Development of postural adjustments during reaching in infants at risk for cerebral palsy from 4 to 18 months
Abstract
Aim
To investigate postural adjustments during reaching in infants at high risk for cerebral palsy (CP).
Method
Observational cohort study in which 25 infants at high risk (11 males, 14 females) and 11 infants with typical development (six males, five females) were assessed at 4, 6, and 18 months corrected age. Reaching movements were elicited during supported and unsupported sitting, while surface electromyography was recorded of arm, neck, and trunk muscles. Percentages of direction-specific adjustments (first level of control), and recruitment patterns and anticipatory activation (second level of control) were calculated. Statistical analyses were performed with a binomial generalized estimating equations model for dichotomous variables and a linear mixed model for continuous variables.
Results
Postural activity of infants at high risk for CP at 4 months was virtually similar to that of infants with typical development. At 18 months, infants at high risk differed from infants with typical development with less direction-specificity (median values 20% vs 58% at trunk and neck level, OR 0.38, 95% CI 0.18–0.82); longer latencies to trunk muscle activation; and less anticipatory activation (41% vs 55%, in any direction-specific muscle, OR 0.53, 95% CI 0.32–0.89). In unsupported sitting, results were largely similar to those in supported sitting.
Interpretation
Infants at high risk for CP grew into a postural deficit: at 18 months they showed delayed development of direction-specificity, and postural dysfunction as evidenced by slower recruitment of postural muscles and less frequent anticipatory activation.
What this paper adds
- In infants at high risk for CP, direction-specificity develops with delay from 6 months onwards.
- Infants at high risk for CP grow into a deficit: at 18 months they have slower recruitment of postural muscles and decreasing anticipatory activation.
This article is commented on by Saavedra on pages 598–599 of this issue.
Abbreviations
-
- NOS
-
- Neurological optimality score
Children with developmental motor disorders such as cerebral palsy (CP) often have problems with postural control that hamper everyday activities. For example, a child with a severe form of CP may never learn to walk or to sit independently, because of an inability to control posture, including that of the trunk. Children with less severe forms of CP are able to sit and walk but may have trouble reaching for and handling objects, as perception of and interaction with objects requires stabilising the head and trunk in space.1 This paper focuses on postural muscle recruitment strategies in infants at high risk for CP.
Previous studies suggest that typical postural development is guided by two principles. The first principle is the concept of the neuronal group selection theory, that motor development is characterized by variation, the presence of a repertoire of strategies, and the development of variability, that is the ability to select situation-specific strategies out of the repertoire.2
The second principle is the organization of postural control into two functional levels (the so-called central pattern generator model).3 The first or basic level consists of direction-specificity, which means, for instance, that when balance is threatened by a forward sway of the body, the muscles on the dorsal side are primarily activated. The second level consists of fine-tuning of the direction-specific adjustments to the specifics of the situation, for example in the number of direction specific muscles that are activated, the order in which they are activated, and the strength of the contraction.3
In infants with typical development, direction-specific postural adjustments during reaching are only present in 40% to 50% of reaches in early infancy, to reach a value of 60% to 80% at 18 months.4 In older children, direction-specificity is consistently present during reaching.5 The second level of postural control in early infancy is mostly characterized by variation. Nevertheless, young infants slightly prefer top-down recruitment when reaching during supported sitting, which changes into a slight preference for bottom-up recruitment at 18 months.4
In children with CP, direction-specificity is generally intact.6-8 Direction-specific postural adjustments have been noted to be entirely absent in the two children studied worldwide, who functioned at Gross Motor Function Classification System (GMFCS)9 level V and did not learn to sit independently.6, 8 However, postural fine-tuning is affected in virtually all children with CP. School-age children with CP have a strong preference for top-down recruitment, and are less able to adapt their postural adjustments to the specifics of the situation such as sitting position or reaching speed.5, 7, 8, 10, 11
Information on postural adjustments of infants developing CP is scarce, as CP cannot be diagnosed reliably before 1.5 to 2 years of age.12 Hadders-Algra et al.6 studied infants between 4 months and 18 months who developed CP and reported the presence of direction-specific postural adjustments during reaching from 15 months onwards. In agreement with the findings in school-age children, these infants had a preference for top-down recruitment.6 In addition, studies investigating non-linear centre of pressure suggest that infants with developmental delay have lower complexity and variation of postural sway,13 which may indicate a reduced motor repertoire.2 Finally, De Graaf-Peters et al.14 studied infants aged 4 to 6 months and reported less direction-specificity in infants at high risk than in infants with typical development. To the best of our knowledge, no longitudinal studies addressed postural control in terms of muscle recruitment strategies of infants at high risk for CP, that is infants who have an abnormal motor development and might benefit from early intervention. The aim of this study was to fill this gap.
We studied postural control during reaching in infants at high risk for CP at 4, 6, and 18 months corrected age. We hypothesized that (1) infants at high risk would display direction-specificity, but would develop consistent use of direction-specificity at a slower pace; (2) infants at high risk would have a preference for top-down recruitment of the postural muscles throughout infancy; and (3) parameters of postural control would be related to motor development, that is lower rates of direction-specificity and/or an unchanging preference for top-down recruitment would be related to a worse neurological condition at 18 months.
Method
Participants
Twenty-five infants (11 males, 14 females) were included in the study at 3 months corrected age. They had been admitted to the neonatal intensive care unit of the University Medical Center Groningen in 2003 to 2005, and were included based on the presence of definitely abnormal general movements at 10 weeks corrected age, indicating a high risk for developmental disorders.15, 16 Gestational age at birth was 25 to 39 weeks (median: 30wks); birthweight 635–3460g (median: 1190g). The infants at high risk participated as controls in a study on the effect of early intervention (for details see Ref. 17). They were assessed longitudinally at 4, 6, and 18 months corrected age. At 18 months the infants were neurologically assessed according to Hempel.18 This resulted in a clinical classification19 and a quantitative measure, the neurological optimality score (NOS) (maximum score 57 points20). Three infants were not assessed at 18 months for logistical reasons.
Postural control data of 11 infants with typical development who were born at term were available (median gestational age: 40.5wks; birthweight: 3000–4000g, median 3463g).4 Power analysis revealed that with these group sizes, a difference in direction-specificity of 25% could be detected with 80% power (α=0.05, mean and SD based on De Graaf-Peters et al.14).
Protocol
A detailed description of the protocol and methods can be found in van Balen et al.4 and in Data S1 (supporting information online). In short, reaching movements were elicited from the infant seated in a supported sitting position (in an infant chair or on their parent's lap). At 18 months, the infants were also assessed in a ‘long-leg’ unsupported sitting position on a flat surface. Electromyography (EMG) was measured continuously with bipolar surface electrodes on the following right-sided muscles: deltoid, pectoralis major, biceps brachii, triceps brachii, neck flexor (sternocleidomastoid), neck extensor, rectus abdominis, thoracal extensor, and lumbar extensor. Deltoid, pectoralis major, biceps brachii, and triceps brachii are referred to as arm muscles; neck flexor, neck extensor, rectus abdominis, thoracal extensor, and lumbar extensor as postural muscles. The sessions were recorded on video, which was time-coupled to the EMG recordings.
Video and EMG analysis
Using the video, we selected arm movements occurring in response to the toy, excluding trials with inappropriate sitting position, attentional state, or reaching movements not involving the right arm. EMG analyses (artefact correction and detection of significant bursts of phasic muscle activity) were carried out with the PedEMG programme (Developmental Neurology, University Medical Center Groningen, the Netherlands; see Ref. 4). The EMG was scanned for activation of the arm muscles from 500ms before visible movement in the video, and the start of the reach was defined as the onset time of the first arm muscle activity that was related to the reaching movement (i.e. the onset of the prime mover, the arm muscle initiating the reach). For the postural muscles, increased activity was included if found within a time window consisting of (1) 100ms before activation of the prime mover, (see Boxum et al.21) and (2) the duration of (the first 1000ms of) the reaching movement. For each trial, we determined the following parameters: (1) direction-specificity; a trial was direction-specific if the dorsal muscle was recruited before the antagonistic ventral muscle or without antagonistic activation. Thus, a trial was direction-specific at trunk level if thoracal extensor and lumbar extensor were recruited before rectus abdominis, or if either thoracal extensor or lumbar extensor was recruited without rectus abdominis recruitment. Direction-specificity at both neck and trunk level entailed direction-specific recruitment of both the trunk and neck muscles in the same trial. (2) The pattern of postural adjustments, that is the specific combination in which direction-specific muscles were activated, either alone or in concert. Specific attention was paid to the occurrence of the ‘complete pattern’, that is the pattern in which all recorded direction-specific muscles recruited. (3) The recruitment latencies of postural muscles, defined as the time interval between the onset of the prime mover and the onset of activity in the postural muscle. For each infant at each age median latency values were calculated. (4) Recruitment order (top-down, bottom-up, or otherwise), which could only be determined when at least two direction-specific muscles showed significant phasic activity. If two muscles were activated within an interval of 20ms, recruitment was considered to be simultaneous. Recruitment of three dorsal muscles with thoracal extensor earlier than neck extensor and lumbar extensor was defined as mixed order recruitment. (5) The presence or absence of anticipatory postural activity at the neck and/or trunk level (i.e. activation starting within 100ms before the prime mover).
Statistics
Statistical modelling was carried out using the Statistical Analytics Software 9.3 (SAS Institute Inc., Cary, NC, USA). For the dichotomous parameters (the presence or absence of direction-specificity, the complete pattern, top-down and bottom-up recruitment, and anticipatory activation), a binomial generalized estimating equations model with repeated measurements was fitted using predictor variables Age, Position, and Group. To take missing data into account, the parameters were modelled as ratios, that is the number of trials with the parameter value ‘true’, divided by the total number of trials. For example, for each child at each age and position, direction-specificity was modelled as the number of direction-specific trials divided by the total number of trials for which direction-specificity could be computed. Continuous variables (the median latencies) were modelled with a linear mixed model with repeated measurements. Finally, a linear mixed model was also used to model the relationship between the postural parameters direction-specificity and anticipatory muscle activity and the NOS. OR (including 95% CI in square brackets) are reported for the statistical analyses; to aid interpretation of the results we also calculated the percentage of trials per infant for each outcome variable (e.g. the percentage of direction-specific trials, the percentage of trials with top-down recruitment, and so on), and report median values of these percentages.
Consent
The infants’ parents gave informed consent and the procedures were approved by the ethics committee of the University Medical Center Groningen.
Results
Table 1 shows the number of infants assessed at each age. Missing data occurred because of non-participation, for logistical reasons (three infants at 18mo); technical issues with video or EMG recording; lack of cooperation of the infants, resulting in an insufficient number of suitable trials; the inability to reach (particularly at 4mo); and difficulties in keeping the electrodes properly attached (especially at 18mo). Missing data were addressed separately for each outcome parameter, that is if neck signals were unavailable for a certain trial because of interference by the infant, data of that trial were included in the analysis of direction-specificity at trunk level but not in the analysis at neck level. This resulted in different n (varying from 9–20 in supported sitting and 4–6 in unsupported sitting) for each outcome parameter (see Figs. 1-3).
| Group | Supported sitting | Unsupported sitting | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Position | 4mo | 6mo | 18mo | 18mo | |||||
| Infants | Trials | Infants | Trials | Infants | Trials | Infants | Trials | ||
| High risk for CP | Chair | 23 | 9 (1–19) | 25 | 14 (5–22) | 16 | 19.5 (5–38) | 11 | 16 (3–23) |
| Lap | 0 | 0 | 0 | 0 | 3 | 15 (8–18) | |||
| Typical development | Chair | 9 | 9 (1–16) | 11 | 11 (7–30) | 2 | 17 (5–29) | 5 | 18 (9–21) |
| Lap | 0 | 0 | 0 | 0 | 8 | 16 (6–24) | |||
- Data are given as number, or median (range).


Preliminary data analysis indicated that EMG activity of the 18-month-old lap-sitting infants (n=3) did not differ from that of their chair-sitting peers (n=16). Therefore, the 18-month-data of chair-sitting and lap-sitting were pooled. Unsupported sitting data at 18 months were analysed separately and compared with supported sitting data.
Longitudinal data in supported sitting
Direction-specificity
In the group of infants at high risk, the percentage of reaches accompanied by direction-specific postural activity at trunk level was 50% to 58% at all ages studied (median values; see Fig. 1). This development differed from that of the infants with typical development, whose direction-specificity at trunk level increased significantly from 55% to 61% at 4 months and 6 months, to 88% at 18 months (OR 3.38, [1.85–6.13]).4 As a result, at 18 months the infants at high risk showed significantly less often direction-specific activity at trunk level than the infants with typical development (OR 0.39, [0.22–0.71]).
At 6 months and 18 months, direction-specificity at neck level seemed to occur less frequently in the group of infants at high risk than in the group of infants with typical development (median values 26% vs 48% at 6mo and 39% vs 48% at 18mo respectively), but the difference was only statistically significant at 6 months (OR 0.56, [0.38–0.81]).
At 4 months the occurrence rate of direction-specific postural activity at both trunk and neck level did not differ significantly between groups (high risk 33%, typical development 36%), but at 6 months and 18 months it was significantly lower in the group of infants at high risk than in the group of infants with typical development (OR at 6mo: 0.42 [0.26–0.69]; median values: high risk 24%, typical development 42%; OR at 18mo: 0.38 [0.18–0.82]; median values: high risk 20%, typical development 58%; see Fig. 1).
Muscle recruitment strategies
In both groups and at all testing ages, the complete pattern was the dominant pattern of activation (median values: high risk 83%, 71% and 81% at 4, 6, and 18mo respectively; typical development 56%, 71%, and 72%).
In both groups recruitment order of the direction-specific muscles varied substantially (Fig. 2). Nevertheless, some differences between groups were found. In infants at high risk, we did not see the pattern that we reported earlier in infants with typical development4 (i.e. recruitment order changing from a slight preference for top-down recruitment in early infancy to a modest preference of bottom-up recruitment at 18mo). As a result, at 4 months infants at high risk less often used top-down recruitment than infants with typical development (OR 0.41 [0.20–0.87], median values: high risk 17%, typical development 37%). A similar outcome seemed to be present at 6 months but we could not demonstrate a statistically significant difference (OR 0.48 [0.22–1.05], median values: high risk 15%, typical development 40%; see Fig. 2). Top-down recruitment increased in the group of infants at high risk between 4 months and 18 months (OR 2.04 [1.23–3.40]) to reach a level similar to that of the infants with typical development at 18 months (high risk 31%, typical development 27%; see Fig. 2).
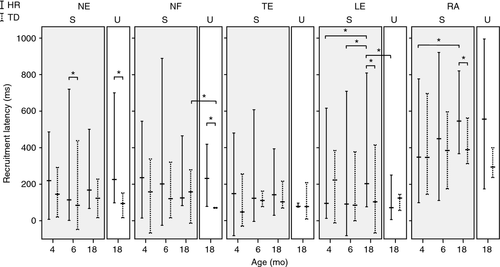
Latencies and anticipatory postural muscle activation
Latencies to postural muscle recruitment varied considerably in both groups, at all ages and in all muscles. At 4 months, median latencies of the postural muscles did not differ significantly between the groups (Fig. 3). At 6 months, neck extensor was recruited more slowly in infants at high risk than in infants with typical development (absolute difference 82ms, [95% CI 4–160ms]). At 18 months, this was the case for lumbar extensor (94ms [17–172ms]) and rectus abdominis (97ms [21–172ms]). In infants at high risk, lumbar extensor recruitment was 89 and 97ms slower at 18 months than at 4 months and 6 months respectively (95% CI [8–170ms] and [21–173ms]) and rectus abdominis recruitment was 100ms [17–183ms] slower at 18 months than at 4 months.
In the infants at high risk, anticipatory postural activation in any of the direction-specific muscles occurred in 38%, 71%, and 41% of trials, at 4, 6, and 18 months respectively (median values; see Fig. 2). In infants with typical development, the corresponding percentages were 60%, 53%, and 55%. Anticipatory activation in 18-month-old infants at high risk was significantly less common than in typically developing age-matched peers (OR 0.53 [0.32–0.89]). The 18-month values of the infants at high risk were also lower than their 6-month values (OR 0.37 [0.18–0.73]). The differences described were mainly because of less frequent anticipatory activation of neck extensor (18mo high risk vs typical development: 24% vs 30%, OR 0.44 [0.25–0.79]; 18mo high risk vs 6mo high risk: 24% vs 33%, OR 0.54 [0.31–0.94]). In the trunk muscles, the pattern looked similar but only the difference between 6 months and 18 months in infants at high risk was significant (18mo high risk vs typical development: 20% vs 31%, OR 0.58 [0.31–1.09], 18mo high risk vs 6mo high risk: 20% vs 33%, OR 0.39 [0.20–0.78]).
Unsupported sitting at 18 months and the effect of sitting position
Direction-specificity
In both groups, there was no significant effect of sitting position on direction-specificity in the trunk (high risk supported 58% vs unsupported 63%; typical development 88% vs 81%), neck (high risk 39% vs 30%; typical development 48% vs 40%), or both neck and trunk simultaneously (high risk 20% vs 11%; typical development 58% vs 50%).
Similar to supported sitting, in the unsupported sitting position 18-month-old infants at high risk showed less often direction-specificity at trunk, at neck, and at both levels than infants with typical development (median values: trunk high risk 63%, typical development 81%, OR 0.40 [0.16–0.99]; neck high risk 30%, typical development 39%, OR 0.66 [0.46–0.93]; both trunk and neck simultaneously high risk 11%, typical development 50%, OR 0.34 [0.13–0.88]). Thus, in agreement with the data from supported sitting, direction-specificity was less likely to occur in infants at high risk than in infants with typical development.
Muscle recruitment strategies
In the unsupported condition, the infants at high risk used the complete pattern less often than the infants with typical development (80% vs 100%, OR 0.17 [0.04–0.66]). In infants with typical development, the complete pattern was used more often in unsupported sitting than in supported sitting (median values 100% vs 72%; OR 6.21 [1.90–20.41]). A similar difference was absent in the infants at high risk (80% vs 81%).
Interestingly, in both groups, preference for a specific recruitment order seemed to be different for the two sitting conditions (Fig. 2). During unsupported sitting, bottom-up recruitment was seen more frequently in infants at high risk than in infants with typical development (high risk 57%, typical development 20%, OR 2.92 [1.13–7.52]). This difference seemed to be mostly caused by a drop in bottom-up recruitment in infants with typical development in unsupported sitting compared with supported sitting (20% vs 42%, OR 0.50 [0.31–0.83]). The limited number of direction-specific trials in the group of infants at high risk meant that the within-group differences were not statistically significant.
Latencies and anticipatory postural muscle activation
Neck extensor and neck flexor recruitment during unsupported sitting was slower in infants at high risk than in infants with typical development (neck extensor by 199ms [86–312ms]; neck flexor by 129ms [47–211ms]; see Fig. 3). In infants at high risk (but not in infants with typical development), lumbar extensor recruitment was 141ms [69–212ms] faster during unsupported sitting than during supported sitting; in infants with typical development (but not in infants at high risk), neck flexor was recruited faster in unsupported sitting than supported sitting, by 86ms [57–115ms].
Anticipatory activation in unsupported sitting was similar to that in supported sitting for both groups, but there were no statistically significant differences between groups.
Relationship to motor development
Of the 23 infants assessed at 18 months, five developed CP (four infants developed a bilateral spastic CP [GMFCS level: II, n=2; III, n=2], and one developed a unilateral spastic CP [GMFCS II]). Of the remaining infants, 16 had complex minor neurological dysfunction and two had a neurological condition within the normal range. The quantitative measure of neurological condition, the NOS, varied from 10 to 47 (median value 27). The postural adjustments of the children with CP did not differ significantly from those without CP. However, the NOS did correlate significantly with direction-specificity at trunk level at 18 months: for every percent increase in direction-specificity at trunk level, the NOS score increased by 0.79 (95% CI [0.37–1.21]). NOS was not associated with the rate of anticipatory muscle activity (data not shown).
Discussion
This study demonstrated that, in early infancy, postural control during reaching while sitting does not differ between infants at high risk for CP and infants with typical development. However, infants at high risk gradually grow into a postural deficit, which at 18 months is expressed by lower rates of direction-specificity, lower rates of anticipatory muscle activation, increasingly longer latencies to postural muscle recruitment, and a lower rate of the complete pattern in the most challenging postural condition of unsupported sitting.
Supported sitting
In accordance with our first and third hypotheses, we found that the age-related increase of direction-specific postural adjustments, which is present in infants with typical development, was absent in infants at high risk, and that lower rates of direction-specificity at 18 months were associated with a worse neurological condition. As consistent direction-specificity during reaching is present in children with CP functioning at GMFCS levels I to IV from preschool age onwards,8 this could be interpreted as a delay in the development of direction-specificity. This is consistent with earlier findings of delayed development of direction-specificity in infants with CP.6 It also implies that a similar delay is found in infants with complex minor neurological dysfunction at 18 months, endorsing the concept that the aetiology of complex minor neurological dysfunction mimics that of CP.22 The delay may reflect impaired selection of optimal motor strategies (impaired ‘variability’),2 which in turn may be caused by deficits in sensory processing.2, 23, 24
However, contrary to our second hypothesis, infants at high risk did not use top-down recruitment more often than did infants with typical development. In fact, at 4 months, top-down recruitment frequency was lower in infants at high risk than in infants with typical development. With age, it rose to a level similar to that of infants with typical development. The increase of top-down recruitment in infants at high risk between 4 months and 18 months may be a delayed onset of the top-down recruitment preference seen in infants with typical development at 4 months. Top-down recruitment may be a good strategy when head stabilization is of primary concern, that is for infants with typical development at 4 months when they start reaching,5 and in children with CP also at a later age because they have deficits in head stability. As head stability is affected in children with CP even in quiet sitting,25 it might be a good strategy to make an effort to stabilize the head in space early during a reaching movement to improve hand–eye coordination, thus making it easier to grasp the object. Bottom-up recruitment is preferred when postural stability of lower body parts is at stake.4, 10 As such, the different recruitment order strategies may be considered adaptive, that is the developmental trajectory of recruitment order is adapted to the postural tasks that are most challenging for the infant at each age.
Interestingly, the latencies to recruitment of the postural muscles and the frequency of anticipatory activation were similar in early infancy. But with increasing age, development of infants at high risk and those with typical development diverged: latencies of postural trunk muscles grew longer in infants at high risk whereas those of infants with typical development did not. As a result, infants at high risk showed less anticipatory activity at 18 months than infants with typical development. Alternatively, the anticipatory activity could have been present but slower in infants at high risk, placing the start of some anticipatory activation before the start of the monitoring window. We could not test this hypothesis as lengthening the window would have introduced a high number of false positives because of movements not related to the reaching movement. Either way, the slower recruitment in infants at high risk at 18 months suggests that postural development was not only characterized by developmental delay in direction-specificity and recruitment order, but also by postural dysfunction. The data suggest that the infants at high risk showed a ‘growing into deficit’ phenomenon.26 This may be a result of changing neuromotor control with age, changing physical characteristics with age that insufficiently adapted neuromotor control cannot adequately address, or a combination of both.
Unsupported sitting at 18 months and the effect of sitting position
As the sitting positions in supported sitting were not comparable between groups at 18 months, it could be argued that the difference in direction-specificity at that age was a result of variations in sitting position, that is lap versus chair. However, in the unsupported sitting position, direction-specificity was also higher in infants with typical development than in infants at high risk. As the lap-sitting and chair-sitting positions were far more alike in terms of postural support, positioning of the limbs, and trunk posture, it is unlikely that the lap-sitting position was the cause of the increased direction-specificity in infants with typical development.
However, while direction-specificity was similar between sitting positions, we did find a difference between the unsupported and the supported sitting position in the prevalence of the complete pattern. Note that we did not find group differences in the occurrence of the complete pattern during supported sitting, supporting our suggestion that chair- and lap-sitting were associated with similar postural challenges. Infants with typical development more often selected the complete pattern in unsupported sitting than in supported sitting, resulting in higher occurrence of this pattern in infants with typical development than in infants at high risk during unsupported sitting. As unsupported sitting is a more demanding posture than supported sitting, increased use of the complete pattern may indicate better variability, that is adaptation of the postural pattern to the specifics of the situation. We also saw adaptation to the sitting position in the recruitment order of infants with typical development: in unsupported sitting, they used less bottom-up recruitment than in supported sitting, which was also less than infants at high risk in the same unsupported sitting position. In the infants at high risk, this adaptation to sitting position was absent.
The strength of our study is the application of a longitudinal approach in a natural setting (i.e. during reaching while sitting) of infants at high risk with a well-documented clinical history and neurological condition. The infants at high risk are representative of infants admitted to a Dutch neonatal intensive care unit and presenting with definitely abnormal general movements around 3 months corrected age. However, our study has several limitations. First, the number of infants with sufficient data for all parameters was relatively small. This was caused mainly by the selection of trials of reaches with direction-specific postural activity for the assessment of the fine-tuning of postural control (muscle activation patterns and recruitment order), and the difficulty of keeping all surface electrodes properly attached during the entire session: infants are aware of the strange situation of the recording and have the ability to explore the presence of electrodes and cables, but they do not have the cognitive ability to understand the information that it is better to leave the recording devices in place. This caused different sample sizes for different parameters, as each parameter required a proper EMG signal of a different combination of muscles. The relatively small sample sizes limit interpretation of the data. On the other hand, it should be realized that this is a common limitation in this type of infant research.27, 28 Second, as only 25% of the infants at high risk developed CP, the validity of the results for infants who will later on be diagnosed with CP is limited. However, as almost all infants at high risk displayed complex neurological dysfunction at 18 months, the results are representative for infants at risk for developmental motor disorders. Third, we took EMG-measurements only on the right side of the body. Contralateral postural adjustments were therefore not taken into account. Consequently, we may have missed some anticipatory postural activity, as it previously has been shown that fast pointing movements in standing school-age children are associated with contralateral anticipatory postural activity.29 However, we assume that the task of infant reaching, which often involves bilateral arm activity and is performed at a slower pace, is less often associated with specific contralateral postural activity. Fourth, the results at the age of 18 months could have been influenced by the slightly different position of the infants at this age. Fifth, studying infants in a natural setting automatically implies limited control of the experimental set-up. As a result, variation in the initial position of the infant and in reaching behaviour may have influenced postural activity. Sixth, it might be argued that infants with definitely abnormal general movements are bound to show atypical postural control. However, the data showed that postural control of the infants at high risk at 4 months – the age closest in time to the abnormal general movements – was only slightly different from that of infants with typical development. The postural deficits of the infants at high risk increased as they grew older.
In conclusion, our data indicate that infants at high risk for CP show impairments at the first level of postural control (reduced direction-specificity) and an altered organization of the second level of control (different recruitment order and less anticipatory muscle activation). The deficits increase with age and may reflect a combination of developmental delay and increasing dysfunction. In particular, the increase in latencies to postural muscle recruitment supports the latter hypothesis. The clinical implication of these findings is a coin with two sides. On the one hand, training of the postural abilities of infants at high risk is recommended by exposing the infants to posturally challenging situations, for instance, by providing just minimal postural support when a sitting infant reaches for a toy. On the other hand, acknowledging that infants at high risk are hampered by postural impairment, adequate postural support is required when the infant is engaged in learning fine motor and cognitive skills. This implies that the provision of postural support in infants at high risk is a continuous search for finding the balance between too much and too little (see also Ref 30).
Acknowledgements
We kindly acknowledge the contribution in data collection and data analysis of Tom van Leussen, Ines Krabben, Janneke Viergever, Victorine B. de Graaf-Peters, PhD, Cornill H. Blauw-Hospers, PhD, Hanneke Bakker, MSc, Leo A. van Eykern, Jeroen van der Eb, PhD, and Michiel Schrier. The study was supported by Stichting Fonds de Gavere, the Johanna KinderFonds, the Cornelia Stichting and the Post-graduate School BCN Groningen. The authors have stated that they had no interests which might be perceived as posing a conflict or bias.




