AFM in cellular and molecular microbiology
Funding information: Fonds De La Recherche Scientifique - FNRS, Grant/Award Number: WELBIO-CR-2015A-05; Horizon 2020 Framework Programme, Grant/Award Number: 693630
Abstract
The unique capabilities of the atomic force microscope (AFM), including super-resolution imaging, piconewton force-sensitivity, nanomanipulation and ability to work under physiological conditions, have offered exciting avenues for cellular and molecular biology research. AFM imaging has helped unravel the fine architectures of microbial cell envelopes at the nanoscale, and how these are altered by antimicrobial treatment. Nanomechanical measurements have shed new light on the elasticity, tensile strength and turgor pressure of single cells. Single-molecule and single-cell force spectroscopy experiments have revealed the forces and dynamics of receptor-ligand interactions, the nanoscale distribution of receptors on the cell surface and the elasticity and adhesiveness of bacterial pili. Importantly, recent force spectroscopy studies have demonstrated that extremely stable bonds are formed between bacterial adhesins and their cognate ligands, originating from a catch bond behaviour allowing the pathogen to reinforce adhesion under shear or tensile stress. Here, we survey how the versatility of AFM has enabled addressing crucial questions in microbiology, with emphasis on bacterial pathogens.
TAKE AWAYS
- AFM topographic imaging unravels the ultrastructure of bacterial envelopes.
- Nanomechanical mapping shows what makes cell envelopes stiff and resistant to drugs.
- Force spectroscopy characterises the molecular forces in pathogen adhesion.
- Stretching pili reveals a wealth of mechanical and adhesive responses.
1 INTRODUCTION
The fundamental principle of atomic force microscopy (AFM) relies on its ability to accurately measure very small forces between a sample and a very sharp tip, typically with an apex of about 20 nanometers (nm). Raster scanning a sample while controlling the position of the tip so as to exert a minimal force allows topographic imaging of the sample with a resolution of a few nm in the horizontal plane and as low as 1 Å in the vertical plane (Dufrêne et al., 2017). In addition, the ultrahigh force sensitivity of AFM (~10 piconewtons [pN]) allows to accurately quantify the forces required to deform the cell envelopes of living bacteria and to break single biomolecular bonds (Alsteens, Müller, & Dufrêne, 2017; Dufrêne, Martínez-Martín, Medalsy, Alsteens, & Müller, 2013; Krieg et al., 2019). This information is obtained from the so called force-distance (FD) curves that are generated as the AFM tip approaches the sample, makes contact with it, pushes on it and finally retracts away. From the approach segment of the FD curve, the amount of indentation and deformation of the sample and its elasticity and stiffness can be derived. The retract portion of the curve may provide information on the specific (e.g., adhesins) and non-specific (e.g., hydrophobicity) adhesive forces between the tip and sample. Here, two complementary AFM approaches have been developed to unravel the adhesive properties of bacteria, from the whole-cell to the single-molecule level. In single-cell force spectroscopy (SCFS), the AFM tip is replaced by a living bacterial cell, immobilised on the cantilever through an adhesive coating; this “bacterial probe” is then brought in contact with a substrate of interest to record FD curves (Beaussart et al., 2014). In single-molecule force spectroscopy (SMFS), the AFM tip is labelled with ligands and allows to probe single molecular interactions with their cognate receptors such as adhesins on the cell surface (Dupres et al., 2005; Lo Giudice, Dumitru, & Alsteens, 2019). Recording FD curves at each pixel as the AFM tip raster scans an area on a sample allows nanoscale mapping of local biophysical properties, such as cell wall elasticity, or the locations of single receptors. AFM, therefore, is much more than a high-resolution microscope capable of capturing the fine ultrastructural details of cell surfaces; it has also emerged as a highly sensitive, multifunctional nanobiophysics tool that is particularly well suited to study the physical properties and interactions of single cells and single molecules. Herein, we review recent advances that have been made in using AFM techniques to address two important questions in molecular and cellular microbiology: (i) what are the ultrastructural and nanomechanical properties of cell envelopes and how do they remodel in response to drugs; (ii) what are the forces and molecular mechanisms driving bacterial adhesion, mediated either by surface adhesins or pili.
2 SEEING THE INVISIBLE
Beyond its very high resolution, AFM topographic imaging has the advantages that live cells are generally imaged in buffer or growth medium and at physiological temperature, and that samples need not be stained or labeled for observation (Figure 1a). For these reasons, AFM topographic imaging has found a great appeal in the investigation of purified cell envelopes (sacculi), whole cells and cells treated with antibiotics (Figure 1).
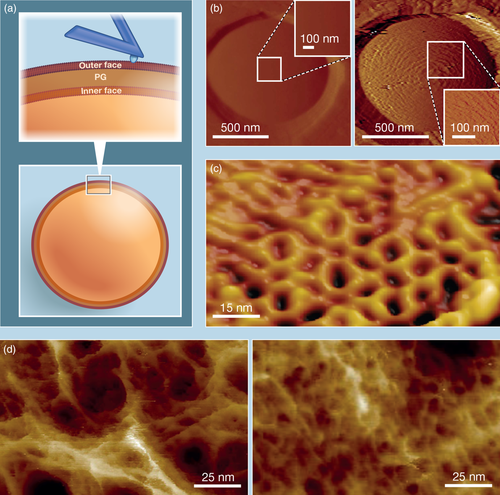
High resolution topographic imaging studies of healthy bacteria have shown that the cell surface may vary considerably based on whether a species is Gram-positive or Gram-negative as well as on the presence or absence of surface molecules such as cell wall polysaccharides or S-layers (Viljoen, Foster, Fantner, Hobbs, & Dufrêne, 2020). In Gram-negatives and mycobacteria that have outer membranes masking the peptidoglycan (PG) layer, the cell surfaces are often relatively featureless and smooth (Alsteens et al., 2008; Mathelié-Guinlet, Asmar, Collet, & Dufrêne, 2020; Vassen et al., 2019). On the contrary, for some Gram-positive species, for which the PG organization is directly accessible to the AFM tip, periodic or concentric-ring features were revealed, highlighting the intrinsic structure of the PG (Francius, Domenech, Mingeot-Leclercq, & Dufrêne, 2008; Pasquina-Lemonche et al., 2020). However, featureless and smooth surfaces were also observed in the case of Gram-positive Lactococcus lactis cells (Andre et al., 2010). Applying a large force during imaging of these cells revealed periodic, concentric bands, which were also observed in mutants devoid of cell wall exopolysaccharides (Figure 1b), indicating that in lactic acid bacteria a smooth outer layer of polysaccharides masks the organised PG structure. In Corynebacterium glutamicum, AFM revealed the ordered hexagonal organization of the crystalline outer S-layer on top of the living cells (Dupres et al., 2009). Notably, these hexagons are approximately 16 nm in diameter therefore showcasing the sub-diffraction limit resolution attainable by AFM on living samples (Figure 1c).
Today, the most detailed insights in the molecular organization of PG have come from the study of purified sacculi. A great advantage of investigating these structures is that both internally- and externally-facing PG are accessible. This has helped, for instance, to unravel a potential mechanism whereby sequential orthogonal division planes are formed in Staphylococcus aureus (Turner et al., 2010). Topographic imaging of the inner face of S. aureus sacculi revealed a ring of thick PG laid down before septation that persists over subsequent cycles of division. Thereby, it provides the cells with an orientation memory of the last division plane and thus a way to determine the next division plane. Work on extracted Escherichia coli sacculi revealed the topography of PG organization at molecular resolution in Gram-negative species: individual PG chains are 1.4 nm wide and parallel chains are separated by approximately 2.7 nm (Turner, Hurd, Cadby, Hobbs, & Foster, 2013; Turner, Mesnage, Hobbs, & Foster, 2018). Although PG chains in these sacculi appeared to be less ordered than previously thought, the sacculi exhibited an averaged orientation around the rod-shaped cell. More recently, the molecular architecture of the Gram-positive cell walls of S. aureus and Bacillus subtilis were revealed by AFM imaging of extracted sacculi and live cells (Pasquina-Lemonche et al., 2020). The surface PG revealed ~60 nm-wide and ~ 20 nm-deep pores resembling a disordered gel, while on the inner faces of sacculi, which are composed of newly synthesised PG, a much denser mesh was observed (Figure 1d). Moreover, in rod-shaped B. subtilis the PG on the inner surface exhibited a circumferential orientation, which was neither the case for S. aureus nor for division septa of either species. By revealing the morphological ultrastructure of their cell wall under native buffer conditions, AFM imaging has brought new insights into the different architectures of Gram-positive and Gram-negative bacteria.
Unique to AFM is the real-time imaging that enables researchers to track cell-surface dynamics with unprecedented resolution. In early work, the structural and physicochemical dynamics of single Aspergillus fumigatus conidia were studied during germination in real-time (Dague, Alsteens, Latgé, & Dufrêne, 2008). AFM investigations revealed the initial presence of a crystalline rodlet layer on the spore surface, which over a 3-hour period gradually gave way to an amorphous structure. Modified AFM tips that expose hydrophobic methyl groups and used to probe similar areas on the germinating spore surfaces revealed a concomitant decrease in surface hydrophobicity, thus suggesting a close structure–function relationship of the cell surface. More recently, the growth of Mycobacterium smegmatis was monitored in real-time over a period of more than 24 hours allowing multiple division cycles. Sinusoidal wave-like features of ~100 nm amplitude were observed on these rod-shaped cells, from which the future site of division could be predicted three generations prior to its occurrence (Eskandarian et al., 2017). Using AFM-based multiparametric imaging to study the mechanics of mycobacterial division, Odermatt et al. also unraveled the temporal dynamics of formation of the pre-cleavage furrow (PCF), a 5 nm-deep, 50 nm-wide narrowing that occurs around the middle of the cell and precedes cell cleavage (Odermatt et al., 2020). Their findings demonstrate how the interplay between the nanomechanical properties of the mycobacterial cell wall and the activity of cell wall remodelling enzymes dictate cleavage and physical separation of newborn cells. Using similar approaches, the same group recently established a new model of biphasic single-cell growth in mycobacteria, where the variability in duration of an initial lag phase in nascent pole growth contributes to the growth asymmetry and phenotypic heterogeneity in daughter cell length that is characteristic of the genus (Hannebelle et al., 2020).
Nowadays, a pertinent question in bacteriology is to understand the effects of antimicrobials on the ultrastructure of bacterial cell envelopes. AFM topographic imaging is a powerful means in answering this question. Focusing on mycobacteria, which normally exhibit smooth, relatively featureless cell surfaces, early AFM imaging studies showed that treatment of Mycobacterium bovis BCG cells with antitubercular drugs lead to a marked increase in the nanoscale roughness of the bacterial surface (Alsteens et al., 2008). Such an increase in surface roughness was also reported for S. aureus when the cell wall was digested by lysostaphin (Francius et al., 2008). Focusing specifically on the effect of ethambutol, which targets synthesis of the arabinogalactan that couples the mycomembrane (mycolic acid-rich outer membrane) to PG, treated cells exhibited concentric striations likely revealing the elusive organization of deeper layers of the mycobacterial cell envelope (Verbelen et al., 2006). Remarkably, technological improvements in high-speed AFM imaging of live cells (Ando, Uchihashi, & Scheuring, 2014) now permit the observation of the effects of drugs or other antimicrobial compounds on cell surface ultrastructure on much shorter time scales (down to milliseconds). This has been applied to investigate the activity of antimicrobial peptides on the E. coli outer membrane and by lysozyme on the B. subtilis cell wall (Fantner, Barbero, Gray, & Belcher, 2010; Nievergelt, Banterle, Andany, Gönczy, & Fantner, 2018; Nievergelt, Brillard, Eskandarian, McKinney, & Fantner, 2018). It will be exciting to see what the future holds for this interesting aspect of antimicrobial activity. AFM imaging will help address key questions like whether antimicrobial-induced surface ultrastructural alterations result from their direct activities or from an indirect stress response.
3 CELL MECHANICS AND ITS ROLE IN DRUG RESISTANCE
Along with morphological characterizations, AFM has been widely used for mechanical investigation of microbes in real time and at the nanoscale, by indenting a sample with the AFM tip (Krieg et al., 2019). Upon initial physical interaction between the tip and the cell surface, a non-linear compression is first observed in the approach FD curve that reflects cell wall elasticity (Velegol & Logan, 2002) (Figure 2a). This regime can be fitted by elastic contact models (e.g., the Hertz model), allowing a measure of the Young's modulus (Touhami, Nysten, & Dufrêne, 2003). When the AFM tip pushes further on the cell, a linear compression is seen that reflects the cellular turgor pressure (Velegol & Logan, 2002). From the slope of this regime, the spring constant of the cell, a measure of its stiffness, can be derived. As mentioned earlier, the spatial distribution of such biophysical properties can be obtained across the cell surface, by recording FD curves in a pixel-by-pixel manner over a defined area (Dufrêne et al., 2013) (Figure 2b).
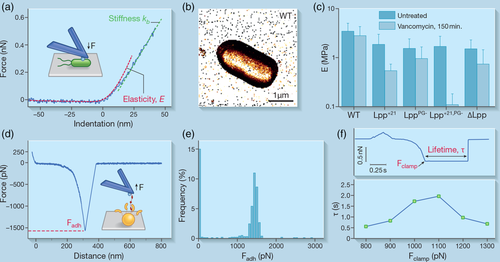
AFM indentation experiments have drawn much attention in the past years because of their ability to assess variations in cell mechanics, following biochemical treatments or changes in the environment. This knowledge is critical to our understanding of bacterial drug resistance and for the design of alternative antimicrobials. Antibacterial treatments often lead to morphological damage and soften the bacterial cell due to their mechanisms of action. For instance, they interfere with the biosynthesis of PG or indirectly cause a leak of intracellular content, as shown for ampicillin treatment in E. coli (Longo et al., 2013). However, for B. subtilis, the opposite effect, that is, a cell stiffening, has also been observed transiently, after short incubation times with synthetic amphipathic agents (Pogoda et al., 2017). This apparent discrepancy is actually intricately dependent on the incubation time, the bacterial species and the nature and properties of the antibacterial compounds (Eaton, Fernandes, Pereira, Pintado, & Xavier Malcata, 2008).
Gram-positive bacteria possess a thick PG layer designed to resist turgor pressure and that defines cellular shape and growth (Silhavy, Kahne, & Walker, 2010). For instance, in response to osmotic challenges, the PG of Group B Streptococcus adapts its physical properties to limit shape changes and potential cellular damage: its net-like arrangement stretches and stiffens to accommodate elevation in internal cellular pressure (Saar Dover, Bitler, Shimoni, Trieu-Cuot, & Shai, 2015). In addition, this mechanical scaffold is the target of many antimicrobials. Investigating S. aureus, Francius et al. demonstrated the cellular damages at the morphological and mechanical level induced by digestion of PG by lysostaphin (Francius et al., 2008). They showed a drastic decrease in the cell wall elasticity (from ~1.8 to ~0.2 MPa) and cell stiffness (from ~0.013 to ~0.006 N/m), consistent with osmotically fragile cells induced by such treatment. The importance of PG crosslinking in modulating bacterial mechanical properties was further evidenced by Loskill et al. (2014) who showed a decrease in S. aureus cell wall stiffness due to a reduction in the level of crosslinking, following the deletion of the gene encoding penicillin-binding protein 4. This illustrates how AFM could resolve some of the underlying mechanisms of bacterial resistance as this protein is essential for β-lactam resistance in some methicillin-resistant S. aureus (MRSA) strains (Memmi, Filipe, Pinho, Fu, & Cheung, 2008).
Despite the multi-layered architecture of Gram-negative bacteria that possess an outer membrane (OM) on top of the PG, it has long been thought that, as for Gram-positive bacteria, PG accounts for most of the mechanical resistance because of its ability to protect the cell from osmotic lysis (Höltje, 1998; Koch, 1988). The OM was thought to serve only as a chemical selectivity barrier. Recently though, Rojas et al. emphasised the critical role played by this outer shell in conferring Gram-negative E. coli its strength and resistance (Rojas et al., 2018). Both lipopolysaccharides and the proteins bridging the PG layer to the OM contribute to the cellular stiffness with mechanical loads being actually balanced between the two structures. This was further confirmed by Mathelié-Guinlet et al. who showed that the Braun's lipoprotein Lpp is crucial in maintaining the optimal elasticity of E. coli cells. Modification of the molecular length of Lpp or removal of its covalent anchorage to the PG led to a ~twofold decrease in cell stiffness (Mathelié-Guinlet, Asmar, et al., 2020) (Figure 2c). To a lesser extent, the membrane lipid composition was also found to affect the mechanical properties of E. coli (Li et al., 2016). Future studies should be devoted to address how the specific components of bacterial membranes influence their contribution in cell mechanics.
Heterogeneity in the composition of the Gram-negative bacterial envelope may be a contributing factor in resistance to antibacterial treatments. Formosa et al. have shown a decrease in the cell wall elasticity of Pseudomonas aeruginosa from ~250 kPa to ~50 kPa and ~25 kPa, when cells were treated respectively with ticarcillin, an antibiotic that inhibits PG synthesis, or tobramycin, an antibiotic (indirectly) perturbing cell wall integrity (Formosa et al., 2012). This suggests, again, the critical role of PG in antibiotic resistance. However, as for untreated cells, other components of the bacterial envelope actively contribute to bacterial sensitivity to some antibiotics. Notably, the dual role of Lpp, as a covalent bridge between the PG and the OM and as a periplasmic spacer plays a part in susceptibility towards vancomycin treatment (Mathelié-Guinlet, Asmar, et al., 2020). Altering one of these functional roles of Lpp drastically decreases the cell stiffness. Markedly, external cell structures, such as pili, that act as permeability barriers by allowing exclusion of harmful substances, can also influence the mechanical properties of E. coli. For instance, cells expressing type I fimbriae were shown to be prone to dramatic softening following antimicrobial peptide treatment (Quilès, Barth, Peric, Fantner, & Francius, 2020). On the other hand, such pilus structures might be lost upon antimicrobial treatment, as shown by da Silva and Teschke (da Silva & Teschke, 2003). Probably the different structures of the bacterial cell envelope are affected by antimicrobial treatments, as illustrated by the action of the PGLa peptide that affects both the OM and PG, with a 20-fold softening of E. coli cells after 5 minutes of treatment, rapidly leading to the permeabilisation of the inner membrane and cell death. Later on, Overton et al. showed that MAG2, another member of the magainin-family of antimicrobial peptides, induces a loss of membrane integrity, as followed by AFM nanoindentation experiments, which is eventually balanced by the cellular homeostasis machinery that helps E. coli cells maintain their turgor pressure (Overton et al., 2020).
Overall, these AFM mechanical studies provide new insights into the resistance of bacteria to the PG-disruptive action of some antibiotics and the membrane-destructive effects of some antimicrobial peptides. In contrast to the cell morphology that might be affected in the long term following a cascade of harmful effects, changes in bacterial elasticity and stiffness, in environments of different osmolarities or containing bactericidal agents, are likely to represent the active short-term responses of bacterial cells.
4 CELL SURFACE ADHESINS, STRONG PLAYERS IN PATHOGENICITY
Apart from their stiff cell walls, microbial pathogens have evolved highly sophisticated surface molecules and organelles to drive interactions with abiotic (medical devices) or biotic (host proteins) surfaces, the initial key step leading to infections. Notably, bacterial adhesins can recognise many components of host-cell surfaces, from proteins of the extracellular matrix such as collagen, elastin, fibronectin (Fn) and fibrinogen (Fg) to some integral host membrane receptors, such as integrins and selectins (Chagnot, Listrat, Astruc, & Desvaux, 2012; Foster, Geoghegan, Ganesh, & Höök, 2014). Getting insights into the mechanisms of cell adhesion could pave the way for the design of novel antiadhesive therapies (Beaussart, Abellán-Flos, El-Kirat-Chatel, Vincent, & Dufrêne, 2016; Geoghegan, Foster, Speziale, & Dufrêne, 2017), as an alternative to fight drug resistant strains (Pizarro-Cerdá & Cossart, 2006). Over the years, AFM has provided a wealth of information on the molecular interactions driving such adhesion, both at the single-molecule and single-cell levels. As we discuss below, much of the recent progress has focused on staphylococcal adhesion, where extremely strong adhesin-ligand interactions have been discovered and are likely to play a role in nosocomial infections involving medical implants (Foster, 2004; Otto, 2009).
One staphylococcal adhesin that has recently drawn much attention is SdrG from Staphylococcus epidermidis, a pathogen leading to nosocomial infections through medical implant colonization (Otto, 2009). Combining SCFS (cell probes vs. Fg-coated substrates) and SMFS (Fg-functionalised AFM tips vs. bacterial cells), Herman et al. reported extremely strong adhesion forces (~2 nN), similar to that of covalent bonds, for single SdrG-Fg complexes (Herman et al., 2014). This unusual strength arises from the specific “dock, lock, and latch” (DLL) mechanism. A target peptide of Fg (~15 residues of the Fg β chain) is first bound and buried between the two immunoglobulin-like domains, N2 and N3, of SdrG, in a screw-like manner, subsequently leading to conformational changes in the adhesin that result in the final “latch” with a strand connecting N3 to N2, locking the peptide in a highly mechanostable state (Bowden et al., 2008; Ponnuraj et al., 2003). The origin of this high mechanostability was further elucidated, by means of SMFS and all-atom steered molecular dynamics, and linked to the formation of a hydrogen bond network between the N2-N3 cleft of SdrG and the Fg peptide backbone (Milles, Schulten, Gaub, & Bernardi, 2018; Milles, Unterauer, Nicolaus, & Gaub, 2018). Other staphylococcal adhesins, such as S. aureus clumping factors ClfA and ClfB, binding their host target proteins (Fg and loricrin, respectively) through the DLL mechanism, or variations of it, have also been shown to mediate strong adhesion, on the order of the prototypical SdrG-Fg interaction (Herman-Bausier et al., 2018; Vitry et al., 2017). Also, ~2 nN binding forces were reported for single interactions between S. aureus protein A and von Willebrand factor, a plasma glycoprotein mediating the pathogen binding to endothelial cell surfaces (Viela et al., 2019). Most of these extremely strong interactions appear to be strengthened by mechanical tension. Increasing the applied tensile load, by varying the AFM tip retraction velocity, we have shown two common features for the above-mentioned adhesins: a shift towards higher forces with increasing tension and a depletion of lower forces favoring a population of high forces (Herman-Bausier et al., 2018; Viela et al., 2019; Vitry et al., 2017). Such signatures highlight the role of shear and tensile stresses in promoting the adhesion of bacterial pathogens to host proteins.
There has also been progress in understanding the beta zipper interaction between Fn-binding adhesins FnBPA and FnBPB of S. aureus and Fn (Foster, 2016). In early work, by grafting Fn on the AFM tip, the Lower group showed that these adhesins are necessary and sufficient for the pathogen's binding to medical devices coated with Fn (Buck et al., 2010). In addition, structural polymorphism of FnBPA increases its affinity for Fn and is associated with clinical isolates from patients with infected cardiovascular devices (Casillas-Ituarte, Lower, Lamlertthon, Fowler, & Lower, 2012; Lower et al., 2011), highlighting the promise of AFM characterizations in biomedical applications. Besides, Prystopiuk et al., (2018) showed that FnBPA mediates bacterial adhesion to soluble Fn via ∼1.5 nN forces, a mechanical stability that pathogens take advantage of to further strengthen their binding to integrins exposed at the surface of endothelial cells (Prystopiuk et al., 2018). High forces, along with a stress-dependent adhesion strength originating from the same mechanism, have also been reported for a closely related adhesin, SpsD, of Staphylococcus pseudintermedius (Viela, Mathelié-Guinlet, Pietrocola, Speziale, & Dufrêne, 2020). These extreme forces result from a tandem β-zipper mechanism. Upon binding to the N-terminal region of Fn, the Fn binding repeat regions of FnBPs transit from a disordered structure into an ordered one by forming additional β-strands along some of the Fn β-sheets (Schwarz-Linek et al., 2003).
External forces tend to sweep bacteria away from their targeted host surfaces but evidence is growing that these mechanical cues can also modulate cellular functions (Dufrêne & Persat, 2020). Intuitively, one could think that adhering bacteria would detach more easily under increasing tensile load, based on the so-called slip bonds their adhesins can form with their cognate ligands (Dembo, Torney, Saxman, Hammer, & Murray, 1988). But, flow chamber assays have shown early-on that bacterial adhesion could actually be enhanced by shear, for example, in the clumping of E. coli and erythrocytes (Brooks & Trust, 1983; Thomas, Trintchina, Forero, Vogel, & Sokurenko, 2002) or the stick-and-roll adhesion of these cells to mannose-coated substrates (Thomas, Nilsson, Forero, Sokurenko, & Vogel, 2004). The so-called catch bonds have been proposed to explain this counterintuitive behaviour: these bonds are able to strengthen in the presence of increasingly high mechanical forces (Dembo et al., 1988). In the past years, AFM experiments, coupled with genetic manipulation and molecular dynamics, have allowed the unravelling of the molecular mechanisms underlying catch bond formation (see review [Thomas, Vogel, & Sokurenko, 2008]). Indeed, by stretching the adhesion complex formed by a receptor and its ligand, at different controlled levels of applied force and by permitting the bond to rupture spontaneously, AFM force clamp experiments allow the determination of the bond lifetime and to investigate its dependence on force, in turn the putative bond strengthening (Marshall et al., 2003). Currently, only a few microbial catch-bonds have been characterised, among which are the complexes formed between the E. coli FimH adhesin and mannose residues. Yakovenko et al. demonstrated that the lifetime of this bond first increases along with the external load between 60 and 100 pN, the threshold above which the bonds eventually slip apart (Yakovenko et al., 2008). This biphasic response to force is typical of catch bonds. This can be explained by the allosteric properties of FimH whose binding pocket for mannose exists as an inactive low-affinity state at low stress but switches to a tight high-affinity conformation under tensile load (Le Trong et al., 2010; Rabbani et al., 2018; Sauer et al., 2016; Thomas et al., 2006). Another catch bond involving allosteric regulation has recently been suggested by Liu et al. (2020). They unraveled how gut microbes modulate cellular adhesion through mechanically stable Dockerin:Cohesin interactions in bacterial cellulosome protein complexes: at high loading rates (high shear) a domain neighboring the Dockerin domain allosterically inhibits the low-force unbinding pathway of the complex, giving rise to a catch binding mechanism and shear-enhanced bacterial adhesion. In a step closer to the complex system of a living bacterium interacting with its host, under physiological conditions, Mathelié-Guinlet et al. recently demonstrated, by means of AFM single-molecule experiments, the first catch bond behaviour for single staphylococcal adhesins on living cells (Mathelié-Guinlet, Viela, et al., 2020) (Figure 2d–f). They proved the long-standing idea that the extreme strength exhibited by some adhesins (~2 nN), notably those involved in DLL interactions, involves a catch-bond mechanism. Due to the extreme tension faced by such adhesins, unlike FimH, it was hypothesised that the force-induced formation of long-lived hydrogen bonds between the ligand and the adhesin, emerging from the DLL steps, might explain a strengthening of the adhesion with force, critically depending on the force propagation as described by the Gaub group (Milles & Gaub, 2020).
In summary, AFM techniques have offered unprecedented possibilities to understand cellular adhesion down to the single-molecule level. Unlike ensemble techniques and traditional affinity assays, AFM provides access to the dynamics of single-molecule and single-cell adhesion interactions, that reflect the actual out-of-equilibrium state of pathogens subjected to physical stress during colonization and infection. The extreme adhesion strength and the catch bond behaviour that some adhesins exhibit are crucial in the ability of bacterial pathogens to tune their adhesion depending on flow conditions. This fundamental knowledge could pave the way for the design of antiadhesive compounds preventing the first step of infection without killing the pathogens, thus decreasing their ability to adapt and develop drug resistance (Geoghegan et al., 2017).
5 BACTERIAL PILI: ULTRATHIN FIBERS WITH MULTIPLE FUNCTIONALITIES
Microorganisms have also developed proteinaceous appendages named pili (singular: pilus) to switch from a planktonic lifestyle to a sedentary or invasive mode. Widespread in the prokaryotic kingdom, pili feature diverse high-order structures and macroscopic shapes and instate contacts of variable distance with abiotic or biological surfaces (Craig, Forest, & Maier, 2019; Telford, Barocchi, Margarit, Rappuoli, & Grandi, 2006). The long filaments (up to 4 μm) that extrude from the cell envelope result from the multi-assembly of major pilin subunits in a helical repetitive motif that is usually decorated with minor (accessory) pilins (Giltner, Nguyen, & Burrows, 2012). At the membrane interfaces, a basal body, formed by several trans-membrane and stabilizing factors, anchors the filament to the cell body. The pilus-surface contacts are usually reversible but can ensure a high resistance to laminar shear forces encountered by bacteria in blood vessels or free aquatic environments. Several pilus types can be categorised depending on their dynamics of elongation and retraction, the overall filament structure and the pilin insertion mode (Figure 3). Even though some pilus types can be found in both Gram-positive and Gram-negative bacteria, a common property of Gram-positive pili is that their pilins are usually covalently bound. While this may also be the case for some Gram-negative pili, these pili are usually more flexible and can alternate cycles of elongation and shrinking to modify their net length.
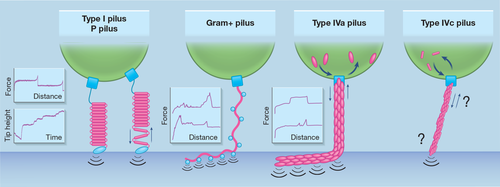
Single-molecule and single-cell AFM experiments, along with optical tweezer approaches, have unveiled various mechanical responses distinctive of pilus type and particularly pilin fold. In the case of type 1 and P pili (a.k.a. fimbriae) of E. coli, the large pilin subunit (FimA and PapA, respectively) adopts a β-barrel secondary fold in which one β-strand of the preceding subunit inserts and instates hydrophobic interactions to complement and stabilise the β-barrel fold of the succeeding subunit in the pilus rod (Phan et al., 2011; Sauer, Pinkner, Waksman, & Hultgren, 2002). This shapes a tertiary solenoid structure that stacks subunits onto each other like an electric coil of 70 Å diameter (Hahn et al., 2002). Under tension, each succeeding subunit will serially break surface interaction with the preceding subunit and progressively uncoil the whole pilus filament (Forero, Yakovenko, Sokurenko, Thomas, & Vogel, 2006). Interestingly, a drop in the tensile force reverts the phenomenon and allows the restacking of FimA or PapA into a cohesive fiber. The discrete coiling and uncoiling events were nicely showcased by SMFS on piliated cells. Ligand-functionalised AFM tips pulling on pili at a constant force (as opposed to more traditional constant velocity) revealed a staircase profile in the tip position versus time curves (Forero et al., 2006). Equilibrium in the type I pilus quaternary structure—when coiling and uncoiling events occur at equal frequencies—resists tensile forces of up to 20 pN. Maintaining a greater constant force of 50 pN as the tip is pulled away resulted in discrete changes in tip position (and hence pilus extension) of ~5 nm, which corresponds to the approximate contour length of a single FimA subunit. This molecular behaviour is also evidenced by characteristic FD curves where pili are stretched at constant retraction speed: a long plateau of constant force (about 60 and 40 pN for type I pili and P pili, respectively) due to the serial pilin subunit unstacking, followed by an abrupt increase in force preceding the rupture point that marks the desorption of adhesins present at the pilus tip (Forero et al., 2006; Lugmaier, Schedin, Kühner, & Benoit, 2008).
Thinner (around 50 Å) than type I and P pili, sortase-dependent pili of Gram-positive bacteria are stiffer and highly resistant to shear force (Telford et al., 2006; Tripathi et al., 2013). This is due to the activity of class C sortases that catalyzes the formation of covalent bonds (isopeptide bridges) between pilin subunits to irreversibly join them together and that attaches the pilus shaft to the lipid II anchor. Besides being comprised of major pilins, the pilus shaft is decorated with ancillary (minor) pilins required for adhesion. In some bacilli, streptococci and lactococci, pili can cluster and assemble in bundles (Hendrickx et al., 2012; Konto-Ghiorghi et al., 2009; Tripathi et al., 2012). Whether this property is common for Gram-positive pili remains to be elucidated. AFM studies on Lactobacillus rhamnosus pili revealed fibers with a helical motif, that can cluster in bundles (Tripathi et al., 2012). The pilus shaft is mainly composed of the major pilin SpaA and is homogeneously decorated with the minor pilin SpaC responsible for adhesion to human mucus (Kankainen et al., 2009). Reminiscent of the type I and P pili, FD curves display a canonical plateau force (Tripathi et al., 2013). However, the molecular behaviour behind this diverges as SpaC-SpaC interactions entail a zipper-like adhesion mechanism of high affinity that explains bundle-like assemblies. Before the last rupture event, tensed pili still in contact with a substrate (hydrophobic surface, mucin or host cell) produce stepped force-distance curves marked by a linear increase in force. This means that they behave like Hookean nanosprings that do not unfold and are capable of sustaining high forces (up to 500 pN for Corynebacterium diphtheriae and Actinomyces oris pili) (Echelman et al., 2016; Tripathi et al., 2013).
Type IV pili (T4P) are the most widespread fibrillar appendage across the bacterial kingdom (Denise, Abby, & Rocha, 2019). To polimerize pilins into a long fiber (up to 10 μm under tensile stress), a cytoplasmic motor consumes ATP to incorporate mature pilin subunits present on the outer leaflet of the plasma membrane to the base of the pilus filament (Craig et al., 2019). This pushes forward the filament that macroscopically elongates. Interestingly, the motorization can revert, sometimes requiring another dedicated ATPase, to remove pilins from the filament driving retraction. The retraction step is as important as the filament extension for the pilus function. For instance, selective deleterious mutations in the retraction motor of Neisseria gonorrhoeae negatively impact virulence and microbiosociability (Hockenberry, Hutchens, Agellon, & So, 2016). Moreover, the iteration of elongation-retraction cycles is used by Pseudomonas as a locomotion mode: twitching motility, where bacteria walk and crawl on solid surfaces (Gibiansky et al., 2010). Optical tweezer and micropillar approaches were used to quantify the pilus retraction force, while AFM revealed characteristic adhesion profiles. In N. gonorrhoeae, the retraction process can generate forces of up to 100 pN and pili can autoaggregate in bundles (containing up to 10 filaments) to act cooperatively at the nanonewton scale (Biais, Ladoux, Higashi, So, & Sheetz, 2008; Clausen, Koomey, & Maier, 2009). Strikingly, the P. aeruginosa pilus features two adhesion profiles in response to mechanical tension (Beaussart et al., 2014; Lu et al., 2015; Sullan et al., 2014). In the first, this pilus produces force plateaus of about 100 pN that suggest a serial desorption mode or a conformational shift induced during the pilus stretching (on the long axis). On the other hand, it alternatively generates linear force peaks typical of nanospring-like extension. With adhesion forces of up to 250 pN, the T4P (subtype a) of P. aeruginosa tolerates shear stresses encountered in physiological environments such as mucosal epithelia or the bloodstream.
Inside the T4P family, the subtype c (T4cP), also known as the Tad pilus, appears as an unusual pilus (Mignolet, Panis, & Viollier, 2018) present in both Gram-positive and Gram-negative bacteria. The small pilin subunits (45 residues) assemble to form the most slender filament (20–30 Å) for which elongation and retraction cycles are powered by a bifunctional ATPase (Ellison et al., 2019). In contrast to naturally superpiliated bacteria (10–50 pili/cell for fimbriae, Gram-positive pili and T4aP), the T4cP of Caulobacter crescentus is only assembled in a low copy number (2–3 at maximum) at one cell pole and is intricately governed by cell stage-specific regulators (Mignolet et al., 2016). AFM studies should shed light on this peculiar pilus and unveil whether structural and topological differences compared with other pili types translate into unique biophysical characteristics and polimerization-depolimerization dynamics.
Pilus biogenesis and dynamics, like for adhesins, represent tantalizing therapeutic targets as they are non-essential and therefore their pharmacological inhibition imposes a low selective pressure while at the same time pili play a crucial role in host-cell adhesion, tissue invasion and infection in general (Duménil, 2019). In this scope, the multiepitope structures of the pilus fiber may be exploited for vaccine development. Alternatively, anti-virulence drugs may replace or complement antibiotics and reduce the emergence of multidrug resistant pathogens. For instance, the Duménil group showed that P4MP4 (1-[(piperidin-4-yl)methyl]piperidin-4-ol) specifically hinders pilus elongation-retraction in Neisseria meningitidis (Aubey et al., 2019). Derivatives of phenothiazine, like trifluoroperazine or thioridazine, act differently to influence pilus function. They impair a sodium pump analogous to the proton pump of the respiratory complex I. Both power the electrochemical gradient at the basis of ATP synthesis. Denis et al. showed that they reduce N. meningitidis adherence on endothelial cells within a few seconds but do not affect bacterial viability (Denis et al., 2019). However, their collateral effects on pilus mechanics and retraction remain elusive. All together, these experiments intimate that pilus inhibitors hold promise to prevent or cure infections caused by piliated microorganisms.
6 CONCLUSION
In summary, AFM has showcased its strengths in the cellular microbiological field as a powerful imaging tool capable of resolving the ultrafine surface structures of bacterial cells while simultaneously characterising the nanomechanical tensile properties of their cell envelopes under optimal growth and under antibiotic stress conditions. The latter shows promise in improving our understanding of antimicrobial modes of action in particular at the single cell level where phenotypic heterogeneity is at play and where the moments preceding cellular death appear to be characterised by changes in cell envelope elastic properties. When it comes to the receptor-ligand interactions of bacterial adhesins binding host factors, AFM is uniquely equipped to study the biophysics, thermodynamics and kinetics of these interactions at a single molecule level. Similarly, AFM force spectroscopy studies have provided detailed insights into the molecular characteristics of appendages such as pili, including their mechanical responses to tensile stress, which have improved our understanding of their structures and their adhesive properties.
ACKNOWLEDGEMENTS
Work at UCLouvain was supported by the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (Grant Agreement No. 693630), the FNRS-WELBIO (Grant No. WELBIO-CR-2015A-05 to Y.D.F the National Fund for Scientific Research (FNRS), and the Research Department of the Communauté française de Belgique (Concerted Research Action).
CONFLICT OF INTEREST
The authors declare no competing interests.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.