Quality of Life in Children with Narcolepsy
Summary
Aims
To evaluate the health-related quality of life (HRQL) and its correlates in children and adolescents with narcolepsy.
Methods
We compared the clinical characteristics of control subjects and patients with primary narcolepsy from data collected at the National Reference Centers for Narcolepsy.
Results
The cohort included 69 control subjects (29 boys) and 117 patients (65 boys; 59 de novo patients). Cataplexy was present in 81% and DQB1*0602 was positive in 91%. The control children were older (13.5 ± 3.2 vs. 11.6 ± 3.1 years, P < 0.001) and less obese (1.4% vs. 60%, P < 0.001). Twenty-five percent of the patients and 15.6% of the control subjects had clinically significant depressive feelings on Children's Depression Inventory (CDI ≥ 16) (NS). Fifty-three narcoleptic and 43 control adolescents, 31 narcoleptic children and 23 control children filled out the HRQL questionnaires as well as 83 parents of patients and 60 parents of control subjects. Narcolepsy seriously impacts HRQL in terms of vitality, physical well-being, relations with friends and leisure activities, especially in adolescents. Depression was the factor that most affected HRQL in both narcoleptic and control subjects. For the control subjects and the narcoleptic patients, when the CDI score was entered into the multivariable regression model adjusted for gender and age, no other continuous independent variable could significantly increase the likelihood of the model. When the CDI score increased by 1, the mean HRQL score decreased by 1.7 for narcoleptic patients and 1.5 for control subjects. Apnea–hypopnoea index, diagnosis delay, disease duration, obesity, the presence of cataplexy or treatment had no effects on HRQL.
Conclusions
Narcoleptic children and adolescents were at high risk for poor HRQL. Depressive symptoms had a major impact on HRQL. We recommend a more thorough assessment and management of psychological health in this population.
Introduction
Classical endpoints such as mortality and symptoms are no longer viewed as the only endpoints when considering the efficacy of medical intervention 1, 2. Health-related quality of life (HRQL) measurement has become an essential outcome both in population health assessment and in clinical improvement 3. Current ability to treat children or adolescents with chronic disease, coupled with the inability to offer absolute cure, raises the issue of the quality of life of these children and adolescents. HRQL measurement may be useful to understand the child's or adolescent's perspective in any assessment of treatment outcome in either routine audit work or clinical trials 1. Narcolepsy with cataplexy is a rare and disabling sleep disorder characterized by excessive daytime sleepiness (EDS) and abnormal rapid eye movement (REM) sleep phenomena, including cataplexy (sudden loss of muscle tone triggered by strong emotions), sleep paralysis, hypnagogic hallucinations and sleep-onset REM periods (SOREMPs) 4, 5. Approximately, half of such patients have symptoms of onset prior to the age of 18 years 6-8. Symptoms are often more severe in children than in adults 8. Narcolepsy is classified as narcolepsy with (NC) and without cataplexy (NwC) 5. NC is caused by a deficiency of hypocretin-1 (also called as orexin) peptides released from the dorso-lateral hypothalamic neurons 9, 10 probably through autoimmune destruction of hypocretin cells 11, 12. As EDS is the major symptom, it undoubtedly has a severe impact on HRQL 13. In children, EDS is sometimes associated with symptoms of motor hyperactivity. The presence of cataplexy could also aggravate psychosocial consequences. However, the respective burden of cataplexy and EDS is not clearly known. Indeed, children with narcolepsy as well as those with EDS alone have shown significantly lower HRQL, higher rates of depression, and behavioral and educational problems compared with controls 14.
Several factors could influence the HRQL of these children, including age at onset 8, diagnosis delay, disease duration, EDS severity, depressive feelings, hyperactivity symptoms, cataplexy 15, dyssomnia, obesity, and concomitant sleep apnea as well as the presence of treatment. The aim of this study was to evaluate the respective impact of these factors on HRQL in a series of children and teenagers with narcolepsy and to compare it with that of control children and adolescents followed in the national reference centers.
Methods
Patients and Control Subjects
This study was based on the data collected on a large cohort of narcoleptic children and control subjects (NARCOBANK). All of these patients were diagnosed with primary narcolepsy after a complete evaluation 5. The children were followed up in the Robert Debré Children's Hospital in Paris (patients n = 65, controls n = 19), the Mother-Children's Hospital in Lyon (patients n = 39, controls n = 14), the Gui-de-Chauliac Adult's Hospital in Montpellier (patients n = 8) and the Pitié-Salpêtrière Adult's Hospital in Paris (patients n = 5, controls n = 36). A description of the patient cohort has already been reported 16. The patient cohort included 117 children (65 boys; 59 de novo patients) with a mean age of 11.6 ± 3.1 years and a median age of 12 years (from 5 to 17 years) on diagnosis. Cataplexy was present in 81%, and DQB1*0602 was positive in 91%. Control subjects were also included in this study. The control children (n = 69, 29 boys) had a mean age of 13.5 ± 3.2 years and a median age of 14 years (from 7 to 17 years). Most of the control children and adolescents were recruited from the children of the nurses and medical staff or their best friends in the authors' departments. This study was approved by the local ethics committees (PHRC AOM07-138). All of the parents signed the written form, and the children gave their informed consent to take part in this research program on their disease which included a long and systematic interview of the patient (and parents) with the neurologist or the pediatrician in charge.
Investigations
The patients had an initial consultation and follow-up visits with the certified sleep specialists (ML, PF, AGP, AR, YD, IA) who were also in charge of treating them. All measurements were collected and analyzed by the research assistants (CI, SL).
Questionnaires
Patients and control subjects completed a standardized sleep questionnaire. Daytime sleepiness was evaluated in 73 patients and 37 controls with the Adapted Epworth Sleepiness Score (AESS), in which the item “falling asleep while in a car stopped in the traffic” was replaced with “falling asleep at school” 17 and in 72 patients and 58 controls with the Pediatric Daytime Sleepiness Scale (PDSS) 18, while 44 patients and 35 controls completed both sleepiness questionnaires. The pathological scores were >10 and >16 in these two questionnaires, respectively.
The severity of cataplexy was evaluated in 87 patients by the Cataplexy Severity Rating Score 1 = moderate weakness, for example, head drop or jaw opening; 2 = can maintain posture with external support; 3 = loses posture and falls to the ground 19. The frequency of cataplexy attacks was also evaluated from 0: less than one episode per year; 1: more than one attack per year; 2: more than one attack per month, 3: more than one episode per week; 4: more than one episode per day 6. No cataplexy was reported by the control subjects.
The Children's Depression Inventory (CDI) was used to assess for symptoms of Major Depressive Disorder (MDD) in 88 patients and 64 controls 20. This questionnaire includes 27 items scoring from 0 to 2. Depression was categorized by the presence and severity of symptoms with a CDI score greater or equal to 16. Although this scale has been widely used in research on sleep and depression 21, it should be noted that the CDI score reflects depressive symptoms rather than providing for each age and sex a clear clinical diagnosis of MDD according to DSM-IV. Ninety-three patients and 66 control subjects filled out the Insomnia Severity Index (ISI) which contains seven questions scored from 0 to 4, with a maximum of 28 and an abnormal score when >10 22. Symptoms associated with hyperactivity and attention deficit 23 were scored by 85 parents of patients and by 61 parents of control subjects using the Conners Parents Rating Scale-Revised (CPRS-R) (48 items) with six subscores: conduct, learning, psychosomatic symptoms, impulsivity, anxiety, and hyperactivity. Moderate to severe symptoms were defined with a cutoff above 65, severe symptoms with a cutoff above 75. Fatigue was scored with the Chalder's fatigue scale 24 in 89 patients and 65 control subjects scored from 0 to 14, with abnormal cutoff score above 10. Parents also answered questions about their childrens' school difficulties, grade repetition, or absenteeism. A status of yes/no was used to categorize these responses.
Health-related quality of life was assessed using a questionnaire adapted for adolescents (VSP-A) 25, for children and parents 26. VSP-A 2, 25, Vécu et Santé Perçue de l'Adolescent, is a self-questionnaire for adolescents from 11 to 18 years, with the following dimensions: psychological and physical well-being, body image, vitality, friends, parents, teachers, medical staff, leisure, school performance, and a global HRQL index (range: 0–100). Lower scores correspond to a poorer quality of life. As the adolescents often did not answer the questions related to their sentimental life, the total score could or not include this dimension. VSP-P can also be filled by the parents to have the perception of the HRQL of their children 27. The VSP-A was adapted for children (8–10 years) (VSP-E) 28, Vécu et Santé Perçue par l'Enfant with nine items: general well-being, energy/vitality, self/body image, relations with friends, parents, medical staff, leisure, school and global HRQL index corresponding to the HRQL index of adolescents minus sentimental life and relations with teachers. These questionnaires were validated in 1057 adolescents with no acute or chronic diseases (mean age 14.8 years, sex ratio 0.9) 2 and 663 healthy children (mean age 10.3 years, sex ratio 0.9) in the French population 26. In our study, 53 narcoleptic and 43 control adolescents, 31 narcoleptic children, and 23 control children filled out these questionnaires as well as 83 parents of patients and 60 parents of control subjects. To compare patients with control subjects, the item interaction with medical staff item was not included in the total HRQL score.
Diagnostic Procedure for Narcolepsy
The sleep- and wake-monitoring procedure included (1) a complete clinical examination; (2) a sleep log of 15 days preceding the sleep laboratory evaluation; (3) a polysomnography with respiratory monitoring from 8 pm to 7 am (n = 109); (4) followed by 4 (n = 48) or 5 (n = 55) standard multiple sleep latency tests (MSLT) at 9 am, 11 am, 1 pm, 3 pm and 5 pm, that were terminated after 20 min if no sleep occurred, and after 15 min asleep if sleep occurred 29. Polysomnographic recordings (PSG) included an electroencephalography (Fp1-A2, C3-A2, O1-A2), left and right electro-oculograms, levator menti surface electromyography, nasal pressure trough cannulae, respiratory efforts using thoracic and abdominal belts, position, ECG, transcutaneous oximetry and end tidal CO2 values (n = 26) during the night. Sleep stages, arousals, and respiratory events were scored visually according to standard pediatric criteria 29. The total sleep time (TST), total sleep period, sleep and REM sleep latencies, the durations and percentages of non-REM sleep (NREM) stage (N1, N2, N3), and REM sleep (R) were determined during night recording as well as the indexes of sleep fragmentation (i.e., the arousal index, respiratory arousal index [RAI]), apnea–hypopnea index, minimal and mean oxygen saturation during sleep, maximum end tidal CO2 values in NREM and REM sleep and percent of CO2 values >50 mmHg during TST. Obstructive hypoventilation was defined by more than 25% of TST with CO2 higher than 50 mmHg 29. Respiratory sensors were removed during MSLT. Mean sleep latency and number of sleep-onset REM periods were calculated on MSLT 29.
Criteria for Idiopathic Narcolepsy
All of the patients met the criteria for idiopathic narcolepsy 5 including: (1) complaints of EDS for at least 3 months; (2) symptoms not better explained by other medical or psychiatric disorders; (3) the absence of secondary narcolepsy; (4) the presence of clear-cut cataplexy and/or (5) mean sleep latency during MSLT <8 min plus two or more sleep-onset REM periods. HLA-DR-DQB1*0602 genotyping was performed in most patients (n = 104). Brain magnetic resonance imaging was also often performed to rule out symptomatic narcolepsy (n = 70) 30.
Hypocretin-1 (orexin-A) was also determined in 20 patients with narcolepsy in duplicate from cerebrospinal fluid (CSF) samples without prior extraction using 125I radioimmunoassay kits from Phoenix Peptide, Inc, according to manufacturer guidelines. The detection limit was 10 pg/mL and intra-assay variability was <10%. CSF hypocretin-1 levels below 110 pg/mL were considered as low, intermediate between 110 and 200, and normal over 200. All values were back-referenced to Stanford reference samples (HHMI Stanford University Center for Narcolepsy, Palo Alto, CA, USA).
Anthropometric Measurements
Height and weight were obtained in each child and body mass index (BMI = weight/height2) was calculated. BMI z-score representing a measure of weight, adjusted for height, sex, and age, relative to a smoothed reference distribution 31, was computed. In medical practice, BMI growth curves are usually used. Obesity was defined by BMI greater than the 97th percentile for age and sex 32, 33. Age at menarche was known in 31 girls. We did not have information about the secondary sexual characteristics at the onset of symptoms to establish the presence of precocious puberty 34. Defined using the 5th percentile of the distribution in the French Health Behavior in school-age children, early menarche was defined as menarche from 9 to 11 years 35. Tanner staging was performed on inclusion in the study 36.
Statistical Analysis
Quantitative variables were expressed as mean and standard deviation. HRQL of the narcoleptic patients was compared with those of control subjects using Wilcoxon tests for quantitative variable because of the non-normality of the data assessed by Shapiro–Wilk test. Fisher's exact test was used for between-group comparisons of dichotomous variables and χ² test for polychromous variables. No imputation of missing data was performed. Bivariate associations were performed to analyze the association between HRQL and the other variables. Owing to missing data, we had a different number of subjects for each couple of variables. We therefore computed the linear coefficient of correlation and the adjusted coefficient of determination just after standardization of the two variables for each couple. To standardize a variable, we took the normal percentile of the rank of each value for a given couple. The coefficient of determination can then be interpreted as the percentage of variability of one variable “explained” by the other expressed in standard units. Package corrplot function was used to represent the coefficient of correlation. Multivariate linear models were adjusted for gender and age (≤10 years vs. >10 years) according to the categorized depression score. Each continuous significant independent variable was then entered into the multivariate linear model. The significance level was set at 5%. Statistical calculations were performed using R language, version 2.15.2 (http://cran.r-project.org/).
Results
Clinical, Polygraphical, and Biological Characteristics of the Population
The control children (n = 69, 29 boys) were older (P < 0.001), less obese (1.4%) (P < 0.001), and ate less during the night (P = 0.02) (Table 1). From the 41 children diagnosed after 2009, 13 narcoleptic patients (31.7%) had received H1N1 vaccination prior the onset of symptoms. Six of the 62 control subjects have received H1N1 vaccination (9.6%) (P = 0.01). Thirteen patients (11%) had an autoimmune disease, including diabetes type 1 (n = 3), psoriasis (n = 2), Crohn's disease (n = 1), lupus (n = 1), retinitis pigmentosa (n = 1), central hypothyroidism (n = 2), HIV infection (n = 1), and rheumatoid arthritis (n = 2). No control subjects had autoimmune diseases (P = 0.010).
| Narcoleptic patients | Control subjects | P | |
|---|---|---|---|
| n (%) | 117 | 69 | |
| Male, n (%) | 65 (55.5) | 29 (42) | 0.10 |
| African origins, n (%) | 37 (31.6) | 19 (29.2) | 0.77 |
| Age at study inclusion, year | 12 (5–17.5) | 14 (7–17.8) | <0.001 |
| Allergy, n (%) | 33 (29.7) | 20 (32.8) | 0.80 |
| Autoimmune disease, n (%) | 13 (11.1) | 0 (0) | 0.01 |
| H1N1 vaccination prior onset, n > 2009 (%) | 13/41 (31.7) | 6/62 (9.6) | 0.01 |
| Body mass index-Z score | 2.36 (−2.34 to 10.82) | 0.85 (−1.64 to 6.49) | <0.001 |
| Obesity, n (%) | 70 (59.8) | 1 (1.4) | <0.001 |
| Night eating, n (%) | 15 (17.4) | 2 (3.4) | 0.02 |
| Age at menarche, year | 11 (9–17) | 12 (10–15) | 0.15 |
| Early menarche <10 years | 8/31 (25.8) | 3/26 (11.5) | 0.30 |
| Cataplexy, n (%) | 95 (81.2) | 0 (0) | <0.001 |
| Hypnagogic hallucinations, n (%) | 47 (40.2) | 2 (2.9) | <0.001 |
| Sleep paralysis, n (%) | 29 (24.8) | 2 (2.9) | <0.001 |
| Dyssomnia, n (%) | 19 (16.2) | 8 (13.6) | 0.44 |
| Somniloquia, n (%) | 85 (72.6) | 29 (45.3) | <0.001 |
| Somnanbulism, n (%) | 24 (20.5) | 8 (12.9) | 0.173 |
| Parasomnia, n (%) | 87 (74.4) | 27 (45) | <0.001 |
| Familial parasomnia, n (%) | 35 (29.9) | 8 (13.6) | <0.001 |
| HLA DQB1*0602, n% | 95/104 (91.3) | ||
| Polysomnography (n = 109) | |||
| Total sleep time, min | 479 (270–795) | ||
| Sleep efficiency,% | 84 (29–98) | ||
| Sleep onset, min | 9 (0–187) | ||
| Rapid eye movement (REM) sleep onset, min | 73 (0–385) | ||
| Multiple sleep latency tests (n = 103) | |||
| Mean sleep latency, min | 4 (0.5–15) | ||
| Sleep onset in REM periods, n | 4 (0–5) | ||
- Data are given for 117 patients and 69 control subjects, except when n is specified. To compare patients and control children, significant levels were given (P). Data were expressed in mean ± SD.
Compared with the control subjects, patients had more hypnagogic hallucinations, sleep paralysis, somniloquia, personal and familial parasomnia. No control children had cataplexia.
Sleepiness, Mood, Fatigue, Hyperactivity, and School Problems
The sleepiness, fatigue, insomnia, mood, hyperactivity scores, and school problems are shown in Table 2. The narcoleptic patients had higher scores on AESS, PDSS, ISI, fatigue, CDI, CPRS-R questionnaires than the control subjects. Twenty-five percent of the patients and 15.6% of the control subjects had clinically significant depressive feelings (CDI ≥ 16) (NS), 7% had a high level of Attention Deficit/Hyperactivity Disorder symptoms (CPRS-R > 75) versus 1.6% in the control group (NS). Forty-one percent of the patients versus 7.5% of the controls reported school difficulties (P = 0.002), while 28% of the patients versus 7.5% of the controls did not pass a grade and repeated it prior to narcolepsy diagnosis (P < 0.001), 30% of the patients versus 8.9% of the controls had absenteeism (P = 0.002).
| Narcoleptic patients | Control subjects | P | |
|---|---|---|---|
| Adapted Epworth sleepiness score (0–24) | 16 (4–23) | 6 (0–13) | <0.001 |
| Score >10, n (%) | 69/73 (94.5) | 5/37 (13.5) | <0.001 |
| Pediatric Daytime Sleepiness Scale (0–32) | 16 (0–27) | 10.5 (1–23) | <0.001 |
| Score >16, n (%) | 38/72 (52.7) | 8/58 (13.8) | <0.001 |
| Fatigue scale | 8 (1–14) | 2 (0–12) | <0.001 |
| Score >10, n (%) | 24/89 (26.9) | 1/65 (1.5) | <0.001 |
| Insomnia Severity Index (0–28) | 12 (1–22) | 5 (0–17) | <0.001 |
| Score >10, n (%) | 59/98 (60.2) | 9/66 (13.6) | <0.001 |
| CDI | 12 (1–33) | 7 (1–27) | <0.001 |
| Score ≥16, n (%) | 22/88 (25) | 10/64 (15.6) | 0.23 |
| Conners Parent Rating scale | 52 (35–120) | 42 (35–82) | <0.001 |
| Hyperactivity score >65, n (%) | 20/85 (23.5) | 6/61 (9.8) | 0.056 |
| Hyperactivity score >75, n (%) | 6/85 (7) | 1/61 (1.6) | 0.26 |
| Quality of life (Parents) | 61.7 (28.5–91), n = 83 | 73.7 (51.2–98.8), n = 60 | <0.001 |
| Quality of life (Adolescents) | 60.4 (25.7–90.7), n = 52 | 69.5 (16.6–93), n = 43 | 0.008 |
| Quality of life (Children) | 58.8 (35.6–90.2), n = 31 | 75.8 (44.9–97), n = 23 | 0.001 |
| Grade repetition | 30/105 (28.6) | 5/66 (7.6) | 0.002 |
| School difficulties | 43/105 (40.9) | 5/66 (7.5) | <0.001 |
| Absenteeism | 31/102 (30.4) | 6/66 (8.9) | 0.002 |
- CDI, Children's Depression Inventory.
- To compare patients and control children, significant levels were given (P). Data were expressed in mean ± SD.
Quality of Life
Fifty-three narcoleptic and 43 control adolescents, 31 narcoleptic children and 23 control children filled out the HRQL questionnaires as well as 83 parents of patients and 60 parents of control subjects (clinical characteristics in Tables S1 and S2). On the other hand, no difference was found between patients and control subjects who filled and those who did not fill the HRQL questionnaire.
Narcoleptic Patients versus Control Subjects
Compared with the control children (n = 23, nine boys, 9.7 ± 4.6 years), the narcoleptic children (n = 31, 18 boys, 8.6 ± 1.7 years) had lower HRQL (P = 0.001) with lower vitality (P = 0.003), general well-being (P = 0.002), poorer self-image (P = 0.03) and tended to have less contact with their parents (P = 0.06) and lower school performances (P = 0.06) (Figure 1A). The narcoleptic adolescents (n = 53, 25 boys, 13.8 ± 2.1 years) had a lower quality of life index (P = 0.008), lower physical well-being (P < 0.001), fewer friends (P = 0.001), and leisure activities (P = 0.006) than the control adolescents (n = 43, 18 boys, 15.5 ± 1.5 years) (Figure 1B).
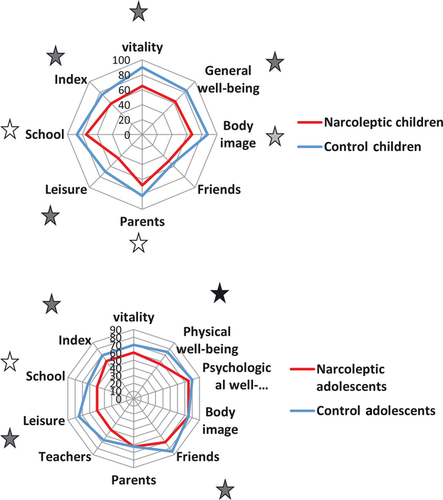
Children versus Adolescents
The narcoleptic adolescents had more contact with their friends (P = 0.003), had more leisure activities (P = 0.005) and lower performances in school (P = 0.034) than the narcoleptic children (Figure 2A). In the control group, these differences already existed between the adolescents and the children. Indeed, the adolescents had more interactions with their friends (P < 0.001), less contact with their parents (P = 0.036), lower school performance (P = 0.004) and vitality (P < 0.001), and tended to have lower general well-being (P = 0.06) than the control children (Figure 2B).

Parents versus Children or Adolescents
The parents of narcoleptic patients had a good perception of the quality of life in their children and adolescents. In the control adolescents, the parents overestimated energy (P = 0.002), psychological well-being (P = 0.022) and the school performance (P = 0.006) of their adolescents. In the control children, the parents underestimated the energy of their children (P = 0.016).
Patient Treatment
Fifty-nine patients were de novo and had never been treated, five patients had stopped their treatment, and 53 had already been treated for a median of 15 months when included in the study. Patients received modafinil (n = 44) and methylphenidate (n = 16) as stimulants, whereas mazindol (n = 3), d-amphetamine (n = 1), and adrafinil (n = 1) were rarely used. These treatments were combined in 13 patients. Venlafaxine (n = 9, 37.5–75 mg/day) was usually used for cataplexy. However, sodium oxybate (n = 3) and mazindol (n = 3) had positive effects on both EDS and cataplectic attacks. Compared with the new patients, treated patients had lower AESS scores (P = 0.049) but not significantly lower PDSS scores (NS).
Concerning HRQL, no differences were found between treated versus nontreated patients. Twenty-seven treated adolescents, 27 treated children, and 50 parents of treated patients filled the HRQL questionnaires. Of these 50 treated patients, 48 received modafinil, 2 methylphenidate, 4 a combination of methylphenidate and modafinil, one mazindol and modafinil.
School Functioning
Both patients and control subjects with school difficulties had lower total HRQL (P < 0.001). We found the same results in the narcoleptic patients (P = 0.039) and tended to reach significance in the control group (P = 0.077). For the whole group, subjects and patients with school difficulties had lower energy (P = 0.001), physical well-being (P = 0.004), psychological well-being (P = 0.003), self-esteem (P = 0.016), relations with friends (P = 0.018), leisure activities (P = 0.017), school performance (P = 0.002) than those without school difficulties. For the patients and control subjects who repeated a year, we found lower energy (P < 0.001), physical well-being (P = 0.007), total HRQL score (P = 0.049) than for those who did not. Patients and control subjects with absenteeism had lower energy (P = 0.006), physical well-being (P < 0.001), psychical well-being (P = 0.006), school performance (P = 0.03), and total HRQL score (P = 0.003) than those who had no absenteeism.
Quality of Life and Covariates
Bivariate Associations
For the narcoleptic patients, the bivariate association between the dimensions of HRQL and continuous covariates are provided in Table S3 and Figure 3. Depression was the factor that most affected the quality of life (53%), followed by behavioral problems (22%), fatigue (14%), hyperactivity (12%), daytime sleepiness (11%), anxiety (11%), and insomnia (7%). In the narcoleptic patients, depressive feelings mostly decreased energy (48%), general well-being (46%), physical (45%), and psychological well-being (40%).
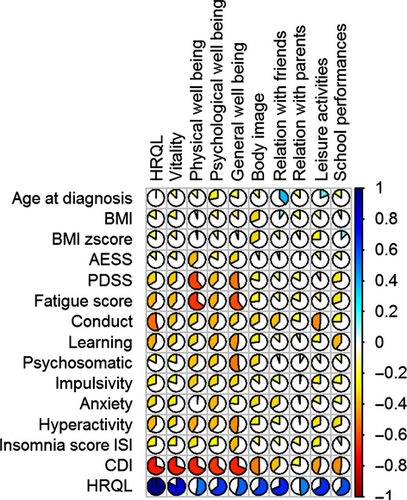
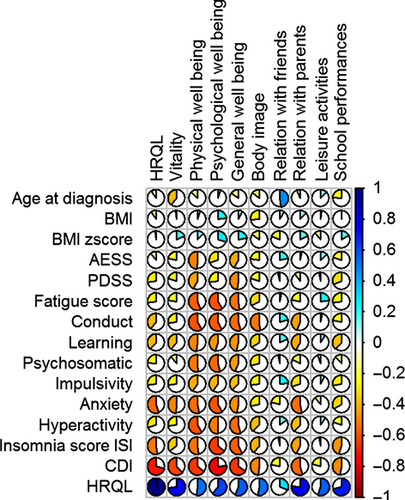
For the control subjects, the bivariate association between the dimensions of HRQL and continuous covariates are provided in Table S4 and Figure 4. Depression was the factor that most affected the quality of life (49%), followed by anxiety (29%), insomnia (26%), behavioral problems (13%), and learning (13%). In the control subjects, depressive feelings mostly decreased energy (36%), general well-being (41%), physical (40%), and psychological well-being (57%).
Multivariable Regression Model
For the narcoleptic patients, when the CDI score was entered into the linear model adjusted for gender and age, no other continuous independent variable could significantly increase the likelihood of the model. Indeed, depressive feelings were associated with fatigue (R2 = 48%), hyperactivity (R2 = 31%), behavioral problems (R2 = 28%), learning difficulties (R2 = 27%), and EDS (PDSS) (R2 = 24%). The retained model with gender, age, and CDI score as independent variables “explained” 56% (adjusted R2 = 0.56) of the variability of the HRQL score (n = 80). In this model, when the CDI score increases by 1, the mean HRQL score decreases by 1.7. The same trend was observed with the energy score as a dependent variable with a larger size effect. When the depression score increased by 1, the mean energy score decreased by 2.5. The same trend was observed with physical and psychological well-being. Obesity, the presence of cataplexy, treatment or comorbidities did not change the associations. For the HRQL score without medical staff interaction, the results were similar (−1.8, P < 0.001).
For the control subjects, we found the same results with a mild intensity. There was no significant effect of gender and age but there was a determinant effect of depression. In the model for control subjects, when the CDI score increases by 1, the mean HRQL score decreases by 1.5. No effect was found for insomnia or anxiety.
When the narcoleptic patients and control subjects were in the same model, we found the effect of depression (−1.76, P < 0.001) as well as the effect of narcolepsy (−5.89, P = 0.001).
Discussion
Narcolepsy impacts HRQL in terms of vitality and physical well-being. As a multidimensional measure, HRQL attempts to identify the most relevant aspects of health, which in adolescence and childhood are both physical and emotional well-being and self-esteem, and social functioning perception with peers, parents and teachers. This questionnaire has been largely validated in the French pediatric population. The characteristics of this subsample were similar to those of the French population attending school: age, gender, grade, or location in socioeconomic area 25. The comparison of the VSP-A scores across health data led to expected results. The narcoleptic patients reported lower HRQL than others on vitality and physical well-being, general HRQL index. These results are consistent with those reported in the literature comparing healthy with sick pediatric patients 2, 37, 38. Narcoleptic patients, especially adolescents reported fewer relations with friends and lower leisure activities than healthy subjects. Even if the social bond with friends persists, lack of vitality causes restrictions of leisure activities. This is particularly true in adolescents. On the other hand, the observed differences were consistent with the expected ones. Compared with narcoleptic children, narcoleptic adolescents had higher scores for the dimensions dealing with relations with friends, leisure and lower scores on school performance.
Only Stores et al. 14 have studied the effect of narcolepsy on HRQL in pediatrics. In the adult population, the health, social, economic consequences of narcolepsy have been evaluated by several authors 39, 40. The accumulation of different clinical symptoms as well as the “social dysfunction” associated with the disease could cause deterioration in physical and psychosocial function as well as emotional health and hence considerably affect the quality of life in those patients 13, 41-44. A similar pattern of impairment of health status was found in our study. In particular, the lowest scores were in the domains of energy/vitality, physical and psychological well-being. Energy/vitality was the first affected dimension of HRQL in our study. Patients with narcolepsy experience EDS with unexpected irresistible sleep episodes. These clinical symptoms could lead to a feeling of physical tiredness, causing reduced energy, fatigue and vitality as documented by the patients. Physical health and lack of vitality limits patients by restricting the type and amount of time spent on home life, social activities, and school performances. Hyperactivity, behavioral problems, fatigue, EDS, and insomnia also negatively influenced HRQL. However, depressive feelings represented the major factor influencing HRQL. This strict relationship between mood symptoms and HRQL has already been found in previous observations in adult 13, 41-46 and pediatric patients 14. In adult patients, the frequency of depressive symptoms ranges from 15% to 56.9% 13, 41-46. Depression could be intrinsic to the pathophysiology of narcolepsy or secondary to the complications of the disease. Indeed, hypocretins modulate stress reactions through stimulation of the hypothalamus–pituitary–adrenal axis, as well as motivated behavior. As a consequence, hypocretin deficiency in NC could also promote mood disorders. The psychosocial impact of narcolepsy and difficulty to cope with sleepiness could also expose patients to the risk of exogenous depression. Behavioral problems, emotional problems with depression, and social difficulties have been reported in 33%, 44%, and 66% of narcoleptic children in the different series 15, 47, 48. Another hypothesis was that depressed subjects may have cognitive biases favoring negative self-related information or interpretations of ambiguous situations 49, 50. Like others, we found that cataplexy did not show any correlation with HRQL scales 42-44. This finding is corroborated by the similarity of HRQL profile in IH (by definition without cataplexy) and narcolepsy found by Stores et al. 14. However, this could be explained by the fact that there were few narcoleptic patients without cataplexy in our series. No effect was found for disease duration for diagnosis delay either. Therapy status (taking drugs for narcolepsy or not) or obesity did not reach any statistically significant correlation with HRQL. Therapy has been reported to not influence the depressive feelings in adult narcoleptic patients and thus the quality of life of these patients 13.
The influence of this pathology on HRQL would appear to be less accentuated in pediatric patients than in the adult population. This phenomenon could also be attributable to the fact that in adult populations, work, and marital situation play a more important role than in the case of adolescents who are attending school. Fifty-nine percent of the adult patients were unemployed 44 and 56% reported difficulties in their private relationships 51-53. Thirteen percent described restrictions in their education because they had been unable to complete the qualification process they had begun.
Our study has several limitations. We report one of the largest series of narcoleptic children that we compared with control subjects. However, these retrospective data came from different reference centers. We used the same procedures except for different daytime sleepiness questionnaires and number of MSLT tests. The cutoff to compare younger to older patients was arbitrarily chosen at 10 years. Indeed, we used the VSA adapted for children, and this questionnaire was validated until 10 years. For adolescents, we used the Vécu et Santé Perçue de l'Adolescent, that is, a self-questionnaire for adolescents from 11 to 18 years. So the cutoff was imposed by the choice of the questionnaires that were validated for these ranges of ages. On the other hand, for HQRL data, the mean age of narcoleptic children was 8.6 ± 1.7 years versus 9.7 ± 1.6 years, and the mean age of adolescent was for narcoleptic patients (13.8 ± 2.1 years) and for control adolescents (15.5 ± 1.5 years).
In this study, only some of our children had CSF hypocretin-1 measurements. Data concerning the socio-economic level was not collected. Secondly, this study was not based on an epidemiological survey of patients with narcolepsy but included patients consecutively seen in highly specialized units. As patients were not included in different levels of care, a bias could be possible toward more severely affected patients and hence a bias toward patients with decreased HRQL. Unfortunately, our study design did not allow us to clearly distinguish different severity states of the disease. Finally, most of the control children and adolescents were recruited from the children of nurses and medical staff or their best friends in the authors' departments. However, the results were similar to those previously obtained in larger, nonselected populations 25, 26. The control subjects were older and less obese than the patients. In the multivariate model, no effect was found for age, sex, or obesity.
In conclusion, the present findings lend weight to the belief that narcoleptic children and adolescents are at high risk of poor HRQL. Depressive symptoms had a major impact on HRQL. Other factors such as hyperactivity, fatigue, EDS, and insomnia also negatively influenced HRQL.
These findings could indicate how such disadvantages might be prevented or minimized to avoid such serious difficulties in adult life. We recommend a more thorough assessment of the psychological health in this population and more aggressive treatment of depression through psychological management and antidepressive therapy at adapted doses. On the other hand, patients with narcolepsy must be encouraged to participate in leisure activities as long as possible to considerably increase the HRQL and well-being in those patients. For school performance, educational teams and school health personnel must be encouraged to set up more adapted educational or medical strategies. Prospective studies are needed to confirm these results and to find the best strategies to manage mood symptoms and to improve HRQL in narcoleptic children.
Conflict of Interest
The authors declare no conflict of interest.




