Triage of LSIL/ASC-US with p16/Ki-67 dual staining and human papillomavirus testing: a 2-year prospective study
Abstract
Objective
To investigate human papillomavirus (HPV) DNA testing and p16/Ki-67 staining for detecting cervical intraepithelial grade 2 or worse (CIN2+) and CIN3 in women referred to colposcopy with minor abnormal cervical cytology low-grade squamous intraepithelial lesions (LSIL) and atypical squamous cells of undermined significance (ASC-US). The clinical performance of both tests was evaluated as stand-alone tests and combined, for detection CIN2+ and CIN3 over 2 years.
Methods
ThinPrep® liquid-based cytology (LBC) specimens were collected from 1349 women with repeat LSIL or ASC-US. HPV DNA was performed using Hybrid Capture. Where adequate material remained (n = 471), p16/Ki-67 overexpression was assessed. Clinical performance for detection of histologically diagnosed CIN2+ and CIN3 was calculated.
Results
Approximately 62.2% of the population were positive for HPV DNA, and 30.4% were positive for p16/Ki-67. p16/Ki-67 showed no significant difference in positivity between LSIL and ASC-US referrals (34.3% versus 28.6%; P = 0.189). Women under 30 years had a higher rate of p16/Ki-67 compared to those over 30 years (36.0% versus 26.6%; P = 0.029). Overall HPV DNA testing produced a high sensitivity for detection of CIN3 of 95.8% compared to 79.2% for p16/Ki-67. In contrast, p16/Ki-67 expression offered a higher specificity, 75.2% versus 40.4% for detection of CIN3. Combining p16/Ki-67 with HPV DNA improved the accuracy in distinguishing between CIN3 and <CIN3. The absolute risk of CIN3 increased from 15.6% in women who were HPV DNA positive to 27% in women positive for HPV DNA and p16/Ki-67. Those negative for HPV DNA and p16/Ki-67 had a low risk of 1.2% of CIN3.
Conclusion
The addition of p16/Ki-67 to HPV DNA testing leads to a more accurate stratification of CIN in women presenting with minor cytological abnormalities.
Abstract
This small national prospective study suggests that the addition of p16 / Ki-67 to HPV DNA could be used to stratify women with recurrent borderline or low grade cytological abnormalities to determine who should attend colposcopy.
Introduction
Based on the known causal relationship between high-risk human papillomavirus (HR-HPV) and cervical cancer,1 HPV testing has become an important tool in developing strategies for cervical cancer screening. It was initially approved as a triage test for minor cytological abnormalities, providing improved detection of cervical intraepithelial neoplasia grade 2 or worse (CIN2+) compared with repeat cytology.2-4 However, despite the utility of HPV testing in triage, concern remains over the reported suboptimal specificity of HPV DNA-based tests.5 This is due to the fact that HPV DNA testing cannot discriminate transforming infections from transient infections of minor clinical relevance. Knowledge of HPV pathophysiology has enabled the identification of a number of biomarkers with the potential to distinguish those at risk of disease progression. Several host cell biomarkers have been evaluated for their potential to improve the diagnostic specificity of cervical screening.6 One of the most promising cellular protein markers to be identified is the cyclin-dependent kinase inhibitor p16INK4A (referred to as p16 hereafter).7-9 However, p16 can be overexpressed in some non-dyskaryotic cells10 and, as a consequence, morphological criteria are needed.11 It is known that increased expression of p16 signals functional activation of E2F mediated by HPV E7. Combining p16 with the proliferation marker, Ki-67 signals HPV transformed cells undergoing proliferation. Studies have reported on the clinical performance of dual staining for p16 and Ki-67 for the detection of CIN2+ and CIN3.12-18 However, longitudinal data are limited on their utility as a triage tool for minor cytological abnormalities LSIL (low-grade squamous intraepithelial lesion) and ASC-US (atypical cells of undetermined significance).
The purpose of this 2-year prospective study was to examine two testing modalities, HPV DNA and p16-Ki-67, with an aim to identify an approach to best manage women with LSIL and ASC-US on cytology.
Materials and methods
Study population
The study setting was Ireland. At commencement of the study, guidelines from CervicalCheck, the National Cervical Screening Program, state the following for referral to colposcopy after minor cytological abnormalities on ThinPrep® liquid-based cytology (LBC): (1) three consecutive ASC-US; (2) two consecutive LSIL; (3) two consecutive cytology samples graded a combination of ASC-US and LSIL; or (4) having any three ThinPrep® LBC test results that are not normal in the previous 10 years without referral to colposcopy.19 Women gave written informed consent to take part in the study at their first visit to colposcopy after referral for repeat LSIL and ASC-US cytology at the National Maternity Hospital, Holles Street, Dublin from October 2008 to July 2011. Women were excluded from the study if they were pregnant, under the age of 18 years or had been treated for CIN in the previous 5 years. All women were assessed by BSCCP (British Society for Colposcopy and Cervical Pathology) trained colposcopists. Women were followed over the period of time they spend under surveillance where they were managed according to the standard protocol of the clinic. This involved follow-up by 6 months cytology after the initial colposcopy visit. If a high-grade lesion was identified at the initial colposcopy or during follow-up, women were brought back within a shorter period, for repeat colposcopy and treatment. Women with persistent minor abnormalities identified at their initial colposcopy (Time 0 months) and up to three or more subsequent follow-up visits (time 18 months), were offered treatment. Women were followed over a 2-year period or until they reached defined study endpoints. Study endpoints included having two consecutive normal cytology results and discharge from the clinic without treatment, or alternatively, having a LLETZ (Large Loop Excision of the Transformation Zone). The majority of women, 90.7% (427/471), had a histological diagnosis over the 2-year follow-up period by punch biopsy and/or LLETZ. A histological diagnosis was based on a standard protocol outlined in the CervicalCheck guidelines. In this study, CIN was diagnosed by a pathologist in daily routine practice to allow test performance to be evaluated in a routine population-based setting. All results were collected and recorded from the participating hospital. The study was approved by The Research Ethics Committee at the National Maternity Hospital, Holles Street, Dublin.
HPV DNA testing
A ThinPrep® LBC specimen was taken for HPV testing and immunocytochemistry (ICC) prior to the first colposcopic examination. Detection of DNA from oncogenic HPV types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68 was performed using Hybrid Capture 2 (HC2) (Qiagen, GmbH, Hilden, Germany), as described by the manufacturer. The RLU/CO negative cut-off value was 1.0. Specimens below this detection limit were considered negative.
p16/Ki-67 dual stain
Cytology slides from residual ThinPrep® material, from the same sample used for HPV testing, were prepared using a T2000 slide processor. The CINtec PLUS® kit (Roche mtm Laboratories AG, Mannheim, Germany) was used for ICC staining of p16 and Ki-67 in accordance with the manufacturer's instructions. A positive result was interpreted as brown cytoplasmic staining for p16 expression and red nuclear staining for Ki-67 expression. The presence of one or more stained epithelial cells, showing simultaneous expression, signified a positive result. All cases were subjected to a pathologist review, blinded to histology and HPV status. All testing on ThinPrep® specimens was performed within 3 months of obtaining the specimen. Results from HPV testing and ICC were not disclosed to the participants or used for patient management.
Statistical analysis
The final outcome was based on the histological grade taken as the worse of the histological findings on punch biopsy or LLETZ during a patient's time at colposcopy. The primary disease endpoint was histologically confirmed CIN2+ and CIN3, diagnosed within 2 years of the first colposcopy visit. While staining for p16 has been recommended to improve the diagnosis of CIN220 it was not possible for this study. We included CIN3 as a clinical endpoint as it is considered the true precursor to cervical cancer. Women who had a normal colposcopy, without biopsy, at the initial or follow-up visits were classified as <CIN2/3. Data were analysed using Minitab statistical software version 16. (Minitab Inc., State College, PA) Confidence intervals were calculated where appropriate. McNemar's test was used to compare differences in disease detection between HPV DNA and p16/Ki-67. A P-value less than 0.05 was considered statistically significant. The clinical performance of both tests, HPV DNA and p16/Ki-67, was assessed by calculating the sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and the relative 95% confidence intervals (CI) for detecting CIN2+ and CIN3 over a 2-year follow-up period in those with a histological diagnosis only. Additional sensitivity, specificity, PPV and NPV were calculated stratified by age and referral cytology.
Results
Among the 1346 women recruited into the study, 1079 had a complete HPV DNA result. Of the 1079, 764 had residual material available to prepare a ThinPrep® slide. As additional tests, not included in this analysis, were also performed on the same samples cellularity was low in some cases and resulted in the exclusion of a further 293 cases. The final study population was 471. All subsequent results are based on this group of 471 women.
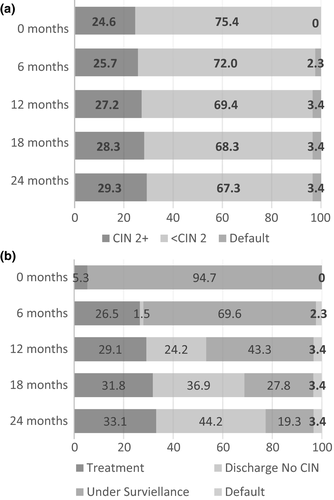
The median age of the population at enrolment was 31 years (interquartile range 27–38). LSIL referral was more common representing 56.3% (265/471) of referrals compared with ASC-US at 43.7% (206/471). Within a 2-year period, 29.3% (138/471) of women had a CIN2+ diagnosis on histology, 65.2% (90/138) of whom were classified as CIN2 and 34.8% (48/138) classified as CIN3. The majority, 83.3% (115/133), of CIN2+ was identified on histology at the first visit, generally after a punch biopsy. The remaining 18 cases were identified at follow-up visits (Figure 1a). Figure 1b illustrates the time at which treatment occurred for high-grade CIN. Within the 2-year follow-up period, a total of 33.1% (156/471) of women received an LLETZ treatment, 87.2% (136/156) to treat a suspected high-grade lesion and 12.8% (20/156) for a persistent low-grade lesion. Two out of 20 women treated for a persistent low-grade lesion had CIN2 on their LLETZ specimen. In total, 22.7% (107/471) of women remained under surveillance after 2 years follow-up. The majority of women who remained under surveillance, 85.0% (91/107), were under the age of 40 years. A further 44.2% (208/471) of the overall population exited the study having had two sequential normal cytology results and no treatment.
The prevalence of p16/Ki-67 and HPV DNA at recruitment indicated p16/Ki-67 over expression was present in 30.4% (95% CI 28.5–32.3) of women, compared with an HPV DNA prevalence rate of 62.2% (95% CI 60.1–64.3). p16/Ki-67 was positive in 34.3% of women referred with LSIL and 28.6% (59/206) referred with ASC-US (P = 0.189). HPV DNA was positive in 71.7% (190/265) of LSIL referrals and 50.5% of ASC-US referrals (P < 0.001). The prevalence across each grade of CIN is shown in Table 1. HPV DNA testing detected a higher proportion of CIN2+ and CIN3, compared with p16/Ki-67 testing (P < 0.001). However, a positive HPV DNA result identified over twice as many women (57.9% and 84.4%) as p16/Ki-67 (27.8% and 73.2%) who, in fact, had no CIN2+ or CIN3 including women with persistent low-grade abnormalities and those discharged with two sequential normal cytology results (Table 1).
| Referral smear | Test | CIN2+ | CIN3 | Persistent low grade* | Discharged No CIN |
|---|---|---|---|---|---|
| LSIL | n | 81 | 35 | 66 | 110 |
| p16/Ki67+ | 62 (76.5%) | 30 (85.7%) | 13 (19.7%) | 16 (14.5%) | |
| DNA+ | 74 (91.4%) | 33 (94.3%) | 44 (66.7%) | 72 (65.5%) | |
| ASC-US | n | 57 | 13 | 39 | 100 |
| p16/Ki67+ | 41 (71.9%) | 8 (61.5%) | 8 (20.5%) | 10 (10.0%) | |
| DNA+ | 54 (94.7%) | 13 (100%) | 21 (53.8%) | 29 (29.0%) | |
| Total | n | 138 | 48 | 105 | 210 |
| p16/Ki67+ | 104 (75.4%) | 38 (79.2%) | 21 (20.0%) | 26 (12.4%) | |
| DNA+ | 128 (92.8%) | 46 (95.8%) | 65 (61.9%) | 101 (48.1%) |
- CIN2+, cervical intraepithelial grade 2 or worse; CIN3, cervical intraepithelial grade 3; LSIL, low-grade squamous intraepithelial lesions; ASC-US, atypical squamous cells of undermined significance.
- * Persistent histological diagnosis of CIN 1/cytology confirmed LSIL or ASC-US. Excludes 18 women who received a LLETZ treatment for a persistent low-grade lesion.
- † Discharged after two sequential normal cytology results at 12, 18 or 24 months and assumed to have no CIN.
Table 2 shows, separately, the clinical performance characteristics of p16/Ki-67 and HPV DNA testing for the detection of CIN2+ and CIN3. The calculations are based on those with a histological diagnosis over 2 years (n = 427). HPV DNA detection demonstrated the highest sensitivity, 92.8% and 95.8%, but with a limited specificity 48.9% and 40.4% for CIN2+ and CIN3, respectively. In comparison, p16/Ki-67 had a lower sensitivity of 75.4% and 79.2%, and higher specificity of 88.3% and 75.2% for CIN2+ and CIN3. p16/Ki-67 demonstrated a significantly higher PPV of 26.6%, for detection of CIN3, compared with 15.4% for the HPV DNA test (P < 0.001). Whereas HPV DNA testing had a significantly higher NPV of 98.8%, compared with p16/Ki-67 at 97.0% (P = 0.01). p16/Ki-67 testing showed comparable sensitivity and specificity in both LSIL and ASC-US referrals, whereas HPV DNA testing had significantly higher sensitivity and specificity in ASC-US compared with LSIL. Table 3 contains the clinical performance, stratified by age. p16/Ki-67 was positive in 36.0% of women under 30 years and 26.6% of women over 30 years (P = 0.029). HPV DNA was positive in 70.9% of women under 30 years and 58.2% of women over 30 years (P = 0.006). Both tests demonstrated a similar sensitivity across both age groups; however, specificity increased in women aged 30 years and older for each test.
| CIN2+ | CIN3 | |||||
|---|---|---|---|---|---|---|
| Sensitivity %(95% CI) | Specificity %(95% CI) | Sensitivity %(95% CI) | Specificity %(95% CI) | PPV %(95%CI) | NPV %(95%CI) | |
| All | ||||||
| HPV DNA | 92.8 (91.6–93.9) | 48.9 (46.3–51.6) | 95.8 (94.7–97.0) | 40.4 (38.1–42.7) | 15.4 (14.0–16.9) | 98.8 (98.7–99.0) |
| p16/Ki-67 | 75.4 (72.3–78.8) | 88.3 (87.2–89.4) | 79.2 (74.5–83.8) | 75.2 (73.4–77.0) | 26.6 (23.4–29.8) | 97.0 (96.6–97.3) |
| LSIL | ||||||
| HPV DNA | 91.4 (89.6–93.1) | 35.3 (32.0–38.6) | 94.5 (92.5–96.1) | 28.9 (26.4–31.5) | 15.7 (13.9–17.5) | 97.3 (96.7–97.9) |
| p16/Ki-67 | 77.8 (74.0–81.5) | 88.6 (87.1–90.1) | 85.7 (81.7–89.8) | 72.7 (70.2–75.2) | 30.6 (26.4–34.8) | 97.3 (96.9–97.7) |
| ASC-US | ||||||
| HPV DNA | 94.7 (93.4–96.0) | 64.4 (60.8–68.1) | 100 (–) | 56.9 (53.3–60.5) | 14.8 (12.1–17.4) | 100 (–) |
| p16/Ki-67 | 71.9 (66.7–77.2) | 87.9 (86.2–89.6) | 71.4 (60.7–82.1) | 78.7 (76.2–81.2) | 17.8 (13.5–22.0) | 96.5 (95.9–97.0) |
- PPV, positive predictive value; NPV, negative predictive value; 95% CI, 95% confidence interval; CIN2+, cervical intraepithelial grade 2 or worse; CIN3, cervical intraepithelial grade 3; LSIL, low grade squamous intraepithelial lesions; ASC-US, atypical squamous cells of undermined significance.
| CIN2+ | CIN3 | |||||
|---|---|---|---|---|---|---|
| Sensitivity %(95% CI) | Specificity %(95% CI) | Sensitivity %(95% CI) | Specificity %(95% CI) | PPV %(95% CI) | NPV %(95% CI) | |
| 18–29 years | ||||||
| HPV DNA | 90.2 (87.9–92.4) | 38.3 (34.2–42.4) | 96.3 (95.0–97.6) | 33.3 (29.9–36.8) | 19.4 (16.8–22.1) | 98.2 (97.7–98.7) |
| p16/Ki-67 | 77.0 (72.6–81.4) | 83.6 (81.2–86.0) | 77.8 (71.3–84.3) | 71.0 (67.8–74.2) | 30.9 (25.8–36.0) | 95.0 (94.2–95.9) |
| 30+ years | ||||||
| HPV DNA | 93.5 (92.2–94.9) | 55.1 (51.7–58.5) | 95.2 (93.3–97.2) | 44.8 (41.8–47.8) | 12.2 (10.6–13.8) | 99.2 (99.0–99.3) |
| p16/Ki-67 | 74.0 (69.7–78.3) | 91.2 (90.1–92.3) | 81.0 (74.4–87.5) | 77.8 (75.7–79.9) | 22.7 (18.7–26.6) | 98.1 (97.8–98.3) |
- PPV, positive predictive value; NPV, negative predictive value; 95% CI, 95% confidence interval; CIN2+, cervical intraepithelial grade 2 or worse; CIN3, cervical intraepithelial grade 3.
From the overall population, 62.2% were positive for HPV DNA, 41.6% of whom had CIN2+ and 15.6% had CIN3 over the study period. There were 30.4% with a positive p16/Ki-67 result, 72.0% had a CIN2+ and 27.3% had a CIN3 diagnosis. The clinical outcome after combined testing of p16/Ki-67 and HPV DNA is shown in Figure 2. From the general population of 471 women, 29.5% had a double-positive test result; 74.1% of these women had CIN2+ and 27.3% had CIN3 diagnosed. Over 2 years, the absolute risk of CIN2+ and CIN3 was 15% and 6.3% in women with a positive HPV result and a negative p16/Ki-67 result. The absolute risk of CIN2+ was 5.4% and CIN3 was 1.2% in women with a negative HPV DNA and negative p16/Ki-67 result.

Discussion
Minor abnormalities, LSIL and ASC-US, represent a large burden at colposcopy. A large proportion of these will not lead to a diagnosis of CIN2+ or CIN3 yet still remain under extensive follow-up. Efforts have been made to manage this by introducing HPV DNA triage of minor abnormal cytology. However, owing to the low specificity, HPV DNA testing can still lead to over referral to colposcopy. In this study, we investigated the potential options for triaging women attending colposcopy after repeat minor abnormal cytology. We found from a population of women attending colposcopy with LSIL and ASC-US on cytology only 29.3% and 10.2% had underlying or subsequent CIN2+ and CIN3 lesions over 2 years. We have shown that a combined HPV DNA and the p16/Ki-67 testing approach could be a potential tool for predicting the diagnosis of CIN2+ and CIN3 in these women.
The two triage modalities, p16/Ki-67 and HPV DNA, were initially explored as stand-alone tests. HPV DNA was over three times higher than p16/Ki-67 in women with persistent low-grade lesions and those discharged with no CIN. Unlike HPV DNA testing, there was no significant difference in the proportion of women with p16/Ki-67 co-expression between LSIL- and ASC-US-referred patients, an important finding considering the reported limited use of HPV DNA triage in LSIL.2, 3 Furthermore, p16/Ki-67 showed only a modest difference in performance with respect to the age of women compared with HPV testing, which showed a substantially reduced specificity in women under the age of 30 years. In contrast, compared with p16/Ki-67, HPV DNA was positive in a significantly higher proportion of CIN2+ and CIN3 (P < 0.001). This highlights the lower sensitivity of p16/Ki-67 compared with HPV DNA testing. Although, sensitivity for p16/Ki-67 in the current study is similar to previous studies.12-18 Generally, PPV appeared low, this is as a result of the low prevalence of CIN3 in this population (10%) and is in line with other studies showing a similar prevalence of CIN3.13, 14, 17 Overall specificity and PPV demonstrated by p16/Ki-67 remained higher than that demonstrated by HPV DNA across all categories. However, as HPV DNA testing outperformed with respect to sensitivity and NPV, we investigated a combined testing approach in order to maintain the high sensitivity of HPV testing and improve specificity with p16/Ki-67.
When combined, 29.5% were found to be positive for both tests, from these 74.1% had CIN2+ and 27.3% CIN3 diagnosed. Combined testing presented the most efficient option for identifying CIN2+ and CIN3 in women with repeat minor abnormal cytology as it identified almost one-third of the population as requiring immediate colposcopy, signified by a double positive result. A negative p16/Ki-67 and negative HPV DNA maintained a high level of reassurance against CIN3 (1.2%) similar to that of a negative HPV test alone. A risk of CIN3 less than 2% has been previously deemed safe to allow a return to routine recall.21 An important consideration in the use of a combined testing approach is how to manage women with discordant results, i.e. who are HPV positive p16/Ki-67 negative. Owing to the risk of CIN2+ and CIN3 in these individuals, it would probably be best advised that they do not return to routine recall but have some form of follow-up or colposcopy referral. An approach of repeat HPV testing of women who are HPV positive cytology negative after 1 year has been previously recommended.22
The strengths of this study are that enrolment was systematic through the Irish national screening programme, CervicalCheck. Women were managed under a standard protocol outlined by CervicalCheck guidelines. These attributes allowed test performance to be evaluated in a routine population-based setting. The consequence of this real-world setting was, however, that some women were not managed according to the protocol, but this probably reflects the day-to-day reality of colposcopy clinics. A limitation of this study is that it focused on a population of women attending colposcopy on the bases of repeat minor cytology rather than a single ASC-US or LSIL, where a secondary test would be applied. However, sensitivity in the present study remains consistent with previous studies.12-18 While a large number of samples were excluded based on low cellularity this is likely because of the fact two or more tests were performed on the samples prior to the ThinPrep® slide been made. In reality, a triage test would be performed after only one HPV test, as such we do not anticipate cellularity to be an issue.
While this work has established that HPV DNA and p16/Ki-67 may play a role in cervical screening programmes, large studies with long-term follow-up are warranted in order to determine an optimal management algorithm. Management of HPV DNA positive p16/Ki-67 negative women will need to be addressed in order to predict the intervals for retesting and return to routine screening. Moreover, further studies investigating triage after an initial minor cytological abnormality and in the context of primary screening with HPV will be important. Currently, triage of primary screened HPV positive women by cytology is recommended; however, a question still remains over how to manage HPV positive cytology-negative women. Dual staining for p16/Ki-67 has been previously shown to help further stratify Pap-negative/HPV positive women that are at the highest risk of underlying high-grade disease.18 In addition, the cost-effective analysis will be an important aspect to help provide guidelines on the delivery and implementation in of such models in cervical screening. While other studies have found p16/Ki-67 superior to HPV E6/E7 mRNA testing by the APTIMA HPV assay,17 further studies on the role of the ATPIMA assay in combination with p16/Ki-67, in addition to a number of positive cells and morphology should be explored.
In conclusion, we have shown that combining HPV DNA-positive women with p16/Ki-67 testing could lead to more accurate stratification of CIN in women presenting with minor cytology, potentially reducing referrals to colposcopy. This strategy is worthy of further evaluation in terms of both clinical effectiveness and cost effectiveness. While adding an additional test to screening may be more costly, introducing further stratification of minor abnormalities, prior to colposcopy rather than at colposcopy, should reduce unnecessary workup and treatment. This will benefit women by reducing the psychosocial effects endured from having repeat abnormal smears and attending colposcopy clinics, in addition to reducing the cost associated with colposcopy visits.23
Acknowledgments
This research was undertaken as part of the CERVIVA, the Irish Cervical Cancer Research Consortium (http://www.cerviva.ie). CERVIVA is funded by the Health Research Board, Ireland, the Irish Cancer Society and Friends of the Coombe. This work is also based upon work supported by and Irish Cancer Society Scholarship. We thank the colposcopy clinic staff at The National Maternity Hospital Dublin for facilitating the study.




