Cerebellar ataxia with normal intellect associated with a homozygous truncating variant in CA8
Abstract
Biallelic pathogenic variants in CA8 cause cerebellar ataxia, mental retardation and dysequilibrium syndrome 3 (CAMRQ3), a rare form of hereditary ataxia characterised by cerebellar hypoplasia/atrophy, variable intellectual disability and often quadrupedal gait. The few cases reported in the medical literature are all caused by pathogenic homozygous or compound heterozygous missense variants in CA8. We report a 9 year-old boy with marked gross motor delay, ataxia and progressive cerebellar atrophy with limited bipedal gait, but without intellectual disability. Singleton whole exome sequencing was performed. A novel homozygous truncating variant in CA8 (c.232C>T) with a predicted premature termination codon at position 78 (p.Arg78*) was identified. Both parents and the proband's healthy sister are heterozygous for the variant. This variant is likely pathogenic and the cause of the condition in this child. Functional evidence in the form of a spontaneous mouse model involving homozygous intragenic deletion of the mouse analogue of CA8 with nonsense-mediated decay and similar clinical features to the proband support pathogenicity. Identification of this truncating variant broadens the genotypic and phenotypic spectrum of CA8-related cerebellar ataxia.
1 INTRODUCTION
The hereditary ataxias, a heterogeneous group of conditions characterised by impaired balance and coordination, are collectively common and confer considerable morbidity across the lifespan.1 Increased availability of next generation sequencing (NGS) has permitted molecular characterisation of many of these conditions and improved understanding of their neuropathophysiological underpinnings. Considerable locus and clinical heterogeneity is now recognised. The existence of an autosomal recessive form of nonprogressive cerebellar ataxia with variable intellectual disability, disturbed equilibrium, cerebro-cerebellar hypoplasia, and loss of bipedal locomotion was described as early as 19402 and further analysed in a large consanguineous Hutterite family in 1981.3 Diagnostic labels of “dysequilibrium” syndrome4 and Unertan syndrome5 have been historically applied, with “cerebellar ataxia, mental retardation and dysequilibrium syndrome” (CAMRQ)6 now in use. Biallelic mutations in four genes have been implicated in CAMRQ, giving rise to four subtypes (CAMRQ1-4) caused by pathogenic variants in VLDLR,7 WDR81,6 CA8,8 and ATP8A2,9 respectively.
CAMRQ1 (MIM 224050), or VLDLR-associated cerebellar hypoplasia (VLDLR-CH), is the most well-described subtype, first described in the Hutterite population due to a founder mutation.10, 11 In addition to the core features, VLDLR-CH causes moderate-to-profound intellectual disability, dysarthria, inferior cerebellar and pontine hypoplasia and gyral simplification, frequently with quadrupedal gait.11 Confirmed mutations in WDR81 and ATP8A2 (causing CAMRQ2 and CAMRQ4) have only been described in a small number of families.6, 9, 12 There are no established genotype-phenotype correlations.
CAMRQ3 (MIM 613227) is caused by biallelic mutations in CA8, a gene that encodes carbonic anhydrase-related peptide 8 (CARP8).8 There are only three published cases, all caused by biallelic missense mutations8, 13, 14 (Table 1). We describe a 9-year-old boy with ataxia and normal intellect who has a homozygous novel truncating variant in CA8, to our knowledge the first published report of this mutational mechanism causing the condition. Our findings expand the phenotypic and genotypic spectrum of CA8-related cerebellar ataxia.
| Family | Nucleotide changea | Predicted protein change | Variant type | Clinical presentation | MRI findings | Source, evidence |
|---|---|---|---|---|---|---|
| 1 | c.298T>C | p.Ser100Pro | Missense | CAMRQ3 Mild ID Cerebellar ataxia, dysarthria Tremor Quadrupedal gait |
n/a | Türkmen et al8: four affected siblings |
| 2 | c.710G>A | p.Arg237Gln | Missense | CAMRQb Ataxia |
Cerebellar hypoplasia | Najmabadi et al14: single proband |
| 3 | c.484G>A | p.Gly162Arg | Missense | CAMRQ3 Mild ID Cerebellar ataxia, dysarthria Delayed/absent walking No quadrupedal gait |
Cerebellar volume loss (possibly progressive) Peritrigonal white matter irregularities PET: hypometabolic cerebellar hemispheres, temporal lobes, mesial cortex |
Kaya at el13: seven affected individuals, three related families |
| 4 | c.232C>T | p.Arg78* | Nonsense | CAMRQ3 Cerebellar ataxia Significant gross motor delay Generalised hypotonia No quadrupedal gait, walking limited to 10-20 steps Normal intellect |
Cerebellar atrophy (vermis, superior cerebellar hemispheres), progressive |
This report |
- Note: CAMRQ3 denotes “cerebellar ataxia, mental retardation and dysequilibrium syndrome 3”; ID, intellectual disability; n/a, not available; PET, positron emission tomography.
- a Transcript NM_004056.4.
- b Limited clinical information available.
2 MATERIALS AND METHODS
2.1 Case report
The 9-year-old male proband is the first child born to healthy nonconsanguineous Caucasian parents. Labour was induced at 35 weeks for spontaneous rupture of membranes after an otherwise uncomplicated pregnancy. He required brief support in the special care nursery for temperature regulation and feeding difficulties. Moderate generalised hypotonia was apparent in infancy, particularly involving the trunk and lower limbs, with severe head-lag that continued until age 5 years. He sat at 14 months and crawled at 18 months. First independent steps were achieved at 6 years with significant cerebellar ataxia. At 9 years, gait is wide-based, slow and limited to 10 to 20 steps, requiring assistance for longer distances. The proband has poor fine motor skills and incoordination with dysmetria. Speech was delayed with his first word at 12 months but no additional language until 24 months. At 9 years speech content and vocabulary is age-appropriate but with reduced velocity and significant dysarthria. He has poor oromotor coordination without bulbar or respiratory compromise. Visual acuity is normal although some tracking and accommodative difficulties are apparent. He attends a mainstream school and does not have an intellectual disability. At no stage was there developmental plateau or regression.
On last examination at age 9 years there were no significant dysmorphic features. His weight and height were on the fifth percentile and head circumference on the 30th percentile. Although nystagmus was not present, he had difficulty with visual tracking and smooth pursuit. He had marked cerebellar dysarthria. His gait was wide based and unsteady, and he showed truncal instability. There was normal tone in his limbs and normal strength. Reflexes in the upper limbs were normal but reflexes in the lower limbs were increased with subtle crossing and an extensor right plantar response. The Romberg test was normal. He had an intention tremor and difficulties with rapid alternating movements.
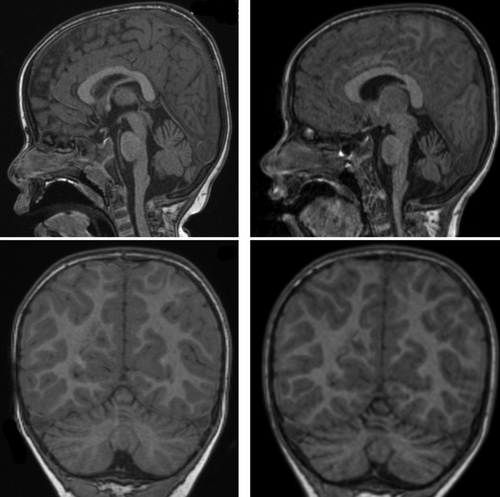
Magnetic resonance imaging (MRI) of the brain was normal at 2 years, but repeat MRI at age 7 years showed cerebellar vermian and hemispheric cerebellar atrophy superiorly with thinning of the folia (Figure 1). Normal investigations included chromosomal microarray, urine and plasma metabolic screens, transferrin isoforms, lysosomal enzymes, mitochondrial and POLG gene panel, mitochondrial deletion/duplication panel and testing for the FXN intron 1 GAA expansion that underlies most Friedreich ataxia.
2.2 Whole exome sequencing
Informed consent was obtained for genomic testing and case report publication, in accordance with institutional requirements. Singleton whole exome sequencing with deletion/duplication analysis was undertaken on the proband. DNA was extracted from peripheral blood using QIAamp DNA blood mini kits (Qiagen, Düsseldorf, Germany) and sample quality confirmed using the Agilent Tape Station Genomic DNA ScreenTape (Agilent Technologies, Santa Clara, California). Sequencing was performed at PerkinElmer Genomics Laboratory (Branford, Connecticut) by massively-parallel sequencing using a targeted sequence capture method (Agilent v6CREv2) followed by NGS of the amplified captured regions (Illumina, San Diego, California). Alignment to reference genome GRCh37 was performed. The DRAGEN (Dynamic Read Analysis for GENomics) Bio-IT Platform (Illumina) generated annotated variant calls within the target region and copy number variation (CNV) analysis was performed using the DRAGEN CNV platform (Illumina). Variants were classified according to the American College of Medical Genetics and Genomics (ACMG) variant classification criteria.15
3 RESULTS
A novel homozygous nonsense variant in exon 2 of CA8 (NM_004056.4[CA8]: c.232C>T) was identified, with predicted substitution of arginine for a premature termination codon (PTC) at position of 78 of the protein (p.Arg78*). This variant has not previously been reported in clinical cases and is absent in population databases (gnomAD, 1000 Genomes). Both healthy parents were heterozygous for the variant by polymerase chain reaction (PCR) amplification and Sanger sequencing, as was the proband's healthy younger sister. There were no additional variants of interest considered compatible with the phenotype of the proband.
4 DISCUSSION
CAMRQ3 is a rare hereditary ataxia reported in only three published families to date. Core features of (usually superior) cerebellar atrophy, ataxia and dysarthria appear to be fully penetrant, while intellectual disability, tremor and loss of bipedal gait are variably reported. This is similar to VLDLR-associated cerebellar hypoplasia. Possible progression of the phenotype has been reported in one individual with CAMRQ3 (Table 1). The proband reported here has not experienced clinical regression but the differing MRI findings between age 2 and 7 may suggest some progression of the phenotype. The small number of individuals reported precludes meaningful conclusions around genotype-phenotype correlation. It would appear, however, that intellectual disability and quadrupedal gait are not necessary in making the diagnosis, and a diagnostic label of “CA8-related cerebellar ataxia” is proposed in place of CAMRQ3 in this child.
There has been some debate regarding the biological mechanisms driving the quadrupedal gait tendency which has historically defined the condition. Quadrupedal locomotion may represent an adaptive compensation for impaired balance and coordination rather than a devolution of bipedality16, 17 although it is generally not seen in other early-onset ataxias. The proband has achieved limited bipedal gait with intensive support and physical therapy, possibly representing a modified phenotype and extending the phenotypic spectrum of the condition.
Given the paucity of truncating CA8 variants in the medical literature and disease databases, additional functional evidence was considered in assigning pathogenicity to the p.(Arg78*) variant. This variant is located in exon 2, predicting nonsense-mediated decay (NMD). Should the transcript escape NMD, there would be loss of the majority of the carbonic anhydrase domain, including a pfam-predicted active site at position 87. There is a spontaneous mouse model in which a biallelic 19 bp deletion in the murine homologue of CA8 results in ataxia and truncal dystonia, providing persuasive evidence for a loss of function mechanism.18 Homozygous wdl mutant mice exhibit reduced CA8 mRNA transcript and protein levels compared to wildtype, indicating NMD and supporting a loss of function mechanism.18
The ataxic phenotype in wdl mice is mediated through disordered granule cell proliferation and circuit patterning of cerebellar Purkinje cells, providing insight into the pathogenesis of the condition.19 Development of paraneoplastic cerebellar degeneration due to anti-CARP8 antibodies targeting Purkinje cells in an individual with malignant melanoma provides additional in vivo evidence that loss of function of this protein results in cerebellar dysfunction.20
We conclude that the p.(Arg78*) variant is the cause of this child's condition. A likely pathogenic classification was applied, based on ACMG criteria,15 in consideration of the variant's absence in population databases (PM2), compelling functional evidence for a loss of function mechanism (PS3) and close concordance of clinical features with those reported in in an animal model. In this family, molecular characterisation has permitted diagnostic confidence and informed reproductive planning. Identification of this truncating variant broadens the mutational spectrum of this rare condition and highlights the value of whole exome sequencing in diagnosis of rare forms of hereditary ataxia.
ACKNOWLEDGEMENTS
The authors wish to acknowledge and value the contributions of clinical and laboratory personnel involved in the care and diagnosis of this patient. We would also like to thank the family of the proband for their time and participation.
CONFLICT OF INTEREST
The authors declare no competing financial interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy restrictions.