Impaired Glymphatic Function in Acute Spontaneous Intracerebral Hemorrhage
Funding: This work was supported by Medicine Health Technology Plan of Zhejiang Province, 2023KY758, Natural Science Foundation of Zhejiang Province, LQ22H090019, National Natural Science Foundation of China, 82101365, 82171274.
Jinsong Cai and Yecheng Dong contributed equally to this work.
ABSTRACT
Background and Aims
Alterations in glymphatic function during the acute phase of acute spontaneous intracerebral hemorrhage (sICH) remain poorly understood. The aim of this study was to investigate whether, compared to healthy controls (HCs), the glymphatic system is impaired in patients with sICH, and to assess its association with hemorrhage and edema severity and outcome.
Methods
Fifty-five sICH patients (including 46 supratentorial sICH and 9 ) and 97 age- and sex-matched HCs underwent conventional MRI and diffusion tensor imaging. The diffusion along the perivascular space (DTI-ALPS) index, serving as a marker for glymphatic function, was computed, with supratentorial cases being categorized into ipsilateral and contralateral ALPS. Volumes of hemorrhage and edema were evaluated using susceptibility-weighted imaging (SWI) and T2-weighted magnetic resonance images, and the relative edema ratio was calculated. Clinical outcomes were categorized as favorable or poor based on a modified Rankin scale score of ≤ 2 or > 2 at 90 days.
Results
sICH patients showed significantly lower DTI-ALPS values on the ipsilateral side compared to the average in the HC group (1.34 ± 0.24 vs. 1.46 ± 0.22, p = 0.003), whereas contralateral DTI-ALPS values in sICH patients did not differ significantly from HCs (1.48 ± 0.21 vs. 1.46 ± 0.22, p = 0.524). The ipsilateral DTI-ALPS was notably associated with both hemorrhage and relative edema volumes (both p < 0.05). A higher ipsilateral DTI-ALPS was independently associated with a favorable outcome at 90 days (odds ratio = 1.686 per 0.1 increase, p = 0.038).
Conclusions
The DTI-ALPS index, which reflects glymphatic functionality, is notably diminished on the ipsilateral side in acute sICH, correlating significantly with increased volumes of hemorrhage and edema. This study suggests that glymphatic dysfunction may contribute to the severity of clinical outcomes, and highlights the potential role of the glymphatic system in the pathophysiology of sICH.
1 Introduction
Spontaneous intracerebral hemorrhage (sICH), the most common type of hemorrhagic stroke, accounts for approximately 15% of all stroke incidents and is associated with a high morbidity and mortality rate, nearing 40% [1, 2]. Despite advancements in the understanding of prognostic factors, risk elements, and the neural tissue's response to hematoma accumulation [3], outcomes for patients remain predominantly unfavorable. Presently, treatment modalities are mainly supportive, with surgical options reserved for cases presenting significant mass effect, herniation, or hydrocephalus [4]. Furthermore, survivors of sICH are at continued risk for delayed neurological injury, driven by inflammatory and cytotoxic responses to the hematoma and its degradation byproducts [5]. This recognition has propelled secondary brain injury to the forefront of therapeutic investigation within the intracerebral hemorrhage research community [6, 7]. Perihematomal edema, indicative of secondary damage, is particularly significant, reflecting the combined effects of thrombin build-up, the influx of inflammatory mediators, and erythrocyte breakdown [8, 9]. Ongoing research is dedicated to deepening the understanding of intracerebral hemorrhage pathophysiology with the aim of pinpointing viable therapeutic targets.
The discovery of the glymphatic system by Iliff et al. marked a significant advancement in our comprehension of cerebral waste clearance mechanisms [10]. This system integrates arterial conduits, aquaporin-4 (AQP4) water channels on astroglial endfeet, and the surrounding perivascular spaces, facilitating the flow of cerebrospinal fluid (CSF) and interstitial fluid, thereby enhancing the removal of metabolic wastes. The entry of CSF into the periarterial spaces enables an exchange with interstitial fluid, assisting in the clearance of amyloid-beta, tau proteins, and other metabolites, as well as proinflammatory factors. Ultimately, these wastes are directed toward lymphatic drainage sites [11]. Notably, the dilatation of perivascular pathways, commonly observed in various pathologies, is critical to the efficacy of this clearance process.
In the realm of cerebrovascular disorders, dysfunction of the glymphatic system plays a critical role in a multitude of detrimental processes, including edema, disruption of the blood–brain barrier, and neuroinflammatory responses [12]. Notably, an observed expansion of perivascular spaces in cases of intracerebral hemorrhage correlates with impaired glymphatic waste clearance, a phenomenon similarly noted in conditions such as traumatic brain injury [13, 14]. Experimental data from rodent studies demonstrate that obstructing glymphatic drainage exacerbates brain edema, inflammation, and neuronal apoptosis, which contribute to neurological impairments in intracerebral hemorrhage. This is mediated by alterations in AQP-4, tumor necrosis factor-alpha (TNF-α), and interleukin-10 (IL-10) levels, thereby highlighting the glymphatic system's protective role under physiological conditions [15].
A recent study employing noninvasive diffusion tensor imaging to assess the perivascular space (DTI-ALPS) identified glymphatic dysfunction on the ipsilateral side of lesions in chronic phase sICH patients [16]. However, alterations in glymphatic function during the acute phase of sICH remain poorly understood. Therefore, the present study aims to evaluate glymphatic function utilizing DTI-ALPS in patients with acute sICH, investigate the factors that influence this function, and explore its association with the severity of hemorrhage and edema, as well as with clinical outcomes.
2 Methods
2.1 Patients
The study was conducted in accordance with the Helsinki Declaration and received approval from the Ethics Committee of the Second Affiliated Hospital of Zhejiang University, School of Medicine (approval number: Yan-20,240,539). Written informed consent was obtained from all subjects or their legal representatives prior to participation. We recruited a convenience sample of 55 hospitalized patients diagnosed with sICH from May 2023 to February 2024, all of whom received conservative management. The inclusion criteria included: (1) age 18 years, (2) verified diagnosis of intraparenchymal hemorrhage by CT or MRI scan, and (3) noncomatose status both 1 week prior to and following MRI administration. Exclusion criteria were established as follows: (1) accompanied by intraventricular hemorrhage or subarachnoid hemorrhage; (2) MRI detection of brain parenchymal lesions other than hemorrhage, (3) secondary causes of ICH such as hemorrhagic transformation of ischemic stroke, aneurysmal, cavernomas, arteriovenous malformations, central venous thrombosis, trauma-related, or tumor, (4) surgical evacuation of hematoma, (5) excessively large hemorrhagic lesions that could interfere with the measurement of DTI metrics, or (6) history of neuropsychiatric disorders. A cohort of 97 healthy volunteers was selected to serve as healthy controls (HCs). Their inclusion criteria were: (1) age over 40; (2) no history of significant cerebrovascular events except lacunar infarction; (3) absence of infarct lesions with restricted diffusion on current diffusion-weighted imaging; (4) absence of intracranial hemorrhage on current susceptibility-weighted imaging; (5) no history of multiple sclerosis, Alzheimer's disease, Parkinson's disease, or head trauma; (6) absence of white matter lesions of nonvascular origin such as immunological-demyelinating, metabolic, toxic, or infectious causes. All participants underwent multimodal MRI. Demographic information and vascular risk factors such as age, gender, and history of hypertension, diabetes, hyperlipidemia, and smoking were recorded. The modified Rankin Scale (mRS) score was recorded 90 days following the onset of hemorrhage. Clinical outcomes were defined as favorable or poor based on a mRS of ≤ 2 or > 2, respectively.
2.2 MRI Protocol
The image acquisition was performed with on a 3.0 T MRI using the system's 20-channel head array coil. DTI images were obtained using a Single-SE diffusion-weighted echo-planar imaging (EPI): TR/TE = 5000/77 ms, FOV = 220 × 220 mm, matri = 128 × 128, b = 1000 s/mm2, voxel size = 1.7 × 1.7 × 2 mm3, 30 directions, thickness = 2 mm, gap = 0.4 mm. Susceptibility weighted imaging (SWI) using 3D gradient recalled echo and minimum intensity projection (mIP) images: TR/TE = 28/20 ms, FOV = 220 × 200 mm, matrix = 320 × 307, voxel size = 0.7 × 0.7 × 2 mm3, thickness = 2 mm without slice gap. T2-weighted TSE: TR/TE = 3800/99 ms, FOV = 230 × 200 mm, matrix = 384 × 320, voxel size = 0.6 × 0.6 × 6 mm3, thickness = 6 mm, gap = 1.2 mm.
2.3 Volume Assessments of Hemorrhage and Edema
SWI and T2-weighted images were analyzed using MRIcron software (available at http://www.nitrc.org/projects/mricron). Hematomas were delineated on SWI images, while brain edema was identified on T2-weighted images by subtracting the volume of hematomas from the total lesion volume. The imaging analysis involved a collaborative effort between an experienced radiologist (C.J.) and a seasoned neurologist (Y.S.), both of whom were blinded to clinical and additional imaging data. Manual corrections were performed jointly, and the final delineation of lesions was established through their consensus. Then the relative edema ratio was determined by calculating the quotient of the edema volume to the hemorrhage volume. The site of the hematoma was categorized as supratentorial (including lobar and deep) or subtentorial.
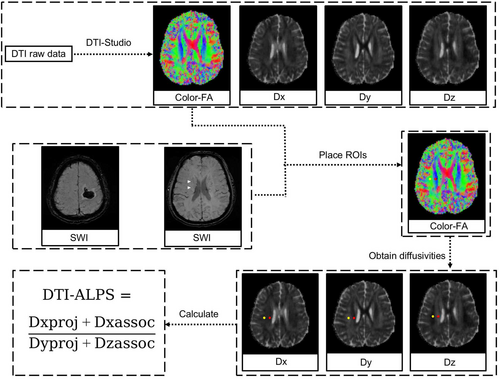
2.4 Calculation of DTI-ALPS
Evaluation of the ALPS index was conducted in accordance with methodologies outlined in previous studies [17-19]. Diffusivity maps along the x-axis (Dx), y-axis (Dy), z-axis (Dz), and color-coded fractional anisotropy (FA) maps were processed using DTI Studio (available at https://www.mristudio.org). Two regions of interest (ROIs), each 5 mm in diameter, were positioned on the color-coded FA map specifically within the projection fibers and the association fibers, located where the direction of the deep medullary veins (DMVs) was perpendicular to the body of the ventricle. The diffusivities in the Dx, Dy, and Dz directions were recorded for each ROI within the projection fibers (Dx_proj, Dy_proj, Dz_proj) and the association fibers (Dx_assoc, Dy_assoc, Dz_assoc), respectively. The ALPS index was then calculated using the formula [(Dx_proj + Dx_assoc) / (Dy_proj + Dz_assoc)]. For patients with supratentorial ICH, cases were categorized into ipsilateral and contralateral ALPS. Figure 1 provides a schematic illustration of the method used for measuring diffusivity with the ALPS index.
2.5 Statistical Analysis
Statistical analyses were performed using IBM SPSS 19.0. The Kolmogorov–Smirnov test was used to assess the distribution patterns of the data. Continuous variables with normal distributions are presented as means ± standard deviations (SD), while other variables are reported as medians with interquartile ranges (IQR). Categorical data are expressed as frequencies (proportions). Group comparisons were conducted using the Student's t-test after confirmation of data normality. Correlations between groups were assessed using either Pearson's or Spearman's correlation tests, depending on the data distribution. Multivariate analyses, including linear and logistic regression, were performed by incorporating factors with a p-value < 0.1 and significant covariates from previously published studies. A p value of < 0.05 was considered statistically significant.
3 Results
3.1 Demographic Characteristics of sICH and HC Participants
In this study, we included 55 sICH patients (25.5% females; mean age = 58 ± 15 years) and 97 age- and sex-matched healthy controls (HCs) (25.8% females; median age = 58 ± 7 years). The average duration of the disease among sICH patients was 5.3 ± 3.1 days. Hemorrhage volumes measured an average of 22.1 ± 20.1 mL, and edema volumes averaged 24.2 ± 26.2 mL. Among them, 46 patients had supratentorial intracerebral hemorrhage, including 16 cases of lobar hemorrhage and 30 cases of deep hemorrhage, while 9 patients had subtentorial intracerebral hemorrhage. Additionally, none of the supratentorial patients had bilateral hemorrhage. The DTI-ALPS on the ipsilateral side was significantly lower in the sICH group compared to the HC group (1.34 ± 0.24 vs. 1.46 ± 0.22, p = 0.003). However, there was no significant difference in DTI-ALPS between the contralateral sides of the sICH and HC groups (1.48 ± 0.21 vs. 1.46 ± 0.22, p = 0.524). Table 1 presents a comparison of demographic and clinical data between the sICH and HC groups.
| HC (n = 97) | sICH (n = 55) | p | |
|---|---|---|---|
| Age | 58 ± 7 | 58 ± 15 | 0.916 |
| Female | 25 (25.8%) | 14 (25.5%) | 1.000 |
| Hypertension | 64 (66.0%) | 40 (72.7%) | 0.469 |
| Diabetes | 17 (17.5%) | 7 (12.7%) | 0.495 |
| Hyperlipemia | 22 (22.7%) | 5 (9.1%) | 0.046 |
| Smoking | 25 (25.8%) | 14 (25.5%) | 1.000 |
| Ipsilateral DTI-ALPS (n = 46) | 1.45 ± 0.22 | 1.34 ± 0.24 | 0.003 |
| Contralateral DTI-ALPS (n = 46) | 1.45 ± 0.22 | 1.48 ± 0.21 | 0.524 |
| Average DTI-ALPS | 1.45 ± 0.22 | 1.42 ± 0.17 | 0.336 |
- Abbreviation: DTI-ALPS, diffusion along the perivascular space.
3.2 Determinants of Ipsilateral and Contralateral DTI-ALPS
The DTI-ALPS value on the ipsilateral side was significantly lower compared to the contralateral side within the sICH group (1.34 ± 0.24 vs. 1.48 ± 0.21, p = 0.003). No significant differences were observed in DTI-ALPS values, both ipsilateral and contralateral, between genders or among patients with or without hypertension, diabetes, hyperlipidemia, or smoking history (all p > 0.05, Table 2). Additionally, there was no significant correlation between age or disease onset duration and DTI-ALPS on either side (all p > 0.05, Figure 2).
| Yes | No | p | |
|---|---|---|---|
| Ipsilateral DTI-ALPS | |||
| Female | 1.39 ± 0.22 | 1.32 ± 0.25 | 0.463 |
| Hypertension | 1.31 ± 0.22 | 1.41 ± 0.32 | 0.256 |
| Diabetes | 1.25 ± 0.22 | 1.35 ± 0.25 | 0.378 |
| Hyperlipemia | 1.29 ± 0.19 | 1.34 ± 0.25 | 0.714 |
| Smoking | 1.32 ± 0.19 | 1.34 ± 0.26 | 0.532 |
| Contralateral DTI-ALPS | |||
| Female | 1.51 ± 0.12 | 1.48 ± 0.23 | 0.717 |
| Hypertension | 1.50 ± 0.22 | 1.44 ± 0.21 | 0.405 |
| Diabetes | 1.49 ± 0.24 | 1.48 ± 0.21 | 0.967 |
| Hyperlipemia | 1.47 ± 0.22 | 1.48 ± 0.22 | 0.925 |
| Smoking | 1.42 ± 0.16 | 1.51 ± 0.23 | 0.325 |

3.3 Impact of Ipsilateral and Contralateral DTI-ALPS on Severity of Hemorrhage and Edema
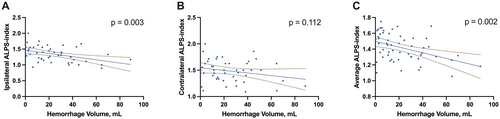
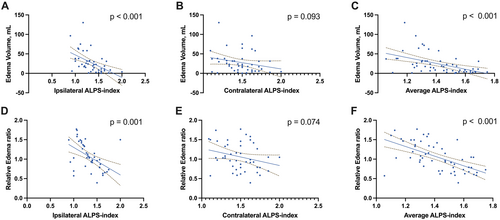
Figure 3 demonstrates that the ipsilateral DTI-ALPS was significantly associated with hemorrhage volumes (r = −0.426, p = 0.003). Figure 4 shows that both the edema volume and the relative edema ratio were significantly correlated with the ipsilateral DTI-ALPS (r = −0.592, p < 0.001; r = −0.489, p = 0.001). No other factors showed significant correlations with either hemorrhage or edema volumes (all p > 0.05, Table 3).
| Yes | No | p | |
|---|---|---|---|
| Hemorrhage and volume | |||
| Female | 17.3 ± 16.1 | 23.7 ± 21.2 | 0.308 |
| Hypertension | 21.9 ± 17.0 | 22.5 ± 27.4 | 0.921 |
| Diabetes | 25.0 ± 21.9 | 21.7 ± 20.0 | 0.684 |
| Hyperlipemia | 14.9 ± 20.7 | 22.8 ± 20.1 | 0.410 |
| Smoking | 19.7 ± 18.5 | 22.9 ± 20.8 | 0.613 |
| Edema volume | |||
| Female | 21.0 ± 22.8 | 25.3 ± 27.5 | 0.601 |
| Hypertension | 23.6 ± 20.5 | 25.8 ± 38.5 | 0.785 |
| Diabetes | 32.4 ± 33.6 | 23.0 ± 25.2 | 0.382 |
| Hyperlipemia | 20.7 ± 30.8 | 24.6 ± 26.1 | 0.755 |
| Smoking | 18.7 ± 19.7 | 26.1 ± 28.1 | 0.366 |
| Pearson r | p | |
|---|---|---|
| Hemorrhage and volume | ||
| Age, year | 0.100 | 0.468 |
| Duration, day | −0.154 | 0.260 |
| Edema volume | ||
| Age, year | 0.212 | 0.120 |
| Duration, day | −0.041 | 0.767 |
3.4 Influence of Ipsilateral DTI-ALPS on Clinical Outcomes in sICH Patients
A total of 39 patients (70.9%) with sICH exhibited favorable clinical outcomes at 90 days postonset. In comparison to those with poor outcomes, patients with favorable outcomes were younger, with smaller volumes of hemorrhage and edema, lower baseline National Institutes of Health Stroke Scale (NIHSS) scores, and lower DTI-ALPS on the ipsilateral side in 46 patients with supratentorial ICH (all p < 0.05, Table 4). After adjusting for age, sex, and baseline NIHSS scores, a higher ipsilateral DTI-ALPS was found to be independently predictive of a favorable outcome at 90 days (odds ratio = 1.686 per 0.1 increase, p = 0.038, Table 5). For the 9 patients with subtentorial ICH, no significant difference in the average DTI-ALPS was observed between those with a favorable outcome at 90 days and those without (p = 0.242).
| mRS 0–2 (n = 39) | mRS 3–6 (n = 16) | p | |
|---|---|---|---|
| Age | 54 ± 14 | 68 ± 13 | 0.001 |
| Female | 8 (20.5) | 6 (37.5) | 0.306 |
| Hypertension | 26 (66.7) | 14 (87.5) | 0.184 |
| Diabetes | 3 (7.7) | 4 (25.0) | 0.175 |
| Hyperlipemia | 4 (10.3) | 1 (6.3) | 1.000 |
| Smoking | 11 (28.2) | 3 (18.8) | 0.734 |
| Basline NIHSS | 3.6 ± 4.7 | 10.6 ± 3.8 | < 0.001 |
| Hemorrhage location | |||
| Deep | 20 (51.3) | 10 (62.5) | 0.500 |
| Lobar | 12 (30.8) | 4 (25.0) | |
| Subtentorial | 7 (17.9) | 2 (12.5) | |
| Hemorrhage volume, mL | 16.8 ± 15.4 | 34.9 ± 24.6 | 0.002 |
| Edema volume, mL | 15.6 ± 14.8 | 45.3 ± 35.4 | < 0.001 |
| Relative edema ratio | 0.97 ± 0.36 | 1.26 ± 0.27 | 0.005 |
| Ipsilateral DTI-ALPS | 1.41 ± 0.24 | 1.16 ± 0.16 | 0.001 |
| Contralateral DTI-ALPS | 1.51 ± 0.21 | 1.43 ± 0.22 | 0.290 |
| Average DTI-ALPS | 1.47 ± 0.17 | 1.30 ± 0.08 | 0.001 |
- Abbreviations: DTI-ALPS, diffusion along the perivascular space; mRS, modified Rankin scale score; NIHSS, National Institutes of Health Stroke Scale.
| Odds ratio | 95% confidence interval | p | |
|---|---|---|---|
| Age | 0.934 | 0.861, 1.014 | 0.101 |
| Sex | 0.251 | 0.026, 2.391 | 0.229 |
| Baseline NIHSS score | 0.833 | 0.679, 1.022 | 0.080 |
| Hemorrhage volume, mL | 0.975 | 0.923, 1.030 | 0.372 |
| Ipsilateral DTI-ALPS, per 0.1 | 1.686 | 1.030, 2.761 | 0.038 |
- Abbreviations: DTI-ALPS, diffusion along the perivascular space; mRS, modified Rankin scale score; NIHSS, National Institutes of Health Stroke Scale.
4 Discussion
The present study advances our understanding of glymphatic dysfunction in acute sICH, utilizing the DTI-ALPS index as a novel biomarker. Our findings reveal a significant reduction in the DTI-ALPS index on the ipsilateral side in sICH patients compared to healthy controls, highlighting disrupted glymphatic function that correlates with hemorrhage severity and edema progression. Notably, the impaired glymphatic clearance identified in our study suggests a potential mechanism linking hemorrhagic brain injury to subsequent edema and poorer clinical outcomes.
Glymphatic dysfunction in cerebrovascular diseases has been implicated in exacerbating secondary brain injury through mechanisms such as neuroinflammation and cytotoxic edema [12]. However, identifying glymphatic system biomarkers is challenging. The DTI-ALPS provides a noninvasive method to assess glymphatic function, showing promise in neurological diseases like Alzheimer's disease and Parkinson's disease, neuroinflammatory diseases like multiple sclerosis and neuromyelitis optica spectrum disorder, and cerebral small vessel disease [19-22]. In our study, the use of DTI-ALPS in acute sICH revealed a significant decrease in glymphatic function on the affected side, highlighting its potential role in hemorrhage pathology. These findings are consistent with previous research on sICH patients with onset times exceeding 2 weeks [16]. Furthermore, the observed correlation between altered DTI-ALPS indices on the lesion side, increased hemorrhage and edema volumes, and poorer clinical outcomes suggests that impaired glymphatic function may contribute to the severity of the condition. While this association does not establish causality, it supports the hypothesis that glymphatic dysfunction could be an important factor influencing the progression and recovery of brain injury following sICH. The glymphatic system, essential for the clearance of metabolic waste and neurotoxic substances, when impaired, may exacerbate the accumulation of hemorrhagic and edematous byproducts, potentially aggravating brain injury and influencing recovery trajectories.
The association between lower ipsilateral DTI-ALPS values and unfavorable clinical outcomes at 90 days further positions this biomarker as a crucial prognostic tool. The extent of glymphatic dysfunction could serve as a biomarker for the severity of acute brain injury and potentially predict long-term outcomes in sICH patients. Notably, our analysis detected no significant differences in DTI-ALPS values across variables such as age, gender, or comorbidities, suggesting that post-sICH glymphatic impairment is primarily driven by the hemorrhagic event rather than by individual predispositions. This insight underscores the utility of DTI-ALPS as a diagnostic and prognostic tool that transcends demographic and clinical factors.
Moreover, the link between glymphatic dysfunction and clinical outcomes underscores the urgency for therapeutic strategies that enhance glymphatic function to mitigate secondary injury and improve recovery. This study found no correlation between the DTI-ALPS index and disease duration, indicating a rapid decline in glymphatic functionality that does not promptly recover. Previous research shows that the DTI-ALPS index increases over time in sICH patients whose symptoms began more than 2 weeks prior, suggesting a delayed recovery of glymphatic system function [16]. These findings imply that the glymphatic system may not effectively recover with conventional clinical treatments in the acute phase, potentially contributing to the challenges of effective intervention. Given the increasing research focus on enhancing glymphatic drainage [23-26], improving glymphatic function could be a promising strategy to treat sICH, particularly in managing secondary edema posthemorrhage. This warrants further exploration into therapeutic interventions that can enhance glymphatic clearance and mitigate the impacts of cerebral hemorrhage.
Several limitations of this study need to be addressed. First, the sample size in our study is modest, particularly for subtentorial patients. The findings should be interpreted with caution and requires confirmation in a larger cohort. Second, while the DTI-ALPS index, derived from perivascular space diffusion, serves as a proxy for glymphatic function, it may not fully correspond to the glymphatic activity confirmed through glymphatic MRI, despite its broad application in various diseases. Third, since glymphatic activity predominantly occurs during sleep, conducting scans during the daytime might not accurately capture complete glymphatic function. Fourth, this initial imaging study did not incorporate pathological or additional biomarkers, highlighting the necessity for more comprehensive future research to validate these findings. Future studies are needed to validate these findings across larger cohorts and to explore potential interventions that could modulate glymphatic function as part of the treatment regimen for sICH.
In conclusion, our study suggests that glymphatic dysfunction may play a significant role in the acute phase of sICH and is associated with clinical severity and outcomes. The DTI-ALPS index presents a promising approach to improving our understanding of sICH pathophysiology and may help identify potential targets for therapies aimed at mitigating secondary brain injury. Further research is needed to establish a clearer cause-effect relationship.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.