame, and there is no evidence for a causal relationship between vaccination and a flare-up of pre-existing autoimmune disease [19-22]. In the MG-ADL and HAQ-DI, a low score indicates better daily functioning, whereas in the I-RODS and MMN RODS, a high score indicates better daily functioning. Secondary outcomes included the association between PROMs and age, sex, body mass index (BMI), and immunosuppressant use. Immunosuppressants also included intravenous or subcutaneous immunoglobulin, which have immunomodulating effects, but for brevity we define them as immunosuppressants. Additional secondary outcomes included the change in PROMs in paired observations from before first vaccination (survey completed before the day of first vaccination) and at first follow-up (survey completed 60–66 days after first vaccination), the proportion of participants with a minimal clinically important difference (MCID) for improvement and deterioration, and if MCID for deterioration was present, the proportion of participants who reported a change in treatment. For these outcomes, we extended the observation period up to 66 days after vaccination instead of up to 60 days as in the primary outcome, as most participants completed the survey during this period and too few participants completed it on exactly day 60 after first vaccination. MCIDs were defined as 2 points for MG-ADL and 4 points on the centile scale for I-RODS [23, 24]. To our knowledge, there is no accepted MCID for the MMN RODS and HAQ-DI for IIM, and therefore analyses related to MCID are not performed on patients with MMN or IIM. A SARS-CoV-2 infection before the first vaccination was defined as either a self-reported positive polymerase chain reaction, antigen test, or a positive SARS-CoV-2 anti-receptor-binding domain antibody test at baseline. Definitions for immunosuppressants, active treatment, and grouping of combination therapies have been described elsewhere [8].
Statistical analysis
As primary analysis, we performed a longitudinal analysis of changes in PROMs over time by constructing uni- and multivariate linear mixed models for the MG-ADL score, I-RODS centile score, and MMN RODS centile score. Due to a strong floor effect, we constructed tobit mixed models for the HAQ-DI score [25]. As fixed effect for the univariate models, we selected any SARS-COV-2 vaccination 8–60 days before the PROMs (in contrast to surveys without prior vaccination or vaccination >60 days before). Surveys with vaccination 0–7 days before were omitted from the model, because these observations may be affected by short-term adverse events. As fixed effects in the multivariate model, we also added age, sex, BMI, and immunosuppressant use to assess potential confounders. As random effect in all models, we added intercepts for the individual participants, thereby controlling for individual differences between patients. For the primary outcome, we report the median PROM score related to vaccination (survey within 8–60 days after vaccination), and the median PROM scores unrelated to vaccination (surveys without prior vaccination, or vaccination >60 days prior to the survey). Also, we report the regression coefficient from the multivariate models for vaccination 8–60 days before a survey, with corresponding 95% confidence interval (CI). For the secondary outcome, we report the regression coefficients from the univariate model, and for age, sex, BMI, and immunosuppressant use with corresponding 95% CI from the multivariate model. In the MMN RODS model, we omitted immunosuppressant use as a fixed effect, as all MMN patients were on immunosuppressants. As secondary analysis, we analyzed paired observations from before first vaccination and at first follow-up. PROM scores from these time points were compared using the Wilcoxon signed-rank test. The proportion of participants with MCID for improvement and deterioration was compared using the McNemar test. Missing data were assumed to be missing at random; no data were imputed. Data analysis was performed using R version 4.3.0 (R foundation for Statistical Computing).
Funding
This study was funded by ZonMw (the Netherlands Organization for Health Research and Development; project number 10430012010009) and the PPP Allowance made available by Top Sector Life Sciences & Health to Samenwerkende Gezondheidsfondsen (SGF) under project number LSHM18055-SGF to stimulate public–private partnerships, with cofinancing by health foundations that are part of the SGF. Both funding bodies had no role in the design, analysis, or reporting of the study.
RESULTS
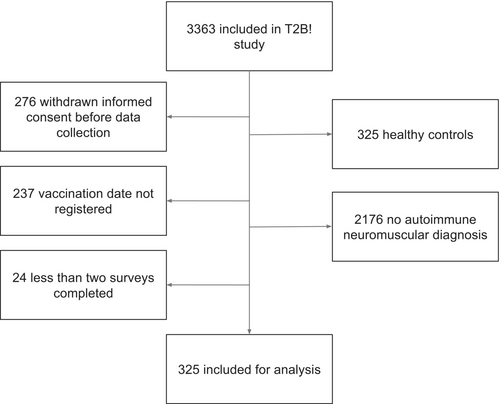
From the cohort of 3363 participants, 325 patients were eligible for this substudy (Figure 1). The median number of completed surveys is six per patient (interquartile range [IQR] = 6–7; Table S1). Autoimmune NMD consisted of 137 (42%) patients with MG, 79 (24%) patients with CIDP, 43 (13%) patients with MMN, and 66 (20%) patients with IIM. Median age was 59 years (IQR = 47–67), 156 (48%) were females, and median BMI was 25.5 (IQR = 22.7–29.1). Median disease duration was 8 years (IQR = 4–14). Of all patients, 267 (82%) were on immunosuppressants, of whom 174 (65%) were on monotherapy and 93 (35%) were on combination therapy. See Table S1 for detailed immunosuppressive treatment per autoimmune NMD. Within the first 60 days after primary immunization, 249 (77%) patients had received two vaccinations, 148 (46%) mRNA-1273 (Moderna), and 131 (40%) BNT162b2 (Pfizer/BioNTech). Overall, 42 (13%) patients had a SARS-CoV-2 infection before first vaccination. Participant characteristics per autoimmune NMD are shown in Table 1.
| Characteristic | MG, n = 137 | CIDP, n = 79 | MMN, n = 43 | IIM, n = 66a |
|---|---|---|---|---|
| Age | ||||
| Years, median (IQR) | 57.0 (45.0–67.0) | 63.0 (53.5–68.5) | 57.0 (48.5–64.0) | 53.5 (41.3–64.5) |
| Sex | ||||
| Female | 78 (56.9) | 28 (35.4) | 8 (18.6) | 42 (63.6) |
| BMI | ||||
| Median (IQR) | 26.3 (23.0–30.8) | 25.5 (23.3–28.2) | 24.0 (22.2–25.2) | 26.1 (22.6–29.3) |
| Disease duration | ||||
| Years, median (IQR) | 8.0 (5.0–18.0) | 6.0 (3.0–10.0) | 11.0 (7.5–18.5) | 5.0 (2.3–9.8) |
| ISP use | ||||
| No ISP | 45 (32.8) | 11 (13.9) | 0 (0) | 2 (3.0) |
| ISP | 92 (67.2) | 68 (86.1) | 43 (100) | 64 (97.0) |
| Monotherapy | 49 (53.3) | 65 (95.6) | 43 (100) | 17 (26.6) |
| Combination therapy | 43 (46.7) | 3 (4.4) | 0 (0) | 47 (73.4) |
| Number of vaccinations within first 60 days of primary immunization | ||||
| 1 | 9 (6.6) | 24 (30.4) | 16 (37.2) | 27 (40.9) |
| 2 | 128 (93.4) | 55 (69.6) | 27 (62.8) | 39 (59.1) |
| Number of vaccinations in primary immunization | ||||
| 1 | 3 (2.2) | 7 (8.9) | 3 (7.0) | 2 (3.0) |
| 2 | 134 (97.8) | 72 (91.1) | 40 (93.0) | 64 (97.0) |
| Vaccine received in primary immunization | ||||
| BNT162b2 (Pfizer/BioNTech) | 8 (5.8) | 55 (69.6) | 24 (55.8) | 44 (66.7) |
| mRNA-1273 (Moderna) | 125 (91.2) | 7 (8.9) | 7 (16.3) | 9 (13.6) |
| ChAdOx1 nCoV-19 (Oxford/AstraZeneca) | 1 (0.7) | 14 (17.7) | 10 (23.3) | 10 (15.) |
| Ad.26.COV2.S (Janssen) | 0 (0) | 3 (3.8) | 2 (4.7) | 2 (3.0) |
| BNT162b2 (Pfizer-BioNTech) or mRNA-1273 (Moderna), not specified | 3 (2.2) | 0 (0) | 0 (0) | 1 (1.5) |
| Number of additional vaccinations | ||||
| 0 | 19 (13.9) | 9 (11.4) | 2 (4.7) | 11 (16.7) |
| 1 | 103 (75.2) | 62 (78.5) | 41 (95.3) | 40 (60.6) |
| 2 | 15 (10.9) | 8 (10.1) | 0 (0) | 15 (22.7) |
| SARS-CoV-2 infection before first vaccination | 16 (11.7) | 9 (11.4) | 8 (18.6) | 9 (13.6) |
| Completed surveys per participant, median (IQR) | 6 (6–6) | 7 (7–8) | 7 (7–8) | 7 (5–8) |
- Note: Table shows participant characteristics per autoimmune neuromuscular disease. Data are given as n (%) except as indicated.
- Abbreviations: BMI, body mass index; CIDP, chronic inflammatory demyelinating polyneuropathy; IIM, idiopathic inflammatory myopathy; IQR, interquartile range; ISP, immunosuppressant; MG, myasthenia gravis; MMN, multifocal motor neuropathy.
- a Twenty-three dermatomyositis, 14 antisynthetase syndrome myositis, 12 immune-mediated necrotizing myopathy, 17 nonspecific/overlap myositis.
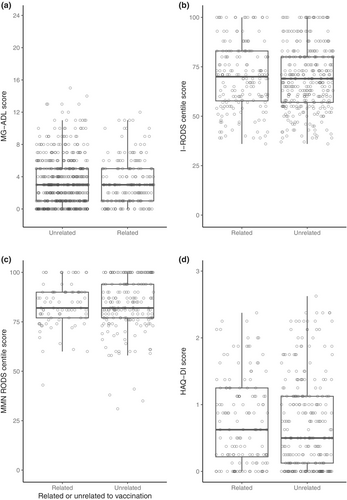
The median PROM scores from surveys related to vaccination versus surveys unrelated to vaccination were for MG-ADL 3 (IQR = 1–5) versus 3 (IQR = 1–5), for I-RODS 70 (IQR = 58–83) versus 69 (IQR = 57–80), for MMN RODS 82 (IQR = 77–90) versus 82 (IQR = 77–94), and for HAQ-DI 0.63 (IQR = 0.22–1.25) versus 0.50 (IQR = 0.13–1.13). See also Figure 2 for an overview of the PROM scores from the longitudinal analysis. The associated differences for vaccination, as regression coefficients from the multivariate model, were 0.01 (95% CI = −0.24 to 0.25) for MG-ADL, 0.47 (95% CI = −0.52 to 1.46) for I-RODS, −0.06 (95% CI = −1.01 to 0.89) for MMN RODS, and 0.04 (95% CI = −0.02 to 0.10) for the HAQ-DI. See also Table 2 for all regression coefficients. Table 2 also shows regression coefficients for other clinical determinants, independent of vaccination. Increasing age was associated with lower I-RODS score (−0.37, 95% CI = −0.68 to −0.07) and higher HAQ-DI score (0.03, 95% CI = 0.02–0.03). Female sex was associated with higher MG-ADL scores (1.58, 95% CI = 0.64–2.52) and HAQ-DI scores (0.21, 95% CI = 0.15–0.27). Increasing BMI was associated with higher HAQ-DI scores with (0.02, 95% CI = 0.01–0.03).
| Dependent variable | ||||
|---|---|---|---|---|
| MG-ADL score | I-RODS centile score | MMN RODS centile score | HAQ-DI score | |
| Univariate | ||||
| Related to vaccinationa | 0.003 (−0.24 to 0.25) | 0.46 (−0.53 to 1.46) | -0.07 (−1.02 to 0.88) | 0.01 (−0.06 to 0.08) |
| Multivariate | ||||
| Related to vaccinationa | 0.01 (−0.24 to 0.25) | 0.47 (−0.52 to 1.46) | −0.06 (−1.01 to 0.89) | 0.04 (−0.02 to 0.10) |
| Age | 0.03 (−0.01 to 0.06) | −0.37 (−0.68 to −0.07)* | −0.34 (−0.72 to 0.04) | 0.03 (0.02 to 0.03)*** |
| Female | 1.58 (0.64 to 2.52)*** | −1.44 (−9.25 to 6.38) | 3.63 (−6.27 to 13.53) | 0.21 (0.15 to 0.27)*** |
| BMI | 0.07 (−0.01 to 0.16) | −0.50 (−1.47 to 0.46) | 0.08 (−1.36 to 1.52) | 0.02 (0.01 to 0.03)*** |
| Immunosuppressant use | 0.08 (−0.89 to 1.05) | 1.74 (−8.20 to 11.67) | 0.13 (−0.29 to 0.55) | |
| Model | LM | LM | LM | Tobit mixed |
| Observations | 717 | 512 | 281 | 395 |
| Related to vaccination | 190 | 180 | 84 | 144 |
| Unrelated to vaccination | 527 | 332 | 197 | 251 |
| Participants | 137 | 79 | 43 | 66 |
- Note: Table shows regression coefficients from the linear and tobit mixed models for all PROMs (uni- and multivariate) and potential clinical determinants.
- Abbreviations: BMI, body mass index; HAQ-DI, Health Assessment Questionnaire–Disability Index; I-RODS, Inflammatory Rasch-Built Overall Disability Scale; LM, linear mixed; MG-ADL, Myasthenia Gravis Activities of Daily Living; MMN RODS, Multifocal Motor Neuropathy Rasch-Built Overall Disability Scale; PROM, patient-reported outcome measure.
- a Related to vaccination is defined as surveys completed within 8–60 days after any SARS-CoV-2 vaccination, and surveys unrelated to vaccination is defined as surveys without prior vaccination, or vaccination >60 days prior to the survey.
- *p < 0.05, ***p < 0.001.
In patients with available paired observations from before first vaccination and 60 days thereafter, median PROM scores did not change, with 3 (IQR = 2–6) versus 3 (IQR = 2–5, p = 0.15) for MG-ADL, 67 (IQR = 60–80) versus 71 (IQR = 61–80, p = 0.72) for I-RODS, 85 (IQR = 81–94) versus 82 (IQR = 79–90, p = 0.22) for MMN RODS, and 0.6 (IQR = 0.1–0.9) versus 0.5 (IQR = 0.3–1.1, p = 0.95) for HAQ-DI. In MG patients, MCID for improvement was observed in 10 (20%) patients, and MCID for deterioration in three (6%, p = 0.10) patients. Of these three patients with deterioration, none reported a change in treatment for their MG. In CIDP patients, MCID for improvement was observed five (17%) patients, and MCID for deterioration in eight (28%, p = 0.58) patients. Of the eight patients with deterioration, two (25%) reported a change in treatment for their CIDP (Table 3).
| PROM score at T0, median (IQR) | PROM score at T60, median (IQR) | MCID improvement, n/N (%) | MCID deterioration, n/N (%) | Treatment change, n/N (%) | |
|---|---|---|---|---|---|
| MG, n = 60 | 3 (2–6) | 3 (2–5) | 10/49 (20.4) | 3/49 (6.1) | 0/3 (0) |
| CIDP, n = 29 | 67 (60–80) | 71 (61–80) | 5/29 (17.2) | 8/29 (27.6) | 2/8 (25.0) |
| MMN, n = 19 | 85 (81–94) | 82.0 (79–90) | NA | NA | NA |
| IIM, n = 31 | 0.6 (0.1–0.9) | 0.5 (0.3–1.1) | NA | NA | NA |
- Note: Table shows median PROMS before first vaccination (T0) and at first follow-up survey, completed 60–66 days after first vaccination (T60). The proportion of MCID for improvement and deterioration is shown for MG-ADL (2 points) and I-RODS (4 points on the centile scale). Treatment change is as reported by the participant.
- Abbreviations: CIDP, chronic inflammatory demyelinating polyneuropathy; IIM, idiopathic inflammatory myopathy; IQR, interquartile range; MCID, minimally clinically important difference; MG, myasthenia gravis; MMN, multifocal motor neuropathy; NA, not available; PROM, patient-reported outcome measure.
DISCUSSION
In our study, we observed no difference in patient-reported daily functioning after SARS-CoV-2 vaccinations in any of the four autoimmune NMDs, through analyzing longitudinal data up to 1 year after first vaccination. Instead, differences in daily functioning were associated with age, female sex, or BMI, depending on the autoimmune NMD. Additionally, we did not observe a change in daily functioning when analyzing paired measurements before first vaccination and 60 days thereafter. For autoimmune NMD and PROMs with a validated MCID (MG-ADL and I-RODS), the proportion of patients with deterioration was comparable to the proportion with improvement. In the case that patients did deteriorate by at least the MCID, a treatment change was reported rarely, indicating no severe deterioration in most.
We used differences in disease-specific PROMs for the selected autoimmune NMD as a proxy marker for changes in disease activity. Previously, we assessed disease activity by asking patients with IMIDs if they felt that their disease activity had changed using a 5-point Likert scale and observed a higher rate of self-reported increased disease activity in patients with autoimmune NMD [4]. Arguably, self-reported changes in disease activity measured on a Likert scale are more subjective than assessing changes in daily functioning using disease-specific PROMs as a proxy for disease activity. PROMs have been developed to measure specific domains and clinimetrically evaluated, and their use is advocated in clinical care and trials. Moreover, for some of the PROMs, MCIDs have been defined that delineate clinically relevant changes, which makes interpretation easier. Despite the lack of an MCID for MMN and IIM, one might conclude from our findings that also in MMN and IIM there is very likely no clinically relevant difference in daily functioning after vaccination, as the observed difference was very small considering the range of the scale, and subsequently not significant in either the regression model or pairwise comparative analysis.
Our findings are consistent with the few available prospective studies on disease activity or disease flare-ups after vaccination in this population. Two studies on MG patients showed no clinically significant worsening of MG symptoms, although follow-up was limited to 1 week after first and second vaccination, which likely is too short [6, 26]. In patients with CIDP and MMN, Baars and colleagues found self-reported increased disease activity in the first 6 weeks after the last vaccination of primary immunization in only 5% and 4% of patients, respectively [5]. However, this rate was not compared to a control group. Doneddu and colleagues found no significantly higher risk of relapse (defined as significant change in PROM scores, clinical evaluation, or adjusting treatment due to clinical worsening) in CIDP and MMN patients after vaccination compared to patients who did not get vaccinated, although these findings should be interpreted with caution due to the small number of nonvaccinated participants [27]. In contrast, the risk of relapse within 3 months after vaccination was 5% compared to 1% before the first vaccination in the same group, leading to a significantly increased relative risk of relapse for CIDP patients but not for MMN patients. In IIM patients, the relapse rate within 3 months after vaccination was comparable for vaccinated and unvaccinated patients, although the unvaccinated group was too small to draw sound conclusions [7]. Likewise, several retrospective studies on short- and long-term safety of SARS-CoV-2 vaccination in MG and IIM patients showed favorable safety profiles [28-36].
Despite the lack of evidence for increased disease activity after SARS-CoV-2 vaccination, fear of deterioration remains among IMID patients, leading to vaccine hesitancy [3]. This has implications for prevention of severe SARS-CoV-2 but may also translate to other vaccinations such as influenza, as observed in patients with MG [37]. Results from this study may aid in alleviating vaccine hesitancy in patients with autoimmune NMD and are all the more important as patients with MG have a higher risk of exacerbation after infection compared to vaccination, thus further highlighting the benefits of vaccination in this group [28].
Strengths of this study include the prospective design and collection of disease-specific PROM scores from a relatively large number of patients with different autoimmune NMDs. Moreover, we assessed disease activity from 8 days or more after vaccination, to prevent short-term adverse events influencing the PROMs. Additionally, the follow-up period of >1 year after first vaccination allowed collection of data on repeated vaccinations. Limitations include the lack of a nonvaccinated control group and the absence of MCIDs for MMN and IIM, as validated MCIDs are important to evaluate the clinical relevance of a difference in PROMs. Also, some of the PROMs reflect disability, whereas others reflect daily living. Also, patients were asked about a treatment change in the surveys, but without specification regarding whether this meant an intensification or reduction of the treatment, and with no verification on this change such as by checking prescription details; thus, we have no objective data on the treatment change. Although it is unlikely that this occurred frequently, some of the treatment changes may have been reduction of treatment and thus would not have been a consequence of increased disease activity. Furthermore, our analysis is at risk of selection bias, as the T2B! study primarily enrolled patients willing to get vaccinated, and patients with high disease activity might have been less willing to participate.
CONCLUSIONS
In our prospective disease-overarching cohort, we observed no difference in patient-reported daily functioning after SARS-CoV-2 vaccinations in autoimmune NMD. These findings confirm the safety of SARS-CoV-2 vaccines for patients with autoimmune NMD regarding disease activity and may aid in alleviating vaccine hesitancy.
AUTHOR CONTRIBUTIONS
Koos P. J. van Dam: Data curation; writing – original draft; formal analysis; validation. Luuk Wieske: Data curation; writing – original draft; formal analysis; validation. Eileen W. Stalman: Data curation; writing – review and editing; validation. Laura Y. L. Kummer: Data curation; writing – review and editing; validation. Anneke J. van der Kooi: Data curation; writing – review and editing. Joost Raaphorst: Data curation; writing – review and editing. Diederik van de Beek: Data curation; writing – review and editing. Jan J. G. M. Verschuuren: Data curation; writing – review and editing. Annabel M. Ruiter: Data curation; writing – review and editing. Esther Brusse: Data curation; writing – review and editing. Pieter A. van Doorn: Data curation; writing – review and editing. Adája E. Baars: Data curation; writing – review and editing. W. Ludo van der Pol: Data curation; writing – review and editing. H. Stephan Goedee: Data curation; writing – review and editing. Anja ten Brinke: Data curation; writing – review and editing. S. Marieke van Ham: Data curation; writing – review and editing. Theo Rispens: Data curation; writing – review and editing. Taco W. Kuijpers: Data curation; writing – review and editing. Filip Eftimov: Data curation; writing – original draft.
ACKNOWLEDGMENTS
We would like to thank ZonMw (the Netherlands Organization for Health Research and Development, grant 10430072010007) and the T2B! partners, including the patient groups and Health Holland, for supporting this study. Also, we would like to thank E. P. Moll van Charante, J. A. Bogaards, and R. A. Scholte for their guidance on the data safety monitoring board.
FUNDING INFORMATION
This study was supported by ZonMw (the Netherlands Organization for Health Research and Development; project number 10430012010009). ZonMw had no role in the design, analysis, or reporting of the study.
CONFLICT OF INTEREST STATEMENT
F.E. and T.W.K. report (governmental) grants from ZonMw to study immune response after SARS-Cov-2 vaccination in autoimmune diseases. F.E. also reports grants from Prinses Beatrix Spierfonds, CSL Behring, Kedrion, Terumo BCT, Grifols, Takeda Pharmaceutical Company, and GBS-CIDP Foundation; consulting fees from UCB Pharma and CSl Behring; and honoraria from Grifols. A.J.v.d.K. reports grants from CSL Behring and participation on an advisory board for Argenx. J.J.G.M.V. reports consulting fees from Argenx, Alexion, and NMD Pharma and is coinventor on patent applications based on MuSK-related research. P.A.v.D. has received research grants from Prinses Beatrix Spierfonds, the Netherlands Organisation for Health Research and Development (ZonMW), Sanquin, Takeda, and Grifols. He is a member of scientific advisory committee/steering committee trials for Annexon, Argenx, Hansa, Octapharma, Sanofi, and Roche. All grants and fees were paid to his institution. H.S.G. is a board member of the Dutch Society of Clinical Neurophysiology (unpaid), reports grants from Prinses Beatrix Spierfonds, and has received speaker fees from Shire/Takeda. W.L.v.d.P. reports grants from Prinses Beatrix Spierfonds, stichting Spieren voor Spieren, Vriendenloterij, and EU Horizon 2020 and has participated on scientific advisory boards for Argenx, Biogen, and Novartis gene therapies. None of the other authors has any conflict of interest to disclose.
Open Research
DATA AVAILABILITY STATEMENT
After publication, anonymized individual participant data and a data dictionary will be made available upon request to the corresponding author to researchers who provide a methodologically sound proposal. Data will be shared through a secure online platform.




