Deep brain stimulation improves electroencephalogram functional connectivity of patients with minimally conscious state
Yuanyuan Dang and Yong Wang contributed equally to this work.
Abstract
Aim
Deep brain stimulation (DBS) is a potential neuromodulatory therapy that enhances recovery from disorders of consciousness, especially minimally conscious state (MCS). This study measured the effects of DBS on the brain and explored the underlying mechanisms of DBS on MCS.
Methods
Nine patients with MCS were recruited for this study. The neuromodulation effects of 100 Hz DBS were explored via cross-control experiments. Coma Recovery Scale-Revised (CRS-R) and EEG were recorded, and corresponding functional connectivity and network parameters were calculated.
Results
Our results showed that 100 Hz DBS could improve the functional connectivity of the whole, local and local–local brain regions, while no significant change in EEG functional connectivity was observed in sham DBS. The whole brain's network parameters (clustering coefficient, path length, and small world characteristic) were significantly improved. In addition, a significant increase in the CRS-R and functional connectivity of three MCS patients who received 100 Hz DBS for 6 months were observed.
Conclusion
This study showed that DBS improved EEG functional connectivity and brain networks, indicating that the long-term use of DBS could improve the level of consciousness of MCS patients.
1 INTRODUCTION
Traumatic brain injury (TBI) is a major cause of death and disability. Although advances in critical care have significantly improved TBI-associated mortality rates, these patients often sustain significant brain dysfunctions. Disorders of consciousness (DOC) are states where consciousness has been altered due to damages to the brain. DOC includes coma, vegetative state/unresponsive wakefulness syndrome (VS/UWS), and minimally conscious state (MCS).1 Studies have found that differences in the underlying pathology of DOC could predict patients' prognosis.2 Although VS patients may show transient periods of eye-opening and sleep–wake cycles, their sleep patterns are significantly disorganized compared to people without DOC.3 The electroencephalogram (EEG) of VS patients may show slow background activity with delta rhythms and even electrocerebral inactivity, while the cortex does not respond to external stimuli.4 MCS patients also show sleep–wake cycles and more sleep patterns, such as spindling activity and REM sleep stage.3, 5 Compared with VS patients, MCS patients show sleep–wake cycles and more sleep patterns, such as spindling activity and REM sleep stage.3, 5 They are more responsive to stimuli, such as auditory stimuli and electrical stimulation,6, 7 show greater activation of cortical metabolic rates, and are more likely to regain consciousness with natural recovery or rehabilitation due to preservation of their brain function.5, 8 Some patients with MCS may even gradually regain consciousness and motor functions after effective rehabilitation and eventually return to society.9
Neuromodulatory interventions have been used in the treatment of patients with DOC.10-12 Transcranial direct current stimulation (tDCS), repeated transcranial magnetic stimulation (rTMS), deep brain stimulation (DBS), spinal cord electrical stimulation (SCS), and vagal nerve stimulation were shown to improve the consciousness level of these patients.13-17 DBS can directly modulate central thalamus nerve activities via electrodes in the thalamus, thus affecting the thalamus-cortex circuit and improving the patients' consciousness.1 Central thalamic DBS aims at regulating the neural circuit of consciousness, and its action site is closer to the core of target of arousal regulation. Theoretically, central thalamic DBS has a higher regulation efficiency than other invasive brain stimulations.18 It is reported that the first DOC patient treated with DBS in 1969 showed improved EEG activity.19 Subsequent follow-up studies showed the effects of DBS over several months on the recovery of consciousness.20, 21 Schiff et al.22 conducted a cross-controlled experiment on an MCS patient and found that central thalamic DBS significantly promoted functional recovery from TBI and improved consciousness. Their study received great attention,22 inspiring many studies to use DBS to improve the consciousness of MCS patients. Kundu et al.23 reviewed the DBS results of DOC patients and observed that the integrity of the reticular structure, cortex and thalamic circuit influenced the effectiveness of DBS. Thus, it is necessary to accurately and objectively measure the regulation effects of DBS to further understand its underlying mechanism.
Electroencephalogram is widely used in the diagnosis of DOC and the measurement of neuromodulation effects. EEG features based on neural oscillation, nonlinear dynamics, and information theory quantify the rhythm, complexity, and coupling integration of brain activity to characterize brain functions.24, 25 Brain functional connectivity describes the synchronous activities and information interaction among different brain regions.26, 27
Theoretical works and clinical evidence suggest that consciousness depends on the capacity of brain regions to interact through functional connectivity. The information integration theory (IIT) asserts that the key explanandum of consciousness comprises of integrated information28 and considers that consciousness emerges from functional connectivity among distributed regions. Clinical studies have reported decreased functional connectivities in the brain during VS,29 anesthesia,30 and non-REM sleep,31 especially in frontoparietal connectivity. In addition, a clear-cut recovery of brain functional connectivity in DOC patients paralleled with the recovery of consciousness. Mutual information is a common assessment method that could address functional connectivity by calculating the interaction probability of two-time series signals. King et al.26 proposed the “weighted symbolic mutual information” (wSMI), a novel measure of mutual information that measures the brain's functional connectivity during DOC. The results showed that VS patients had significantly lower brain functional connectivity than MCS patients, especially long-distance connectivity. Li et al.27 proposed the permutation conditional mutual information (PCMI) and used it to measure the changes in brain information interaction during anesthesia. Liang et al.25 proposed genuine permutation cross mutual information (G_PCMI) to explore anesthesia mechanisms and found that the loss of consciousness caused by anesthesia was due to reduced information sharing across brain regions. The above studies not only illustrate the importance of functional connectivity in the study of consciousness but also provide evidence supporting IIT.
To study the modulation mechanism of DBS in MCS patients, we used EEG to measure cortical functional connectivity changes in MCS patients. The results provide evidence explaining the modulation mechanism of DBS and electrophysiological indicators to evaluate the modulation effects of DBS.
2 METHODS
2.1 Subjects
Patients with DOC were selected for central thalamic DBS treatment from July 2017 to July 2019 at the Seventh Medical Center of PLA General Hospital. The study inclusion criteria were (1) DOC occurred after a severe brain injury. (2) The duration of stable DOC was >3 months (for patients with traumatic injuries, the period was extended to >6 month). Stable DOC was defined as no significant improvement or deterioration of consciousness for more than 4 weeks. (3) MCS was diagnosed based on the JFK Coma Recovery Scale-Revised (CRS-R). Patients were excluded if they had severe brain deformation that rendered the thalamus unrecognizable, chronic neurological diseases independent from the original injury, untreated hydrocephalus, or other severe extraneurological diseases that could reduce their life expectancy to <1 year. In total, nine consecutive MCS patients were eligible for this study.
In total, nine consecutive MCS patients (five males and four females) were eligible for this study. The median age of the whole cohort was 34.3 (range, 11–49) years. The time after injury ranges from 6 to 12 months. The details regarding patient characteristics, etiology, injured brain areas, and CRS-R scores are shown in Table 1. All patients received a thorough preoperative evaluation. An experienced clinician obtained the CRS-R score of the patients after repeated assessments. The family members of the patients were informed of the details of the study, including the operation process, DBS postoperative care, and DBS parameter selection process, and provided signed consent for participation. The study was approved by the Ethics Committee of the Seventh Medical Center of PLA General Hospital (approval no.: No. 2017-33).
| Patient number | Group | Age (years) | Sex | Etiology | Injured brain sites | Post-injury (months) | CRS-R |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 25 | Male | Trauma | Extensive subcortical white matter | 6 | 12 (2-3-3-1-0-3)a |
| 2 | 2 | 35 | Female | Hemorrhage | Left parietal cortex | 6 | 9 (1-3-2-1-0-2) |
| 3 | 2 | 52 | Male | Hemorrhage | Left basal ganglia | 6 | 9 (1-3-2-1-0-2) |
| 4 | 1 | 35 | Male | Hemorrhage | Brain stem | 7 | 9 (0-3-3-1-0-2) |
| 5 | 1 | 45 | Female | Anoxic | Extensive cortex | 7 | 12 (2-3-3-2-0-2) |
| 6 | 2 | 49 | Male | Trauma | Extensive subcortical white matter | 12 | 12 (3-1-5-1-0-2) |
| 7 | 1 | 11 | Female | Trauma | Extensive subcortical white matter | 8 | 9 (1-3-2-1-0-2) |
| 8 | 1 | 26 | Female | Anoxic | Extensive cortex | 8 | 14 (2-4-5-1-0-2) |
| 9 | 2 | 31 | Male | Trauma | Extensive subcortical white matter | 6 | 9 (1-3-2-1-0-2) |
- a The score of each subscale (auditory, visual, motor, oromotor/verbal, communication, and arousal) are shown in parentheses; CRS-R, JFK Coma Recovery Scale-Revised.
2.2 Surgery
Preoperative 3D-T1 MRI scan was performed under general anesthesia perioperatively. The bilateral centromedian-parafascicular nuclei complexes (CM-Pf) were targeted for all the patients. Surgery was performed under general anesthesia. Quadripolar electrode leads (L302, PINS) were implanted with the guidance of the Leksell stereotactic headframe. Intraoperative CT was used to confirm the accuracy of electrode implantation.
2.3 Study design
A randomized sham-controlled crossover study was conducted to study the short-term effects of DBS. The nine patients were randomly divided into two groups (Table 1). In the first group, five patients received 100 Hz DBS22 and sham DBS on two separate days separated by 24 h. The same treatment was given to the second group but in reversed order (no. of patients = 4). The other DBS parameters included 15 min of stimulation duration, a DBS pulse width of 120 μs and a 3.0 V voltage.22, 32 CRS-R and resting state EEG (15 min) were recorded before and immediately after DBS. The patients' follow-up outcomes were recorded 6 months after the DBS operation using the CRS-R scale to assess the long-term effects of DBS. The study design is shown in Figure 1.
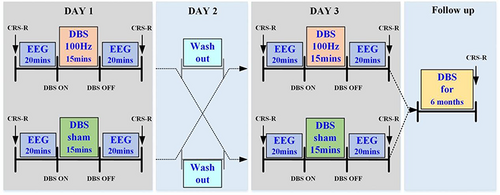
2.4 CRS-R
CRS-R is a stimulus–response measurement that indirectly represents the functions of corresponding brain structures and circuits through behavioral responses.33, 34 The scores range from 0 to 23 and are grouped into six subscores quantifying auditory, visual, motor, oromotor-verbal, communication, and arousal processes. CRS-R is widely used to assess the level of consciousness and can differentiate patients with MCS from those with VS/UWS. Patients who met the diagnostic criteria of MCS were selected for surgery. An experienced neurosurgeon measured the patient's CRS-R through in-hospital evaluations or home visits.
2.5 Neurophysiology
A 32-lead amplifier (Brian Product, Germany) was used to record the EEG data, filtered (0.1–500 Hz) and sampled at 1000 Hz at 16-bit resolution. The brain activity was obtained via 32 Ag/Cl electrode caps and displayed in real time through a BP viewer. The patients were required to keep their eyes open and rest, and the experiment stopped when they started sweating or felt uncomfortable. To ensure data quality, the impedance was kept below 5 KΩ.
2.6 EEG preprocess and analysis
EEG preprocessing was performed using EEGLAB of Matlab R2012a (Mathworks). The EEG data were verified, bad channels were removed, and data down-sampling was performed at 250 Hz, with a band-pass filter set at 1–45 Hz. Then, the data were segmented into epochs of 4 s. Artifactual epochs with voltages exceeding 100 μV were rejected following visual inspections. At least 45 artifact-free epochs were retained for each data. Residual electromyographic (EMG) and electrooculographic (EOG) activity was removed by independent component analysis (ICA). Artifactual ICs were visually rejected based on time course, spectrum, and sensor topography. The EMG component showed higher frequency power, high local scalp source, and obvious spiking activity. The EOG components were symmetrical or asymmetrical, temporarily sparse, and showed intense activities in the prefrontal lobe. Lastly, the average reference data was also obtained.
2.7 Genuine permutation cross mutual information
- A phase space reconstruction procedure was performed using the EEG data of two channels, xt and yt, t = 1, 2,…n. The vectors Xt[xt, xt+τ,…xt++mτ,] and Yt[yt, yt+τ,…yt++mτ,] were constructed with embedded dimension m and the time delay τ. In this study, m = 3, τ = 1.
- Xt and Yt were arranged in ascending order as symbols of vectors: Sn and Sq, respectively. Theoretically, there were m! different types of symbolic vectors.
- Based on the marginal probability distribution functions p(x) and p(y), the entropy of xt and yt were respectively defined as
- The joint entropy H(X,Y) was calculated based on the joint probability function, using the following equation:
- The permutation cross-mutual information (PCMI) of xt and yt was defined as
- The iterative amplitude-adjusted Fourier transform method was used to generate surrogate data, and . This study created 50 surrogate data and calculated their PCMI and .
- G_PCMI was calculated based on the comparison between the original PCMI () and . The Wilcoxon signed-rank test was used to compare and . If H0 = 1 and p < 0.001, then, G_PCMI = PCMIoriginal, otherwise, G_PCMI = 0.
- A 32 × 32 symmetric connectivity matrix of G_PCMI was obtained between 0 and 1. All G_PCMI values were sorted in ascending order, and the 70th largest value was used as the threshold. Values below this threshold were set to zero.
- The average G_PCMI values of different brain regions, including whole brain, local brain, and across-brain regions, were calculated. The local brain regions included the frontal lobe (FP1, FP2, FZ, F3, F4, F7, F8, FC1, FC2, FC5, and FC6), central lobe (CZ, C3, C4, T3, T4, CP1, CP2, CP5, and CP6), and parietal lobe (PZ, P3, P4, P7, P8, PO3, PO4, PO7, PO8, Oz, O1, and O2). The across-brain regions include the frontal-central region, central-parietal region, and frontal–parietal region.
2.8 Network topological measures
We used the binarized G_PCMI matrices based on the 70th largest G_PCMI value to construct the brain network.35 Each node in the network corresponded to an electrode, and the G_PCMI between electrodes acted as edge weights. We calculated metrics from graph theory to characterize the brain network, including clustering coefficient, average path length, and small-world characteristics.
Here, N represents the total number of nodes in the brain network.
2.9 Statistical analysis
Statistical tests were used to compare the significance of between- and within-group differences based on the G_PCMI and network metrics. The Kolmogorov–Smirnov test was performed to test the normality of the data. A paired t-test was used to evaluate within-group differences in EEG metrics before and after dual stimulation. Two-sample t-tests were performed to identify significant differences in EEG metrics between groups. Post hoc paired t-test adjusted for multiple comparisons using the false discovery rate method. Statistical significance was set at p < 0.05.
3 RESULTS
3.1 CRS-R scores
The CRS-R of patients before and after DBS was recorded. The data showed that CRS-R did not change after 15 min of 100 Hz DBS or sham DBS (p > 0.05). After 6 months of DBS, the results still showed no improvement in CRS-R in both groups (p > 0.05). However, the CRS-R scores of P4, P7, and P9 increased, with an increase from 9 (2-1-3-1-0-2) to 12 (2-3-3-2-0-2) for P4, 8 (1-1-3-1-0-2) to 11 (1-3-4-1-0-2) for P7, and 9 (1-1-3-1-0-3) to 16 (1-5-5-1-1-3) for P9.
3.2 EEG
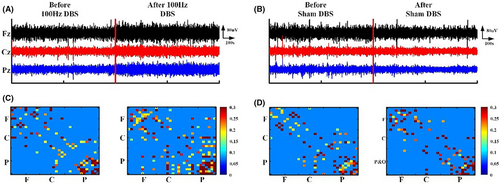
The normal distribution of EEG metrics was evaluated using the Kolmogorov–Smirnov test (all p > 0.2). Figure 2 shows the EEG outcome before and after 100 Hz DBS and sham DBS for P9 (Figure 2). The raw EEG of P9 changed in amplitude. The functional connectivity of local regions (frontal and parietal lobe) and local–local regions (frontal–parietal lobe) increased for P9 patients after 100 Hz DBS (Figure 2A,C). However, almost no changes were detected with sham DBS (Figure 2B,D).
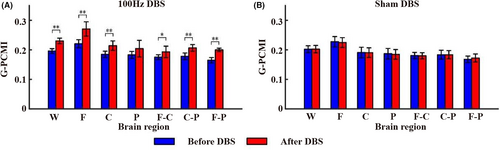
The G_PCMI before and after 100 Hz DBS and sham DBS are shown in Figure 3. The results showed that the G_PCMI of the whole (p < 0.005), local (frontal and central lobe, p < 0.005) and local–local (frontal-central lobe, p < 0.05; frontal–parietal lobe and central-parietal lobe, p < 0.005) brain regions enhanced after 15 min of 100 Hz DBS (Figure 3A). Comparatively, 15 min of sham DBS showed no significant changes in brain functional connectivity (p > 0.05; Figure 3B). See Supplementary file (Data S1) for detailed data of the G_PCMI. Next, we calculated the correlation between G_PCMI and baseline CRS-R at different time points in different brain regions. Before 100 Hz DBS, the G_PCMI of the whole brain, parietal lobe, and frontoparietal lobe was significantly correlated with baseline CRS-R (p < 0.05). After 100 Hz DBS, the G_PCMI of the whole brain and the frontal-central lobe were significantly correlated with the baseline CRS-R (p < 0.05). The change rate of G_PCMI in the frontal-central lobe and frontoparietal lobe was significantly correlated with baseline CRS-R (p < 0.05; Table 2).
| W | F | C | P | F-C | C-P | F-P | |
|---|---|---|---|---|---|---|---|
| T0 | 0.67 | 0.64 | 0.46 | 0.79 | 0.56 | 0.54 | 0.83 |
| T1 | 0.70 | 0.60 | 0.66 | 0.54 | 0.75 | 0.48 | 0.47 |
| T01 | 0.48 | 0.42 | 0.50 | 0.47 | 0.76 | 0.43 | 0.71 |
- Abbreviations: T0, before DBS; T1, after DBS; T01, changes before and after DBS; W, whole brain; F, frontal lobe, C, central lobe, P, parietal lobe, F-C, the means frontal-central lobe; C-P, central-parietal lobe; F-P means frontal–parietal lobe; CRS-R, JFK Coma Recovery Scale-Revised; EEG, electroencephalogram; Bold represents p < 0.05.
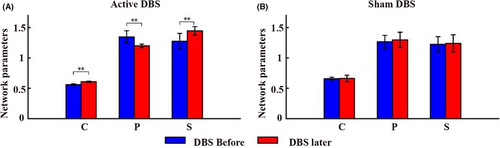
Figure 4 shows the changes in network parameters of the whole brain before and after 100 Hz DBS and sham DBS (Figure 4). After 100 Hz DBS, a significant increase in clustering coefficient and small world characteristic was observed (p < 0.005), the average path length between network nodes decreased significantly (p < 0.005; Figure 4A), and there were no significant changes in network parameters from pre- to post-sham stimulation (p > 0.05; Figure 4B). See Supplementary file (Data S1) for detailed data of the network parameters. We then calculated the correlation between network parameters and baseline CRS-R at different time points in different brain regions. The results showed that before and after 100 Hz DBS, the three network parameters were significantly correlated with the baseline CRS-R (p < 0.05). In addition, the correlation coefficients after 100 Hz DBS were higher than before 100 Hz DBS (Table 3).
| CC | PL | SW | |
|---|---|---|---|
| T0 | 0.87 | −0.67 | 0.86 |
| T1 | 0.96 | −0.86 | 0.88 |
- Abbreviations: T0, before DBS; T1, after DBS; CC, clustering coefficient; PL, the average path length; SW, small world; Bold represents (p < 0.05).
All patients received 100 Hz DBS for 6 months. The stimulation started at 8:00 and was turned off at 20:00. After 6 months of 100 Hz DBS, the consciousness level of P4, P7, and P9 patients improved. Figures 5 and S1-S2 show the changes in functional connectivity and CRS-R scores for these patients before and after 15 min DBS and after 6 months' DBS, and the results at the three time points suggest that the frontal and frontoparietal connectivity were enhanced in the three patients.
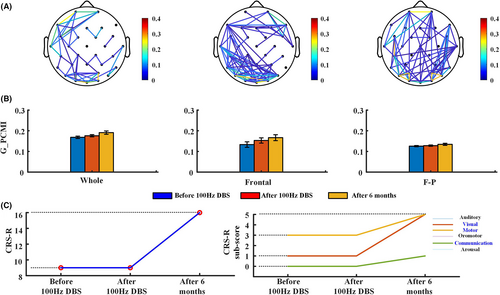
No changes were observed in the CRS-R scores of the patients before and after DBS. After 6 months of 100 Hz DBS, the functional connectivity of P9 patients was enhanced (Figure 5A,B), and the CRS-R increased by seven points (Figure 5C). P9 showed new behaviors in visual, motor, and communication functions. See Supplementary file (Data S1) for detailed data of the G_PCMI. P9 demonstrated the ability to recognize different objects (green ball and black pencil) and had lower accuracy in responding to autobiography questions via yes/no blinking. P9 also showed higher level behaviors related to MCS (automatic motor response). After 6 months of 100 Hz DBS in P7 patients, the whole and frontal–frontal functional connectivity improved (Figure S1A,B) and the CRS-R increased by three points (Figure S1C). P7 showed novel behaviors in visual and motor functions, demonstrated the ability to perform smooth eye movement by following a moving green ball, and showed higher level motor behavior (object manipulation). Comparatively, the brain functional connectivity of P4 improved significantly (Figure S2A,B) and the CRS-R increased by three points (Figure S2C). P4 also showed visual function (visual pursuit) behaviors for the first time.
4 DISCUSSION
In this study, G_PCMI was used to measure the changes in functional connectivity in MCS patients before and after DBS and to explore the underlying mechanism of DBS. The results show: (1) G_PCMI in whole, local, and local–local brain regions significantly improved after 15 min of 100 Hz DBS, (2) the change rate of G_PCMI wassignificantly correlated with the baseline CRS-R, (3) the network parameters (clustering coefficient, path length and small world characteristic) of the whole brain was significantly improved, and (4) the network parameters after 100 Hz DBS demonstrated higher correlation coefficients than prior to100 Hz DBS, while no significant changes were observed after sham DBS. After 6 months of 100 Hz DBS, three patients demonstrated some behaviors related to consciousness, indicating that DBS might have long-term effects on consciousness recovery.
Patients with DOC lose or have decreased consciousness due to severe brain injury. Previous literature indicated that exploring the mechanism of DOC could be effective in helping these patients to recover consciousness. Schiff et al.18 proposed the “cortical-subcortical structure-thalamus cortex” neural circuit to explain the mechanism of DOC, in which the thalamus was the “commander” of the consciousness circuit. Brain injury can lead to impairment in thalamus functions, resulting in a block of thalamus afferent regulation to the striatum and cortex and attenuation of the whole brain activity. Research on the mechanism of DOC provides a theoretical basis for the neuromodulation of DOC.36 DBS can modulate the thalamus's activity and promote consciousness recovery.21, 22 Relevant studies have confirmed that DBS can improve the activity of the thalamus, thus promoting the recovery of the brain metabolism and brain structures associated with the thalamus,22 such as the cortex. The cerebral cortex is an important part of the conscious neural circuit.18, 37, 38 Sleep and anesthesia studies have confirmed that the cortex can reflect the changes in thalamus activity with different consciousness states.39, 40 fMRI and EEG studies have shown that the cerebral cortex's functional connectivity is an important symbol of consciousness.37, 38 Tononi et al.28 proposed the integration theory and stated that the interaction between the thalamus and the cortex might be the basis of consciousness. Similarly, Dehaene et al.41 proposed the global neuronal workspace theory and believed that the cooperative work of the whole brain is key to the formation of consciousness.
This study used functional connectivity to investigate the effects of DBS on DOC. The results showed the effects of DBS on cortical connectivity, including whole, local, and local–local brain regions. A close relationship between the frontal cortex and the thalamus was previously reported.18, 42 In addition, the frontal cortex can affect other cortical activities through cortex-cortex circuits. These results suggest that DBS could modulate brain processes through the thalamus-cortex circuits. Moreover, the recovery of consciousness is accompanied by improved cortical activity,42 especially in the frontal cortex. Our findings are consistent with previous research suggesting that noninvasive neuromodulatory interventions such as tDCS and rTMS could promote consciousness recovery by targeting the frontal cortex.13, 43 Similarly, impairment in cross-region networks might lead to loss of consciousness, especially in the frontoparietal network.29, 44 fMRI showed the correlation between frontal–parietal connectivity and consciousness level in anesthesia, sleep, and DOC.29-31 Laurey et al.45 research on DOC showed that the recovery of the frontal–parietal network represents a sign of improved consciousness. A study showed that tDCS could improve patients' consciousness by modulating the frontal–parietal connectivity. Our study confirmed the modulation effects of DBS on brain functional connectivity in patients with consciousness disorders.
Our results also showed changes in brain network characteristics from pre- to post-DBS. After DBS, the clustering coefficient between the nodes of the whole brain network was enhanced, the characteristic distance of communication between nodes became smaller, and the small world characteristic of the whole brain was enhanced, indicating that the communication efficiency of the brain network was improved. Brain network is the basis of brain cognitive activities. In recent years, brain networks have been used to investigate the mechanism of consciousness. Anesthesia research showed the generation and disappearance of consciousness based on the network topology. Lee et al.46 found that the average path of the whole brain became longer in an anesthesia state due to propofol; that is, the disappearance of consciousness was related to the slow communication of brain network nodes. Liang et al.25 further confirmed that the loss of consciousness caused by propofol was due to the reduction of information exchange efficiency of brain network nodes at the cortical level. However, the brain network characteristics related to the loss of consciousness caused by brain injury are still unknown. Achard47 and Crone48 reported that the brain network characteristics in patients with consciousness disorder were lower than in normal people. Chennu et al.35 found a correlation between brain network characteristics and prognosis of DOC patients. This study confirmed the consciousness effects of DBS on the patients by enhancing the connection of brain network nodes, speed of information exchange, and efficiency of brain information exchange to improve consciousness activities.
DBS promoted consciousness recovery and improved visual pursuit and object manipulation in three patients. After 15 min of DBS, the functional connectivity of MCS patients enhanced, but their behavior did not improve. Schiff et al.22 reported that a patient could follow commands when DBS was on but failed to respond to sham DBS. It showed that 100 Hz DBS could improve the patient's consciousness, but the results in a follow-up study were different. Considering the individual differences of patients in the two studies, the differences in the degree of brain injury might lead to different outcomes. Brain imaging studies confirmed that MCS could retain more complete brain function and thalamus-cortical circuit, making the patients have more complex behavior. Given the relatively complete structure of MCS, such as the thalamus and frontal cortex, MCS patients have a better prognosis and are more likely to benefit from neuromodulation, such as tDCS, rTMS, and SCS. In addition, ERP and TMS-EEG studies confirmed that MCS had more brain response activity. The results of this study show that the brain functional connectivity of MCS significantly improved after DBS. On the one hand, it confirmed that the DBS could effectively modulate MCS patients' brain activity. On the other hand, combined with the prognosis of MCS patients, it shows that the functional connectivity characterized by G_PCMI could be a marker of modulation effects in DBS.
There were some limitations to this study. Although all patients completed the CRS-R score at 6 months follow-up, EEG results of long-term DBS were obtained in only three MCS patients. Despite our inclusion criteria, it was hard to disentangle spontaneous recovery from the DBS effect for long-term follow-up, especially for TBI patients. Future studies with a larger sample size will be needed to strengthen the power of analysis of this study.
5 CONCLUSION
DBS enhanced brain functional connectivity and brain network characteristics, indicating that long-term effects of DBS could improve the consciousness level of MCS patients.
CONFLICT OF INTEREST
All the authors declare that they have no conflict of interest.
FUNDING INFORMATION
This research was supported by grants from the National Natural Science Foundation of China (81771128) and the Key R&D Program of Guangdong Province, China (2018B030339001).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.




