Hereditary spastic paraplegia is a novel phenotype for germline de novo ATP1A1 mutation
Abstract
Dominant mutations in ATP1A1, encoding the alpha-1 isoform of the Na+/K+-ATPase, have been recently reported to cause an axonal to intermediate type of Charcot-Marie-Tooth disease (ie, CMT2DD) and a syndrome with hypomagnesemia, intractable seizures and severe intellectual disability. Here, we describe the first case of hereditary spastic paraplegia (HSP) caused by a novel de novo (p.L337P) variant in ATP1A1. We provide evidence for the causative role of this variant with functional and homology modeling studies. This finding expands the phenotypic spectrum of the ATP1A1-related disorders, adds a piece to the larger genetic puzzle of HSP, and increases knowledge on the molecular mechanisms underlying inherited axonopathies (ie, CMT and HSP).
1 INTRODUCTION
ATP1A1 encodes the α1 isoform of the Na+, K+-ATPase, a protein responsible for active transport of three sodium ions (out) and two potassium ions (in) across the plasma membrane, powered by the hydrolysis of ATP. This ion pump maintains the electrochemical cellular gradient, which is essential for functions such as osmoregulation, ion transport, and electrical excitability of nerve and muscle. The Na+, K+-ATPase is a heterodimer composed by α and β subunits. The catalytic α subunit binds the two ions as well as ATP. Four α isoforms are encoded by four distinct genes (ATP1A1-4). ATP1A1 is expressed in most tissues, particularly in kidneys and the nervous system.1 Germline dominant variants in ATP1A1 cause an axonal to intermediate type of Charcot-Marie-Tooth disease (ie, CMT2DD)2 and a syndrome with hypomagnesemia, intractable seizures and severe intellectual disability (ie, HOMGSMR2),3 while somatic mutations have been discovered in aldosterone-producing adenomas.4
We describe the first case of congenital-onset, complex, hereditary spastic paraplegia (HSP) caused by a de novo (p.L337P) ATP1A1 mutation identified via whole-exome sequencing (WES). Evidence for the causative role of this variant is provided in functional and homology modeling studies.
2 MATERIALS AND METHODS
2.1 Clinical description
We describe a 4.5-year-old boy, first son of non-consanguineous healthy parents of Romanian origin with no familial history of neurological disorders. He was born after an uneventful pregnancy by spontaneous delivery. The Apgar score was of 9 at 1′ and 10 and 5′. A motor developmental delay appeared in the first year: he was able to sit autonomously at the age of 12 months and started to walk at 18 months with abnormal gait and frequent falls. He came to our attention at the age of 2.5 years. Neurological examination revealed spastic gait with pyramidal signs at the lower limbs (ie, increased tonus and osteo-tendineous reflexes, ankle clonus, bilateral Babinski sign); eye contact was normal, but language was limited to 3 to 5 simple words. Irritability and attention deficit together with hyperactivity and temper tantrum were noticed. He also suffered from a sleep disorder with frequent nocturnal awakening, treated by slow-release melatonin. Head circumference was 48.5 cm (3%-10%). Brain and spine magnetic resonance imaging were normal. Motor evoked potential revealed increased central conduction time at lower limbs; electroencephalogram and nerve conduction study of upper and lower limbs was unrevealing. A diagnosis of sporadic congenital-onset (ie, first year of age), complex form of spastic paraplegia was made. Neuropsychological assessment at 3 years 10 months of age confirmed the presence of developmental delay with moderate severity (eg, developmental quotient <50 and developmental age equivalent of 29 months, by using “Griffiths Mental Development Scales, third version”; adaptive composite score 42 by using “Vineland Adaptive Behavior Scales, second version”).
2.2 Next-generation sequencing (NGS) analysis and Sanger sequencing
Genomic DNA was extracted from peripheral blood by using NucleoSpin tissue, according to the manufacturer's protocol (Macherey-Nagel, Germany). Nextera custom enrichment panel (Illumina) was first carried out covering promoters, coding regions, exon-intron boundaries, and untranslated regions of all known HSP genes as previously described.5 Successively, a WES was conducted on the proband and his parents by using xGen Exome research panel v1.0 kit (IDT) for exome regions enrichment and Illumina platform for sequencing analysis. WES data were processed using an in-house implemented pipeline as previously reported,6 and filtering strategy is summarized in Supporting Information. Sanger sequencing was used to validate and to check variant segregation in the family and to analyze all ATP1A1 (NM_000701) exons (ie, whole ATP1A1 sequencing) in a group of undiagnosed patients with sporadic pediatric-onset simplex or complex HSP. Lastly, a search for individuals who harbored variants in the same candidate disease gene was performed on GeneMatcher (http://www.genematcher.org).7 Informed consent was obtained from all parents of participating subjects. The research has been conducted in accordance with the Declaration of Helsinki.
2.3 Homology modeling
Homology modeling of the human sodium/potassium-transporting ATPase subunit alpha-1 (ATP1A1, NCBI accession NP_000692.2) in the residue interval 30 to 1023 was made with the SCWRL4 software8 employing as the template the crystal structure of the homolog from S. scrofa (PDB 3WGU), which shares 99% amino acid identity. The pairwise sequence alignment used for the modeling is available as Supporting Information. The Na+ ions and ADP co-crystallized with the pig ATP1A1 protein were kept in same binding positions in the human ATP1A1 model.
2.4 Ouabain survival assay
U2OS cells were transfected with plasmids encoding full-length human ATP1A1 (WT-ATP1A1) and an ATP1A1-ouabain–insensitive construct that harbors two mutations that confer ouabain resistance (Oua-WT).2 The patient mutation (p.L337P) was generated by site-directed mutagenesis using the Q5 site-directed mutagenesis kit (New England Biolabs) using the ATP1A1-oubain–insensitive plasmid and the following primers: 5′-CTCCAGATTTCACAAATGAAA-3′ (forward) and 5′-CGGCACATTGGCTACGAT-3′ (reverse). U2OS cells were transfected to express WT-ATP1A1, Oua-WT, and selected mutants (Oua-p.L337P and Oua-p.D811A, the latter as a control parameter) using Lipofectamine 3000 (ThermoFisher). Cells were treated the following day with 5 μM ouabain for 72 hours. Cell viability assays were quantified using the CellTiter-Glo assay (Promega). Finally, immunofluorescence of HEK293T cells transfected with either WT-Oua-ATP1A1 or L337P-Oua-ATP1A1 was performed (see Supporting Information for methods).
3 RESULTS
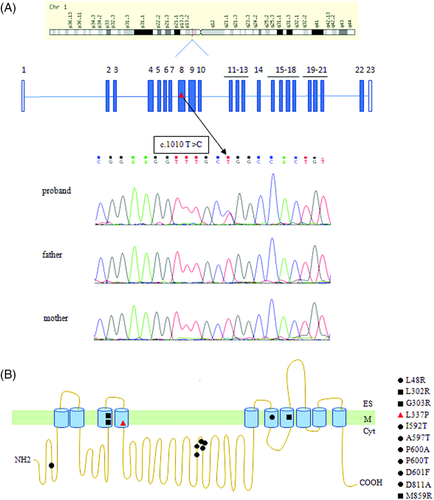
Our filtering strategy of WES led to the identification of a heterozygous missense (c.1010T>C, p.L337P) substitution in ATP1A1 as the top candidate. Sanger sequencing validated the variant, and segregation analysis in parents confirmed its de novo origin (Figure 1A). This variant is not reported in any public (gnomAD, ExAC) and in-house (GENESIS)9 databases and is predicted to be damaging by multiple bioinformatics tools.
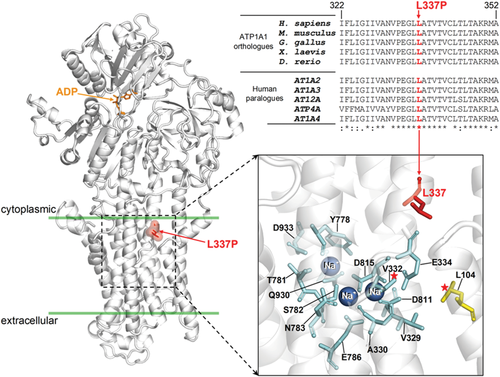
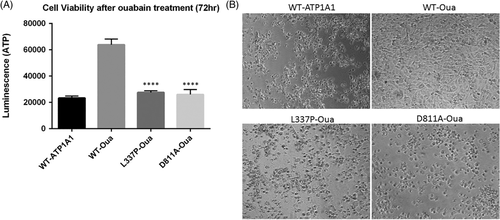
Homology modeling showed that the p.L337P variant affects a highly conserved leucine in the transmembrane region of the ATPase (Figure 2) nearby the sites of occlusion of the three Na+ ions.10 Ouabain survival assay showed that the cells transfected with L337P-Oua had a significant decrease in cell viability compared to cells transfected with WT-Oua (n = 8; t test P < .0001 compared to WT-Oua) (Figure 3A,B). This suggests a detrimental functional effect due to the mutation. Immunofluorescence of transfected HEK293T cells showed predominant localization of both WT and mutant (p.L337P) forms in the plasma membrane with some diffused cytoplasmic staining as a result of overexpression (Supplementary Figure S1). Sanger sequencing of ATP1A1 in 24 undiagnosed HSP patients discovered no further variants. No additional individuals harboring ATP1A1 variants who matched the observed phenotype of our patient were identified on GeneMatcher.
4 DISCUSSION
HSPs are clinically and genetically heterogeneous degenerative disorders of the upper motor neurons with over 90 HSP-causing genes known.11 The introduction of NGS methodologies has increased both molecular diagnosis and identification of ultra-rare or new genes, but a consistent percentage of patients remain undiagnosed.5, 12-14 We identified a novel de novo ATP1A1 variant in a child with complex HSP, significantly expanding the known phenotypic spectrum. Homology modeling of ATP1A1 allowed us to propose that the p.L337 to proline replacement alters the conformation of the proximal sodium-binding residues impairing their function. Indeed, functional studies carried out on the somatic variants p.L104R and p.V332G, both falling nearby the p.L337P variant, showed the lack of K+-stimulated pumping, and, at physiological membrane potentials, a marked ouabain-sensitive and voltage-dependent inward current that was partly inhibited by K+.15 The pathological role of the p.L337P variant is also supported by the ouabain survival assay, since transfected cells died in the presence of ouabain, even though they harbored the mutations that confer ouabain insensitivity. The association with HSP phenotype enlarges the ATP1A1 clinical spectrum, which includes CMT2DD and HOMGSMR2.
Interestingly, our patient manifested central nervous system involvement in the form of spastic paraplegia—a novel finding for all the Na+, K+-ATPases-related conditions—coupled with moderate psychomotor delay and behavior disorder. These two latter manifestations overlap that is observed in HOMGFSMR2, in which very early-onset (ie, first days or months of life) resistant seizures and hypomagnesemia are followed by global severe developmental delay and behavior disorder that may include autistic features.3 Our case has normal serum magnesium level, and, at moment, he does not suffer from epilepsy. Clinical and neurophysiological signs of peripheral neuropathy are absent as well at his present age. Taken all together, these data indicate that phenotype of our patient is distinct from HOMGFSMR2 as well as from the late-onset cases of neuropathy in CMT2DD.2
Even before ATP1A1, the pleiotropic effect of mutations in the Na+, K+-ATPase proteins had been found for ATP1A3, that in the nervous system is exclusively expressed in neurons and whose mutations cause alternating hemiplegia of childhood, rapid-onset dystonia-parkinsonism, CAPOS syndrome16 and overlapping phenotypes17, 18 and to a less extent for ATP1A2, whose mutations cause familial hemiplegic or basilar migraine.19 Moreover, recessive mutations in ATP13A2, a lysosomal P5-type transport ATPase, involved in cellular protection from heavy metal ions, α-synuclein, and mitochondrial toxicity, may cause a spectrum of pyramidal-extrapyramidal degenerative disorders ranging from Kufor-Rakeb syndrome/PARK9, neuronal ceroid lipofuscinosis and spastic paraplegia type 78.20
The term “inherited axonopathies” refers to disorders with length-dependent axonal degeneration as CMT type 2 (ie, axonal type) and HSP.21 Recently, it has been demonstrated how CMT2 and HSP disease proteins are significantly more linked than expected.11 Mutations in few HSP genes (eg, ATL-1, BSCL2, NIPA1, REEP1, KIF1A, TFG, and rarely SPAST) may be associated with peripheral axonal or mixed neuropathy in addition to involvement of corticospinal pathway; furthermore, variants in KIF5A cause CMT2, HSP or a mixed phenotype.21 This new observation increases the group of genes underlying inherited axonopathies. ATP1A1 is the main ATP1A paralog present in most of the motor and sensory axons of the ventral and dorsal roots, and it is also expressed in motor neurons of the ventral horn as well as peripheral nerve axons and Schwann cells.2 The localization of ATP1A1 allows understanding the possibility of either central or peripheral nervous system involvement, but the reason why a patient develops one or the other phenotype is currently unknown. The distribution of the mutations along the different protein domains does not allow to shed light on the mechanisms (Figure 1B), and it is likely that other genetic, epigenetic, or environmental modifiers could play a role in determining the phenotype.
5 CONCLUSIONS
We describe the first case of HSP caused by de novo heterozygous ATP1A1 variant. Screening of this gene should be implemented in patients with HSP. Since the clinical spectrum of conditions associated with ATP1A1 mutations appears to be broad, the term “ATP1A1-related disorders” is suggested.
CONFLICT OF INTEREST
The authors have no conflict of interest to disclose.
ACKNOWLEDGEMENTS
We thank the patient's family for participating in this study. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. S.Z. received support from the National Institutes of Health.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.