Genotype-phenotype analysis of selective failure of tooth eruption—A systematic review
Abstract
Tooth eruption is an important and unique biological process during craniofacial development. Both the genetic and environmental factors can interfere with this process. Here we aimed to find the failure pattern of tooth eruption among five genetic diseases. Both systematic review and meta-analysis were used to identify the genotype–phenotype associations of unerupted teeth. The meta-analysis was based on the characteristics of abnormal tooth eruption in 223 patients with the mutations in PTH1R, RUNX2, COL1A1/2, CLCN7, and FAM20A respectively. We found all the patients presented selective failure of tooth eruption (SFTE). Primary failure of eruption patients with PTH1R mutations showed primary or isolated SFTE1 in the first and second molars (59.3% and 52% respectively). RUNX2 related cleidocranial dysplasia usually had SFTE2 in canines and premolars, while COL1A1/2 related osteogenesis imperfecta mostly caused SFTE3 in the maxillary second molars (22.9%). In CLCN7 related osteopetrosis, the second molars and mandibular first molars were the most affected. While FAM20A related enamel renal syndrome most caused SFTE5 in the second molars (86.2%) and maxillary canines. In conclusion, the SFTE was the common characteristics of most genetic diseases with abnormal isolated or syndromic tooth eruption. The selective pattern of unerupted teeth was gene-dependent. Here we recommend SFTE to classify those genetic unerupted teeth and guide for precise molecular diagnosis and treatment.
1 INTRODUCTION
Tooth eruption is a unique biological process in which teeth move from the intraosseous to the functional position of occlusion, and that occurs simultaneously with tooth root formation, which is critical for the normal development of dentition and the face of humans and mammals. Disturbance in tooth eruption can directly influence the fundamental functions of the craniofacial structure, such as growth and development of the face, mastication, phonetic, and esthetics.1
Impacted teeth and embedded teeth are major types of unerupted teeth, and maybe caused by local environmental factors, or systematic factors such as genetic factors. Failure of tooth eruption is usually characterized by the embedded teeth with a “clear” eruption path or tooth failing to erupt after removal of impacted factors.2 The treatment effects on unerupted teeth vary a lot and some unerupted teeth could not be rescued by orthodontic treatment in certain genetic disorders.3 For this reason, systematically clinical and molecular classification is essential. Unerupted teeth have been reported in a variety of genetic diseases, and may occur as an isolated disorder (primary failure of tooth eruption) or as a symptom of syndromes such as cleidocranial dysplasia (CCD), osteogenesis imperfecta (OI), osteopetrosis, enamel renal syndrome (ERS), otodental dysplasia, and osteoglophonic dysplasia. Mutations in PTH1R, RUNX2, COL1A1/2, CLCN7, and FAM20A4-8 have been associated with unerupted teeth. However, only a few studies have explored and characterized the specific genotype–phenotype relationship of unerupted teeth. Selectivity has been reported in inherited tooth agenesis, and is characterized by the mutations in a certain gene resulting in a higher incidence of missing specific tooth.9 It remains unclear whether the selectivity also exists in abnormal tooth eruption.
The aim of this study was to identify the genotype–phenotype associations in abnormal erupted teeth, illustrate the selectivity characteristics in five kinds of genetic diseases with unerupted teeth from a retrospective view, and suggest the selectivity concept and classification for the failure of tooth eruption.
2 RESULTS
2.1 Genetic unerupted teeth
Failure of tooth eruption and impacted teeth were two main types of genetic unerupted teeth, which are closely related to causative genes. Table 1 lists the genetic disorders with unerupted teeth based on OMIM database. Thirty-two types of genetic disorders were reported to cause unerupted teeth. Among these diseases, primary failure of eruption (PFE) occurs as an isolated failure of tooth eruption, and other diseases accompanied with oral or systematic anomalies. Nineteen (59.4%) genetic disorders are closely associated with bone development. And three ones are involved in dental malformation such as amelogenesis imperfecta.
| Genetic diseases | OMIM number | Responsible gene | |
|---|---|---|---|
| a) | Primary failure of eruption | 125350 | PTH1R |
| b) | Amelogenesis imperfecta | 104500, 104510, 104530, 130900, 204650, 204700, 301200, 301201, 612529, 613211, 614832, 615887, 616221, 616270, 617217 | LAMB3, ITGB6, AMTN, AMBN, ENAM, ODAPH, FAM83H, RELT, MMP20, GPR68, SLC24A4, WDR72, SP6, DLX3, FAM20A, ACP4, KLK4, AMELX, AI1E2 |
| Dentin dysplasia | 125400, 125420 | DSPP | |
| Otodental dysplasia | 166750 | FGF3, FADD | |
| c) | Cleidocranial dysplasia | 119600 | RUNX2 |
| Osteoglophonic dysplasia | 166250 | FGFR1 | |
| Osteogenesis imperfecta | 166210, 259420 | COL1A1, COL1A2 | |
| Osteopetrosis | 259700, 611490, 259720, 615085, 259730, 259710, 611497, 612301 | TCIRG1, CLCN7, OSTM1, and so forth | |
| Apert syndrome | 101200 | FGFR2 | |
| Carpenter syndrome | 201000, 614976 | RAB23, MEGF8 | |
| Cherubism | 118400 | SH3BP2 | |
| Osseous heteroplasia, progressive | 166350 | GNAS | |
| Hallermann–Streiff syndrome | 234100 | - | |
| Spondylocarpotarsal synostosis syndrome | 272460 | FLNB | |
| d) | Eiken syndrome | 600002 | PTH1R |
| Gorlin syndrome | 109400 | PTCH2, PTCH1, SUFU | |
| Elsahy–Waters syndrome | 211380 | CDH11 | |
| Hyper-IgE recurrent infection syndrome | 147060 | STAT3 | |
| Splenogonadal fusion limb defect | 183300 | - | |
| GAPO syndrome | 230740 | ANTXR1 | |
| Costello syndrome | 218040 | HRAS | |
| Aarskog syndrome | 100050, 305400 | FGD1 | |
| SHORT syndrome | 269880 | PIK3R1 | |
| e) | Gardner syndrome | 175100 | APC |
| Hereditary gingival fibromatosis | 135300, 605544, 609955, 611010, 617626 | SOS1, REST | |
| Singleton–Merten syndromes | 182250 | IFIH1 | |
| Marbach–Rustad progeroid syndrome | 619322 | LEMD2 | |
| Microphthalmia, syndromic 3 | 206900 | SOX2 | |
| Brachydactyly, type E | 113300 | HOXD13 | |
| Microphthalmia, syndromic 2 | 300166 | BCOR | |
| Rutherfurd syndrome | - | - | |
| Junctional epidermolysis bullosa | - | COL17A1, ITGA6, ITGB4, LAMA3, LAMB3, LAMC2 |
Twenty-four genes associated with failure of tooth eruption have been identified from NCBI and PubMed databases (Table 2), among which the correlation between PTH1R and PFE has been widely studied. Here, we mainly focused on five causative mutated genes and related genetic diseases with eruption failure: PTH1R related PFE, RUNX2 related CCD, COL1A1 or COL1A2 related OI, CLCN7 related osteopetrosis and FAM20A related enamel-renal-syndrome.
| Gene | Gene/locus MIM number | Involved diseases | References |
|---|---|---|---|
| PTH1R | 168468 | Primary failure of eruption Eiken syndrome |
|
| RUNX2 | 600211 | Cleidocranial dysplasia | 12 |
| COL1A1 | 120150 | Osteogenesis imperfecta | 13 |
| COL1A2 | 120160 | ||
| CLCN7 | 602727 | Osteopetrosis | 7 |
| FAM20A | 611062 | Enamel-renal-syndrome (Amelogenesis imperfecta, type IG) |
8 |
| FAM83H | 611927 | Amelogenesis imperfecta, type IIIA | 14 |
| MMP20 | 604629 | Amelogenesis imperfecta, type IIA2 | 15 |
| COL17A1 | 113811 | Amelogenesis imperfecta | 16 |
| CLDN16a | 603959 | Amelogenesis imperfecta | 17 |
| FGFR1 | 136350 | Osteoglophonic dysplasia | 18 |
| LTBP3 | 602090 | Dental anomalies and short stature (DASS) | 19 |
| GNAS | 139320 | N/A | 20 |
| KMT2Ca | 606833 | Primary failure of tooth eruption | 21 |
| GUSB | 611499 | Mucopolysaccharidosis type VII | 22 |
| PTHLH | 168470 | N/A | - |
| HMGA2 | 192240 | N/A | - |
| VEGFA | 192240 | N/A | - |
| BMP4 | 112262 | N/A | - |
| KDR | 191306 | N/A | - |
| TNFSF11 | 602642 | N/A | - |
| VCL | 193065 | N/A | - |
| TEX14 | 605792 | N/A | - |
| ADAMTS5 | 605007 | N/A | - |
- Abbreviation: N/A, not applicable.
- a The gene requires further validation. “-” The gene was reported by NCBI (http://www.ncbi.nlm.nih.gov/gene/?term=failure+of+tooth+eruption#).
2.2 Characteristics for five genetic diseases
PFE is a rare autosomal dominant disorder characterized by incomplete tooth eruption despite the presence of a clear eruption pathway. Typical symptoms of PFE usually include failure of eruption of permanent molars (or/and deciduous infraoccluded molars), open bite and reduction of vertical growth of the alveolar bone in the affected regions. The orthodontic force applied to the unerupted teeth usually leads to ankylosis. In severe cases, prosthetic treatment and surgical approaches such as segmental osteotomy or/and distraction osteogenesis are necessary to improve malocclusion.3
CCD is a rare autosomal dominant skeletal disorder, characterized by patent fontanelles, wide cranial sutures, hypoplasia of clavicles, short stature, and multiple dental anomalies such as supernumerary teeth, retention of primary dentition, impacted teeth, and failure of tooth eruption. Timing of treatment is important for CCD patients. Removing the primary and supernumerary teeth together with the bone covering the impacted teeth in early age can facilitate the spontaneous eruption of impacted teeth. For adults, combined surgical-orthodontic treatment can achieve a nearly complete dentition and stable occlusal contact.23
OI is a phenotypically and genetically heterogeneous heritable connective tissue disorder characterized by bone fragility, growth deficiency, skeletal deformity, craniofacial, and dental malformations (such as dentinogenesis imperfecta type I). Some patients exhibited failure of tooth eruption. The classical types I–IV of OI are dominantly inherited disorders mainly caused by COL1A1 or COL1A2 mutations.
Osteopetrosis, a group of rare genetic bone disorders, is characterized by increased bone mass and density due to failure in bone resorption. Dental anomalies in osteopetrosis patients include delayed or failure of tooth eruption, impacted teeth, and root dysplasia.
The ERS, a rare autosomal recessive disorder, is characterized by amelogenesis imperfecta, failed or delayed eruption and gingival fibromatosis. ERS is caused by the mutations in FAM20A gene.
2.3 Clinical classification for failure of tooth eruption
We group failure of tooth eruption into four groups according to their clinical characteristics, impact factors, prognosis and outcome (Figure 1). Type ① and ② exist in all types of genetic unerupted teeth, and type ③ exists in osteopetrosis patients with CLCN7 mutations and enamel-renal-syndrome with mutated FAM20A gene, while type ④ most occurred in CCD patients with RUNX2 mutations.
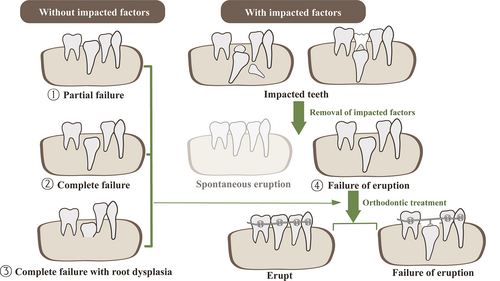
Generally, the unerupted PFE teeth have a higher ratio of orthodontic treatment failure, while the unerupted CCD teeth may be successfully rescued by orthodontic treatment. The outcomes of orthodontic treatment for other three genetic diseases were rarely reported.
2.4 Meta-analysis
2.4.1 General information
In total, 79 articles describing 223 patients were included. The 223 patients include 109 males, 101 females and 13 unstated ones. All patients were over 12 years old and the average age was 20.9 years old (11 PFE patients were in mixed dentition and affected teeth were not included). There were 62 PFE patients with PTH1R mutations, 50 CCD patients with RUNX2 mutations, 85 OI patients with mutations in COL1A1 or COL1A2 gene, 6 osteopetrosis patients with CLCN7 mutations and 20 ERS patients with FAM20A mutations (Table 3). Of 212 patients with permanent dentition, 203 (95.8%) had failure of tooth eruption without impacted factors, 70 (33.0%) had impacted teeth, and 13 (6.1%) had tooth root dysplasia.
| Genetic diseases | Mutated genes | Patients (n) | Unerupted teeth (n) | Failure of tooth eruption | Impacted teeth | Supernumerary teeth | Root dysplasia | Amelogenesis imperfecta |
|---|---|---|---|---|---|---|---|---|
| Primary failure of eruption (PFE) | PTH1R | 62 | 375 | Yes | - | - | - | - |
| Cleidocranial dysplasia (CCD) | RUNX2 | 50 | 665 | Yes | Yes | Yes | Yes | - |
| Osteogenesis imperfecta (OI) | COL1A1 COL1A2 |
85 | 73 | Yes | N/A | - | - | - |
| Osteopetrosis | CLCN7 | 6 | 42 | Yes | Yes | - | Yes | - |
| Enamel renal syndrome (ERS) | FAM20A | 20 | 209 | Yes | Yes | - | Yes | Yes |
- Abbreviation: N/A, not applicable.
2.4.2 Unerupted teeth
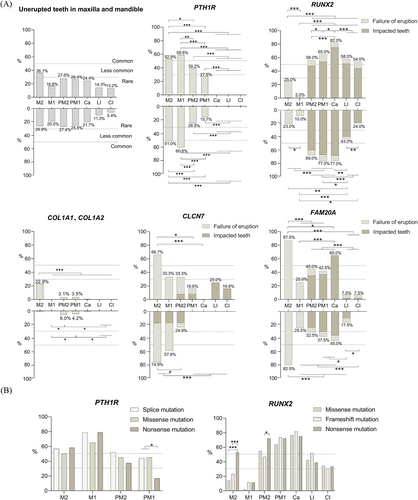
In total, there were 1364 unerupted teeth, including 1327 permanent teeth and 37 primary teeth. Generally, prevalence of unerupted teeth caused by the five genetic disorders was rare (most under 30%) (Figure 2). The 1327 permanent teeth consist of 678 (51.1%) teeth failing to erupt, 649 (48.9%) ones with impacted factors, and 54 (4.1%) had tooth root dysplasia, with the upper teeth being more affected than lower teeth (53.4% vs. 46.6%). The average number of unerupted teeth was 6.7 for mutations of PTH1R, 13.3 for RUNX2, 0.8 for COL1A1 or COL1A/2, 7.0 for CLCN7, and 10.5 for FAM20A. The top three types of teeth that were affected were maxM2 (36.1%), mandM2 (28.9%), maxPM2 (27.6%) and mandPM2 (27.4%). In contrast, MandCI (5.4%), and MandLI (11.3%) were least affected.
2.4.3 Genotype–phenotype in PFE
Seventeen articles reported 62 PFE patients with 24 PTH1R mutations. Among the 62 patients, 51 (82.5%) had permanent dentition (age range 13–62 years), while 11 (17.7%) were in mixed dentition (age range 5–12 years).
In permanent dentition, 338 teeth were unerupted and the number of unerupted teeth ranged from 1 to 16 (mean, 6.7). The 51 patients had failure of tooth eruption. The first and second molars were the most affected. A high percentage of unerupted teeth was noted for mandM1 (60.8%), maxM1 (58.8%), maxM2 (52.9%), and mandM2 (51.0%). The anterior teeth were not affected. All the affected teeth had clear eruption pathway while failed to erupt. Almost all patients (n = 49, 96%) had at least one affected first molar and most patients (n = 46, 90%) also had affected second molars. Additionally, the first molars were the most affected in type II and III PFE (n = 16, 84.2%), and that teeth distal to the first molars were more susceptible to failure of eruption (especially the second molars).24 In primary or mixed dentition, 37 teeth were affected and only primary molars failed to erupt. We highlighted the genotype–phenotype correlation by categorizing the patients based on their radiographic and genetic findings in Table 4 and Figure 2.
| Permanent dentition (N = 51) | Mixed dentition (N = 11) | |
|---|---|---|
| Age (years) | 13–62 | 6–12 |
| Gender (n) | ||
| Male | 22 | 4 |
| Female | 23 | 4 |
| Unstated | 6 | 3 |
| Prevalence of affected molars, n (%) | ||
| First molar | 121 (35.7) | - |
| Second molar | 106 (31.3) | - |
| First primary molar | - | 14 (37.8) |
| Second primary molar | - | 23 (62.2) |
| Affected anterior teeth | No | No |
| Classification of PFE, n (%) | ||
| Type I | 8 (29.6) | 1 |
| Type II | 10 (37.0) | 6 (36.4) |
| Type III | 9 (33.3) | 4 (63.6) |
| Unknown | 24 | - |
| Patients with affected vertical growth of the alveolar bone around affected teeth (n) | 51 | 9 |
| Type of PTH1R variants (n) | ||
| Splice-site mutation | 26 | - |
| Missense mutation | 5 | 1 |
| Nonsense mutation | 6 | - |
| Intronic mutation | 3 | - |
| Synonymous variant | - | 1 |
| Indel | 3 | 3 |
| Not available | 8 | 6 |
The prevalence of unerupted teeth was also significantly associated with the type of PTH1R variants. Six types of PTH1R variants in the 51 affected individuals were reported, with splice, missense, and nonsense mutation being most common ones. We found nonsense and splice variants were significantly associated with unerupted first molars. Nonsense mutations had a lower incidence of unerupted first premolars than the other variants.
2.4.4 Genotype–phenotype in CCD
Thirty-nine articles reported 45 mutations of RUNX2 in 50 CCD patients, including 22 males, 25 females, and 3 unstated ones (age range 12–49 years). The number of unerupted teeth ranged from 1 to 23 (mean, 13.3). Of 50 patients, 49 (98%) had impacted teeth, 41 (82%) had failure of eruption, and one (2%) had root dysplasia.
A total of 665 teeth did not erupt, among which 128 teeth failed to erupt and 537 teeth were impacted. The most prevalent unerupted teeth were canines (79.5%), the first and second premolars (71.0%, 62.5% respectively), with supernumerary teeth and/or retained deciduous teeth often occurred in those regions. While the first and second molars were the teeth least frequently affected (6.0%, 24.0% respectively). The second molars had the highest incidence of failure of eruption (24%), but had the least incidence of impacted teeth (1.0%). Of 50 patients, 46 (92%) had supernumerary teeth. Premolar and canine were regions most frequently affected by supernumerary teeth (found in 76.1% and 69.6% of patients respectively). The molar and incisor region were least affected by supernumerary teeth (found in 10.9% and 28.3% of patients respectively) (Figure 2).
Forty-five RUNX2 mutations included missense (n = 16), frameshift (n = 15), nonsense (n = 9), duplication (n = 3), splice-site (n = 1), intron (n = 1) mutations, and 5 patients had no information on the molecular consequence of RUNX2 variants involved. Interestingly, various RUNX2 mutations had different phenotype of unerupted teeth (Figure 2B). Nonsense mutations had a higher incidence of unerupted second molars than missense and frameshift mutations (p ≤ 0.001). Nine (18%) CCD patients had deletions in RUNX2 gene. A previous study indicated that the length of deletion had little effect on the CCD phenotype.25
The incidence of failure of tooth eruption in CCD patients might be largely underestimated due to the presence of impacted factors. From our study, impacted factors such as retained deciduous teeth and supernumerary teeth often occurred in premolar and canine regions, which were similar to findings of a recent study.26 Another study showed the incidence of spontaneous eruption after removal of impacted factors was highest for the maxillary and mandibular incisors and lowest for the mandibular canines and premolars. The incidence of unerupted teeth due to impacted factors seemed to be more common in incisors than in other teeth.27
2.4.5 Genotype–phenotype in OI
A total of 85 OI patients with COL1A1 or COL1A2 mutations were evaluated, with 47 males and 38 females (age range 15–43 years). Generally, unerupted teeth in patients with COL1A1/2 mutations were rare (under 30%). On average, each OI patient had 0.8 unerupted teeth (most teeth failed to erupt without impacted factors). Of 73 unerupted teeth, the maxillary second molars (22.9%) were the teeth most frequently affected (p < 0.001) (Figure 2).
The various COL1A1/2 variants directly affected the phenotype of eruption anomaly. A previous study indicated all COL1A1/2 variants presented phenotype of unerupted maxillary second molars except for α1 haploinsufficiency, which only exhibited unerupted second premolars. Unerupted mandibular premolars and maxillary second molars were more common among patients with α1 glycine substitution than those with α2 glycine substitutions.28
2.4.6 Genotype–phenotype in osteopetrosis
Six osteopetrosis patients with CLCN7 mutations in seven articles were included (3 males and 3 females, age range 15–38 years). The number of unerupted teeth ranged from 2 to 15 (mean, 7). All patients (n = 6) had failure of eruption with root dysplasia and four had impacted teeth. Of 42 unerupted teeth, 39 (92.3%) had root dysplasia, 29 (69%) failed to erupt, and 13 (30.1%) were impacted. Molars were mainly affected in osteopetrosis patients with CLCN7 mutations: The most prevalent unerupted teeth in total were mandM2 (74.9%), maxM2 (66.7%), and mandM1 (57.8%). Failure of tooth eruption was highest in maxM2 (66.7%), mandM2 (58.3%), while affected anterior teeth and the first premolars were rare (Figure 2).
2.4.7 Genotype–phenotype in ERS
Twenty ERS patients with FAM20A mutations included 11 males, 8 females, and 1 unstated one, from 12 to 29 years old, with 209 unerupted teeth in total. The number of unerupted teeth ranged from 2 to 18 (mean, 10.5). The most prevalent unerupted teeth were the second molars and canines: maxM2 (87.5%), mandM2 (82.5%), and mandCa (65%). All patients (n = 20, 100%) had failure of tooth eruption, 16 (80.0%) had impacted teeth and 6 (30.0%) showed tooth root anomalies, with 110 teeth (52.6%) failing to erupt, 99 teeth (47.4%) being impacted and 15 teeth (7.1%) with short or atypical shape of root. The incidence of failure of eruption was highest in maxM2 (87.5%), mandM2 (82.5%) (Figure 2).
2.5 Selectivity in unerupted teeth
2.5.1 Tooth position
Mutations in a certain gene result in a higher incidence of unerupted teeth in specific position: PTH1R mutations most caused unerupted first (59.3%) and second molars (52.0%). RUNX2 mutations had a higher incidence of unerupted teeth in the canines (79.5%), the first premolars (71.0%) and second premolars (62.5%). Additionally, distribution of supernumerary teeth in CCD patients with RUNX2 mutations also showed selectivity which usually occurred in premolar and canine region. While COL1A1 and COL1A2 mutations most affected the maxillary second molars (22.9%). Mutated CLCN7 genes most caused unerupted teeth in the second molars (70.8%) and mandibular first molars (57.8%), while FAM20A mutations most affected the second molars (86.2%) and maxillary canines (65.0%).
Additionally, the highest incidence of unerupted second molars and first molars occurred in FAM20A and PTH1R mutations respectively. While RUNX2 mutations showed the highest incidence of unerupted premolars, canines and incisors.
2.5.2 Type of failure of tooth eruption
According to clinical classification, the ratio of four types of failure of tooth eruption was different in the above genetic diseases. In PFE and OI patients, complete or partial failure of tooth eruption (type ① and ②) were dominant, while CCD patients had higher ratio of failure of tooth eruption after removal of impacted factors (type ④). And the supernumerary teeth, found in 92% of CCD patients, were not found in other four diseases. Failure of tooth eruption with root dysplasia (type ③) can be found in osteopetrosis and ERS patients and were highest in former. Root dysplasia in osteopetrosis patients frequently affected root formation and the root length. While atypical root shape than the root length was found in some ERS patients (Figure 1 and Table 3).
2.6 Molecular classification for failure of tooth eruption
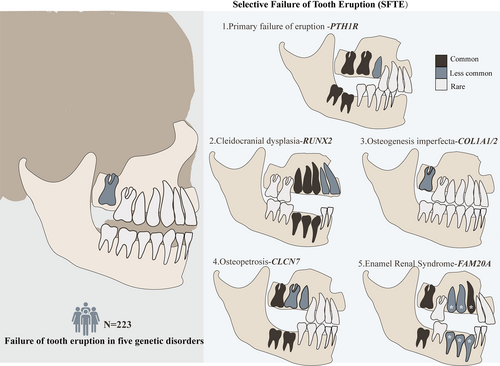
Although there were significant differences in the distribution of unerupted teeth among the five genetic diseases, the common characteristic among the diseases was that failure of tooth eruption in the specific tooth was caused by particular genes or molecules. As a result, the phenotype was termed as selective failure of tooth eruption (SFTE). For the first time, we used SFTE to show the role of genetic factors in unerupted teeth and further classified the phenotype into five types, that is, SFTE1 ~ 5 caused by mutations in PTH1R, RUNX2, COL1A1/2, CLCN7, and FAM20A genes, respectively. The specific genotype–phenotype profiles for each of the genes implicated in failure of tooth eruption were described in the following sections.
Generally, the maxillary second molars had the highest incidence of failure of tooth eruption. The SFTE1 ~ 5 types were characterized based on their unique genotypes and phenotype: PTH1R related SFTE 1 affected posterior teeth, especially in the first and second molar. RUNX2 related SFTE 2 may affect premolars and canines, and could also affect incisors and second molars. COL1A1/2 related SFTE 3 most caused eruption failure of maxillary second molars. CLCN7 related SFTE 4 most affected the second molars and mandibular first molar. FAM20A related SFTE5 may affect the second molars and canines. According to a previous study, frequently impacted teeth in SFTE2 fail to erupt even after removal of impacted factors27 (The incidence of failure of tooth eruption after removal of impacted factors was unknown in SFTE 5) (Figure 3).
3 DISCUSSION
To our knowledge, this is the first systematic review and meta-analysis of genetic unerupted teeth case reports from literature review. Unerupted tooth is caused by local environmental factors, or systematic factors such as genetic factors. Over 30 types of genetic disorders has been reported to cause unerupted teeth, which are closely associated with bone development and tooth formation. In general population, the first molars and canines relatively have the lowest and highest rate of eruption anomalies respectively. The prevalence of unerupted premolars and second molars is low (about 1.6% and 2.3% respectively).29 However, patients with genetic disorders usually had a higher incidence of eruption anomalies in specific tooth and closely related to causative mutated genes. Very few reports have focused on the selective phenotype in genetic unerupted teeth. Selectivity has been reported in inherited tooth agenesis, and is characterized by mutations in specific genes that increase the incidence of missing specific teeth.
Here, we highlight the selective pattern of unerupted teeth and causative mutated genes. We recommend SFTE to classify those genetic unerupted teeth, guide for precise molecular diagnosis and treatment. Selectivity in tooth position, type of unerupted teeth was found in genetic unerupted teeth, which were characterized based on their unique genotypes-phenotype profiles and has been described in Section 2.
Meanwhile, there are not a reasonable number of case reports for exploring gene-phenotype relationships in CLCN7 related SFTE4. Previous studies showed clcn7− deficient zebrafish had unerupted 3V1 and 5V1 teeth.7 The finding better supported the selective phenotype of SFTE 4 in humans, while further investigations are required.
3.1 Possible mechanism of selectivity
A variety of factors including genetic and molecular regulation as well as environmental aspects contribute to selectivity in unerupted teeth. Genetic factors usually include the effects of various mutated genes and the inheritance pattern. And molecular regulation may be associated with bone remodeling, spatiotemporal regulation, local environment, and other potential factors. While, environmental factors involve the physical obstacles and lack of space.
3.1.1 Causative mutated genes and inheritance pattern
Different causative mutated genes in the same or different genetic diseases play significant roles in selective phenotype in unerupted teeth. In terms of tooth position and type of unerupted teeth in SFTE 1 ~ 5 caused by five mutated genes were significantly different. While various mutated genes in the same diseases such as PFE also exhibited different phenotypes.
Inheritance pattern and dose-dependent effects of related causative mutated genes may also contribute to the selectivity. Our previous study revealed tooth phenotype was more severe and frequent in autosomal recessive osteopetrosis than in autosomal dominant osteopetrosis patients.7 Other studies showed the PFE phenotype (preferentially affecting posterior teeth) may be the result of dose-dependent inactivation of PTH1R. Patients carrying pathogenic or likely pathogenic PTH1R variants mostly had classic PFE than benign ones.30, 10 In our study, PTH1R and RUNX2 mutations also showed dose-dependent effects, with lower incidence of unerupted first premolars and higher incidence of unerupted second molars in nonsense mutations than other mutations respectively.
3.1.2 Bone remodeling, spatiotemporal regulation, and local microenvironment
Previous research focused on the molecules in the two main aspects of tooth eruption: the formation of tooth eruption pathway and eruptive force, a coordinated process in which the dental follicle interacts with both osteoclasts and osteoblasts. And bone remodeling, the selective deposition and resorption of bone, which is controlled by osteoclasts and osteoblasts, may be an important cause of selectivity of unerupted teeth.31
The common pathogenic foundation of SFTE 1–4 might be unbalanced bone remodeling during the tooth eruption, which further results in abnormal formation of the eruption pathway and eruptive force of teeth. PTH1R mutations affect the development of dental follicle cells resulting in anomalous periodontal tissues and alveolar bone formation, which further caused impaired eruptive force.32, 33 RUNX2 mutations impair osteogenic differentiation and osteoclast-inductive capacity of dental follicle cells, leading to imbalanced bone remodeling.5 COL1A1/2 mutations directly affected bone formation and eruptive force.34 CLCN7 mutations lead to osteoclast dysfunction which directly affects bone resorption and formation of the eruption pathway.35
However, different from bone remodeling, FAM20A related SFTE5 highlighted the abnormal enamel development and its regulation for tooth eruption. Interestingly, it is proposed previously that a “clock” existed in the enamel organ that regulated the cellular events of tooth eruption. The stellate reticulum, adjacent to the dental follicle, is the major component of the enamel organ. And it is speculated the secretion of molecules such as TGF-β1 and/or IL-1 with their subsequent effect on the dental follicle that initiates eruption.31
Biological tooth eruption critically follows a time sequence and can only occur in specific regions. Signaling pathways such as Wnt and Hh play important roles in the process, and may directly or indirectly regulate SFTE molecules.36, 37 Secondly, various mediators in the regional microenvironment (such as RANK, OPG and CSF-1, TGF-β, IGF, VEGF, and CTSK), are significant for regulating the tooth eruption and maintaining tissue homeostasis. The spatiotemporal pattern and relative abundance of various mediators during eruption are key determinants of site-specific activity of dental follicle cells, osteoclast, and osteoblast in bone surrounding the tooth.31, 38, 39 Disruption in spatiotemporal regulation and homeostasis of regional microenvironment during the tooth eruption may cause SFTE.
3.1.3 Regional impacted factors
The incidence of unerupted teeth with impacted factors or without sufficient space significantly increased when one showed impaired eruptive force, and differed in the tooth position. According to a previous study, CCD patients had a reduced spontaneous eruption of unerupted teeth after removal of impacted factors, which was lower in the mandibular canines and premolars than incisors.27
4 CONCLUSION
Our systematic review and meta-analysis provides strong evidence for selective pattern of failure of tooth eruption and causative mutated genes. We recommend SFTE to classify those genetic unerupted teeth, might better guide for precise molecular diagnosis, treatment, and prognosis. Failure of tooth eruption should be considered a clinical sign of an underlying genetic diseases and not only as an isolated disease. It is suggested doctors exploit dental exam findings as an accessible and useful tool to predict genetic diseases and their mutated candidate genes.
The five types of SFTE and their pathogenic genes discussed in this study only represent a small proportion of all the SFTE types. Over a third of PFE patients do not carry any PTH1R mutations. Other four types of genetic diseases with unerupted teeth sometimes occurred without common mutant genes as well. We believe there are more pathogenic genes involved in unerupted teeth in a selective way. And more studies are required to further explore the genotype–phenotype correlation and specific molecular mechanisms for SFTE observed in this study. Animal models such as deficient Fgfr2 mice with unerupted maxillary incisors (early erupted teeth) and clcn7- deficient zebrafish with affected 3V1 and 5V1 teeth (late erupted teeth), might be used for the further mechanistic studies.7, 40
5 METHODS AND SUBJECTS
OMIM, NCBI, and PubMed databases were used in searching for genetic disorders with unerupted teeth and genes associated with failure of tooth eruption respectively. The following key words were used in OMIM database: “unerupted tooth,” “failure of eruption,” “failure of tooth eruption,” “embedded tooth,” and “impacted tooth.” In NCBI database, the following key words were used: “failure of tooth eruption” (Homo sapiens). In PubMed database, the following key words were used: “failure of tooth eruption,” “failed eruption,” “failed erupted teeth,” “gene,” “mutation,” “mutated,” “variant” (Filters: Humans).
Systematic literature review was conducted using the PubMed on the distributions of unerupted teeth in patients with five mutated genes (Figure S1). The following key words were used in the search strategy: “Primary failure of tooth eruption,” “PTH1R,” “CCD,” “RUNX2,” “OI,” “COL1A1, COL1A2,” “Osteopetrosis,” “CLCN7,” “Amelogenesis imperfecta,” “FAM20A,” “tooth,” “teeth,” “dental,” “oral.” We then retrieved articles published before 2023 that matched the key words. Articles showing detailed dental phenotype and genetic background were included in the study.
The number of affected patients and percentages of unerupted teeth of patients with each gene mutation for the maxillary and mandibular arches were calculated to determine phenotype–genotype correlations (excluding the third molars). The occurrence of unerupted teeth was divided into three groups: “common,” more than 50%; “less common,” 30% to 50%; and “rare,” less than 30%. Kruskal Wallis test, chi square test, and modification were performed to compare data using SPSS.26. P values were calculated (*p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001).
AUTHOR CONTRIBUTIONS
Xinyue Guo contributed to conception, design, data acquisition, analysis, drafted, and critically revised the manuscript; Xiaohong Duan contributed to conception, design, funding acquisition and critically revised the manuscript. All authors gave their final approval and agree to be accountable for all aspects of the work.
ACKNOWLEDGMENTS
This work was supported by Key R&D plan of Shaanxi Province, Grant numbers: 2021ZDLSF02-13, National Natural Science Foundation of China, Grant/Award numbers: 81974145, 81771052; National Clinical Research Center for Oral Diseases, Grant numbers: Key Project LCA202013.
CONFLICT OF INTEREST STATEMENT
The authors declare no competing interests.
Open Research
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are available within its supplementary materials.




