Infectious issues of therapeutic monoclonal antibodies in multiple sclerosis and neuromyelitis optica spectrum disorders
Abstract
Various effective monoclonal antibodies (mAbs) have been approved for both multiple sclerosis (MS) and anti-aquaporin-4-seropositive neuromyelitis optica spectrum disorders worldwide, including in Japan. As these newer mAbs have distinct modes of action that effectively suppress the recurrence of inflammation and slow disability progression, they can modulate and interfere with the protective immune response against pathogens, resulting in various infectious complications. Among various mAbs, natalizumab (NTZ) has the highest risk of causing progressive multifocal leukoencephalopathy (PML), a rare but fatal opportunistic brain infection caused by John Cunningham polyomavirus. Switching from NTZ to B-cell-depleting mAbs, such as ocrelizumab, is also a possible risk factor for PML development. Alemtuzumab carries the risk of reactivation of varicella-zoster virus (VZV); therefore, prophylactic acyclovir treatment is required. NTZ has also been associated with VZV reactivation. Eculizumab can cause severe meningococcal infection due to Neisseria meningitis, and vaccination prior to treatment induction is required. Attention to the reactivation of hepatitis B or Mycobacterium tuberculosis is also needed during mAb therapy. Additionally, in the era of severe acute respiratory syndrome coronavirus 2 infection (COVID-19), the risk for of developing severe COVID-19 may be associated with some mAbs, such as B-cell-depleting agents. Thorough understanding and mitigation strategies for infectious risks are essential.
1 INTRODUCTION
Multiple sclerosis (MS) and neuromyelitis optica spectrum disorders (NMOSD) are autoimmune-mediated inflammatory and degenerative diseases that affect the central nervous system (CNS).1, 2 MS begins with a relapsing–remitting course termed relapsing remitting MS (RRMS), followed by insidious worsening disability without clinically apparent relapses, known as secondary progressive MS.3
NMOSD are characterized by recurrent optic neuritis, acute myelitis, and brain syndromes. They are divided into anti-aquaporin-4(AQP-4)-seropositive NMOSD (seropositive NMOSD) and anti-AQP4-seronegative NMOSD (seronegative NMOSD).4
In the last 15 y, new disease-modifying drugs (DMDs) have become available for MS, which has prompted a change in treatment algorithms.5, 6 Additionally, since 2019 three monoclonal antibodies (mAbs) have been approved for seropositive NMOSD worldwide, including in Japan. mAbs used to treat MS and seropositive NMOSD and their action mechanisms are shown in Table 1. Some drugs such as ocrelizumab (OCR) and alemtuzumab (ATZ) have not been approved in Japan. Rituximab (RTX) is widely used for MS and NMOSD in Western countries, and in 2022 it has been approved for NMOSD, including in Japan.
| Drug | Indications | Modes of action | Infectious complications | Recommended evaluations before treatment induction |
|---|---|---|---|---|
| MS | ||||
Natalizumab (NTZ) |
RRMS | Binds to the α4 integrin subunit of α4β1 integrin, And blocks leukocytes migration via the BBB into the CNS Induces T-cell lymphocyte apoptosis |
PML Herpes virus reactivation |
Serum JCV titers and brain MRI Exclusion of active and latent infections (including HIV, HBV, HCV) Tuberculosis test Herpes viral infections (the history of infections and anti-VZV IgG titer)a |
| Alemtuzumab (ATZ) | RRMS |
Directs against CD52, expressed at high levels on T cells and B cells, and depletes them | PML (not reported in MS patients) Listeria-associated infections Herpes virus including CMV reactivation Hepatitis B reactivation Mycobacterium tuberculosis infection Others: upper respiratory infections |
Exclusion of active and latent infections (including HIV, HBV, HCV) Herpes viral infectionsa →if negative, vaccination might be recommended Tuberculosis test Chest radiography |
Ocrelizumab (OCR) |
RRMS PPMS |
Depletes circulating immature and mature B cells, but spares CD20-negative plasma cells Antibody-dependent cellular cytotoxicity |
PML (almost carry-over; previously treated with NTZ or fingolimod) Herpes virus reactivation Hepatitis B reactivation COVID-19? |
Exclusion of active and latent infections (including HIV, HBV, HCV) Herpes viral infectionsa Brain MRI |
Ofatumumab (OFAB) |
RRMS SPMS |
Depletes circulating CD-20-positive B cells Complement-dependent cellular cytotoxicity |
COVID-19? | Exclusion of active and latent infections (including HIV, HBV, HCV) Blood examinations (CBC, blood biochemistry) |
| Seropositive NMOSD | ||||
Eculizumab (ECZ) |
Relapse prevention |
Inhibits the terminal complement protein C5 and prevents the cleavage into C5a and C5b |
Meningococcal infection (Neisseria Meningitis) Other encapsulated bacteria |
Exclusion of active and latent infections (including HIV, HBV, HCV) Blood examinations (CBC, blood biochemistry) |
Satralizumab (SAT) |
Relapse prevention |
Binds to membrane-bound and soluble IL-6 receptors, and blocks the IL-6 signaling pathways |
Bacterial pneumonia Mycobacterium tuberculosis infection Atypical mycobacteriosis Hepatitis B reactivation |
Exclusion of active and latent infections (including HIV, HBV, HCV) Blood examinations (CBC, blood biochemistry) Tuberculosis test Herpes virus infectionsa Chest radiography |
Inebilizumab (INEB) |
Relapse prevention | Binds and depletes CD19-positive B cells, including plasma blasts | Pneumonia | Exclusion of active and latent infections (including HIV, HBV, HCV) Blood examinations (CBC, blood biochemistry |
Rituximab (RTX) |
NMOSD (approved) MS (not approved) |
Binds and depletes CD20-positive B cells |
PML (very rare in MS and NMOSD) COVID-19? Hepatitis B (rare in rheumatoid diseases) Hepatitis C reactivation |
Exclusion of active and latent infections (including HIV, HBV, HCV) Blood examinations (CBC, blood biochemistry) Brain MRI |
- MS, multiple sclerosis; NMOSD, neuromyelitis optica spectrum disorders; RRMS, relapse-remitting multiple sclerosis; PPMS, primary progressive multiple sclerosis; SPMS, secondary progressive multiple sclerosis; PML, progressive multifocal leukoencephalopathy; CMV, cytomegalovirus; COVID-19, severe acute respiratory syndrome coronavirus 2 infection; JCV, John Cunningham virus; HIV, human immunodeficiency virus; HBV, hepatitis B virus; HCV, hepatitis C virus; VZV, varicella zoster virus; CNS, central nervous system; BBB, blood–brain barrier.
- a Taking history of previous infections and evaluation of anti-VZV IgG titers.
Compared with the first-generation DMDs for MS (interferon beta and glatiramer acetate) and conventional immunosuppressive drugs for NMOSD, these newer mAbs are more effective in preventing the recurrence of inflammation and reducing disability progression. These mAbs have distinct immunological action mechanisms that can modulate and interfere with the protective immune response against various pathogens, resulting in various infectious complications (Table 1). Some complications, such as progressive multifocal leukoencephalopathy (PML) and reactivation of the herpes virus or hepatis viruses, can be life-threatening. Additionally, since the beginning of 2020 we have been experiencing a pandemic of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (COVID-19). The risk of developing COVID-19 may increase under immunosuppressive treatments. Recently, several groups of MS experts have published clinically relevant, evidence-based recommendations of general screening for viral, bacterial, and fungal infections, and vaccination before starting or switching DMDs, including mAbs, as well as mitigation and surveillance strategies for infectious complications during DMDs treatment. These recommendations could also be applied to NMOSD treatments.7-10
Here, I review particularly important infectious complications associated with treating MS and NMOSD with antimonoclonal agents.
2 VIRAL INFECTIONS
2.1 Progressive multifocal leukoencephalopathy
PML is a rare but fatal opportunistic brain infection caused by the John Cunningham polyomavirus (JCV). The development of PML is associated with cell-mediated immune system impairment.11 For the last two decades, PML has been known as an opportunistic infection of pharmacological immunosuppressive conditions in patients treated with mAbs for MS and other autoimmune diseases.
Natalizumab (NTZ), a humanized anti-α4β1-integrin mAb,12 was the first mAb approved for RRMS in 2004. NTZ effectively reduced the clinical relapse rate and disability progression.9, 13 Among the approved mAbs for MS and NMOSD, the incidence of PML is the highest with NTZ.14, 15 In 2005, the first two NTZ-associated PML (NTZ-PML) cases were reported in patients with MS taking NTZ in combination with interferon-β,16, 17 which prompted a transient market withdrawal. As of February 2022, there were 884 confirmed PML cases among 251,119 patients exposed to NTZ (Biogen, Cambridge, MA, USA, data on file).18
Patients with NTZ-PML are not systemically immunosuppressed, and inadequate immune surveillance is the major explanation for the mechanism.19 PML risk increased with previous immunosuppressant exposure (more than 2 y before NTZ treatment) and the presence of serum anti-JCV antibodies.20 Among patients who were anti-JCV antibody-negative at baseline in the AFIRM12 and STRATIFY-1 trials,21 97% remained negative or below an index threshold of 1.5 over 18 mo.22
The clinical stage of NTZ-PML and characteristic magnetic resonance imaging (MRI) findings are presented in Table 2. In NTZ-PML, favorable prognostic outcomes are expected when PML is diagnosed in the early asymptomatic period.23 Generally, the survival rate of NTZ-PML is better than that of most other PML.24 In addition, Dong-Si et al23 reported that mortality in symptomatic patients was 24.6%, whereas that in asymptomatic patients was 3.3%. Brain MRI is a vital tool for PML diagnosis.19 Moreover, NTZ-PML can be diagnosed using routine MRI scans before any neurological symptoms develop.25-28 Nonenhancing punctate patterns on T2-weighted/FLAIR images of the cerebral subcortical U-fiber region are observed in presymptomatic or asymptomatic NTZ-PML.29, 30 However, a confirmed diagnosis may be difficult when MRI changes suggestive of PML are observed, but CSF JCV-PCR results are negative during the same period.19 In 2016, the European Medical Agency confirmed initial recommendations for the early diagnosis of PML aimed at minimizing CNS injury and avoiding severe disability.31 They also stratified the risk of PML by the index value of the serum anti-JCV antibodies. Major et al advocated a protocol for surveillance in patients with NTZ-treated MS, and emphasized the need for more frequent MRI scans in patients with a higher anti-JCV antibody index and longer duration of treatment.19, 32 The protocol for the surveillance of NTZ-PML is summarized in Table 3. Currently, there are no effective drugs for the treatment of NTZ-PML. Mefloquine and mirtazapine are used to treat PML under clinical settings in Japan, although their effectiveness has not been proven. In a recent study, the efficacy of filgrastim, a granulocyte-colony stimulating factor, for treating NTZ-PML has been reported; 17 patients survived 2 y after the onset of PML.33
| Stage | Symptoms | JCV-DNA detection in CSF | MRI findings |
|---|---|---|---|
| Presymptomatic PML | No obvious symptoms |
Small and solitary subcortical lesions on DWI and FLAIR images (FLIAR image is more sensitive than T2 image) (DWI image could be useful for distinguishing between PML lesions and MS relapse) Punctate pattern of cerebral subcortical region, brainstem and cerebellum May be associated with Gd-enhancement |
|
Symptomatic PML |
Cognitive impairments Aphasia Visual disturbance Hemiparesis Ataxia, etc. (All subacute) |
May be low levels in NTZ-PML (25- >1,000,000 copies/mL) |
Enlarging subcortical lesions on FLAIR and T2 images T1 image presents low signal intensity Gd-enhancement is considered rare Observed no mass effect |
PML with IRIS |
Worsening of speech and disability Fever, Headache Convulsion, etc. |
Vary but may be declining levels (Not detection to >20,000 copies/mL) |
Enlargement of the lesions and mass effects Contrast enhancement is often recognized |
Post-PML |
Residual disability Progressive cognitive impairments, etc. |
Often undetectable But may be still detectable |
No contrast enhancement and no mass effects Brain atrophy progression |
- PML, progressive multifocal leukoencephalopathy; NTZ, natalizumab; NTZ-PML, natalizumab-associated progressive multifocal leukoencephalopathy; PML JCV, John Cunningham polyomavirus; DWI, diffusion-weighted image; FLAIR, fluid-attenuated inversion recovery; MS, multiple sclerosis; Gd, gadolinium.
| PML risk (/1000)a | Recommended frequency for anti-JCV antibody titer monitoring | Recommended frequency for MRI | Remarks (about MRI sequence) | |
|---|---|---|---|---|
| Anti-JCV negative | Every 6 mo | Annually | Conventional sequencesb | |
| Anti-JCV positive, no prior immunosuppressant | ||||
| ・Anti-JCV titer index <0.9 | ||||
| Treatment duration 1–72 mo | 0.6–1.0 | Every 6 mo | Annually | Conventional sequencesb |
| ・Anti-JCV antibody titer 0.9–1.5 | ||||
| Treatment duration 1–36 | 0.8–1.0 | Every 6 mo | Annually | Conventional sequencesb |
| Treatment duration 37–72 mo | 2–3 | Further test is not required | Every 3–4 mo | DWI image is recommended |
| ・Anti-JCV antibody index >1.5 | ||||
| Treatment duration 1–24 mo | 0.2–0.9 | Further test is not required | Annually | Conventional sequencesb |
| Treatment duration 25–72 mo | 3–10 | Further test is not required | Every 3–4 mo | DWI image is recommended |
| Anti-JCV antibody positive, prior immunosuppressant | ||||
| Treatment duration 1–24 mo | 0.3–0.4 | Further test is not required§ | Annually | Conventional sequencesb |
| Treatment duration 25–72 mo | 4–8 | Further test is not required§ | Every 3–4 mo | DWI image is recommended |
- The patients with anti-JCV index >0.9 should receive MRI scans annually in the first 24 or 36 mo after induction, then every 3–4 mo afterwards. Frequency of anti-JCV antibodies and MRI evaluations should be increased as appropriate.
- §For patients with prior immunosuppressant treatment, no significant difference was observed in median index between non-PML and PML patients26.
- PML, progressive multifocal leukoencephalopathy; NTZ, natalizumab; MS, multiple sclerosis; JCV, John Cunningham polyomavirus; DWI, diffusion-weighted image.
- a Risk estimates per 1000 NTZ-PML, from a report of the EMA26.
- b Fluid-attenuated inversion recovery and T2-weighted images.
The correlation between CSF JCV viral load and outcome has also been described; a higher JCV-DNA copy number in CSF at diagnosis resulted in worse outcomes.34, 35 Generally, the CSF JCV-DNA load at PML diagnosis tends to be low in NTZ-PML patients.24
In almost all cases of NTZ-PML, immune reconstitution inflammatory syndrome (IRIS) occurs to some degree upon discontinuation of NTZ, within days to weeks after plasma exchange (PLEX), which is often used to accelerate the clearance of active drugs.36 On MRI examination, contrast-enhancing lesions are the most common earliest signs of IRIS following NTZ-PML.37 In a histological study, abundant perivascular and parenchymal CD8-positive T-cell infiltration was observed in patients with IRIS following NTZ-PML.38 The incidence of PML-IRIS among patients treated with NTZ was higher than in other patients with PML.39 PLEX and immunoabsorption have been used to rapidly remove NTZ, which results in restored lymphocyte trafficking into the CNS. Paradoxically, the effective removal of NTZ and restoration of cellular immunity can worsen the neurological deficit. Tan et al40 reported that early-onset PML-IRIS resulted in worse survival and neurological outcomes in 28 patients with NTZ-PML. From an examination of 372 NTZ-PML cases, Dong-Si et al23 reported that more than 80% of asymptomatic patients with PML were treated with PLEX, and 66.7% of them developed IRIS. Recently, it has been suggested that there is no evidence for the beneficial effects of PLEX in NTZ-PML.41 Intravenous pulse corticosteroid therapy can lead to favorable functional outcomes.40 However, early corticosteroid use had no effect on the subsequent progression of IRIS in early-PML-IRIS.40 The efficacy of maraviroc, a C-C chemokine receptor type 5 blocker, on PML or PML-IRIS associated with immunosuppressive agents including NTZ, has not yet been proven.42
Today, emerging evidence suggests that extended interval dosing (EID) NTZ (infusion every 6 wk) might decrease the risk of PML among patients who were positive for JCV antibodies and had received prior immunosuppressants compared to standard interval dosing (SID) (every 4 wk).43 NTZ effectiveness was maintained when switched to EID from SID after more than 1-y treatment.44 However, it should be noted that the risk of PML cannot be completely eliminated with EID, and vigilance is required even under this regimen.45
Compared to NTZ, the risk of PML was lower with other mAbs.14 Nevertheless, anti-CD-20 mAbs that selectively deplete B-cells, such as RTX and OCR, may be associated with PML development. B-cell depletion reduces antibody production, modulates antigen presentation, and diminishes the proinflammatory reaction of the T-cell,46 which may increase the risk of PML. However, the relationship between RTX and PML remains unclear. Although the onset of PML has been described in RTX-treated patients with rheumatoid arthritis, ANCA-associated vasculitis, and systemic lupus erythematosus (SLE), the incidence was only 1/30,000.47 RTX-induced PML in patients with MS is very rare. Although the study sample was small, no patients developed PML on a clinical trial of RTX for NMOSD in Japan (RIN-1 study).48 To date, 10 cases of confirmed PML after treatment with OCR for MS have been reported.49-51 Nine out of 10 cases had carry-over PML: eight patients had previously been treated with NTZ, and one with fingolimod. In all eight cases, the duration of NTZ treatment prior to OCR was 22–120 mo. The interval between the last NTZ and the first OCR infusion was 43–98 d, and the onset of PML was within 6 mo of the last NTZ infusion. All patients had tested positive for serum JCV antibodies and had clinical worsening symptoms or MRI findings compatible with PML before OCR initiation. Notably, one patient reported by Patel et al50 developed PML under OCR monotherapy. The PML of all 10 of the above-referenced cases was nonfatal. The switch from NTZ to B-cell depleting therapy should be performed with extreme caution after adequate clinical and MRI evaluation. Regarding ofatumumab (OFAB), the onset of PML has not been reported during administration for MS and NMOSD. Regarding inebilizumab (INEB), there has been one death due to CNS lesions in the open-label extension of the N-MOmentum study, in which the possibility of PML could not be ruled out.52
ATZ is an mAb against CD52 that depletes B and T cells.53 To date, no cases of PML have been reported after ATZ administration for MS,14 although they have been reported in patients with lymphocytic leukemia or post-organ transplantation underlying conditions known to predispose to PML. Berger et al classified ATZ based on PML risk as Class III.14
As mentioned previously, the anti-JCV antibody index is commonly used to evaluate the risk of developing PML. However, since OCR/RTX and ATZ deplete B-lymphocytes from peripheral circulation, monitoring the anti-JCV index might not be reliable for predicting the risk of PML.54 In fact, the anti-JCV antibody index was reduced after ATZ and RTX/OCR treatment.55
2.1 Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection
The first case of SARS-CoV-2 infection (COVID-19) was reported in Wuhan, China, in December 2019.56 After its rapid spread within China, outbreaks have occurred in almost all countries worldwide. The global mean mortality of COVID-19 is estimated to be 3%, but mortality increases with age, obesity, and comorbidities, such as diabetes, chronic heart disease, severe asthma, and immunosuppressive conditions. Current evidence shows that simply having MS does not increase the risk of developing COVID-19.57 In the first worldwide meta-analysis of 101,462 MS patients from 1029 articles, the rate of hospitalization and intensive care unit (ICU) admission was low among patients with MS; 4 out of 570 suspected/confirmed COVID-19 patients died, which was lower than the rate in the healthy population.58 Moreno-Torres et al59 reported that among 5641 Spanish patients with MS from February to May 2020, 219 patients (3.9%) developed COVID-19, and this incidence was not greater than that in the general Spanish population. They also reported no association between the risk of onset or severity of COVID-19 and the use of specific DMDs.59 Similarly, COVID-19 clinical outcomes were not associated with exposure to different DMDs in a population-based MD cohort in Austria.60
However, there have been reports that treatment with B-cell mAbs, especially RTX, may increase the severity of the COVID-19 clinical course.61-65 From their analysis, Safavi et al65 reported that being on B-cell mAbs was associated with a 2.6-fold increase in the risk of developing COVID-19 compared to treatment with NTZ and fingolimod. In a large cohort of patients with MS and COVID-19 from 28 countries, Simpson-Yap et al63 reported that RTX was associated with a higher risk of hospitalization, ICU admission, and the need for artificial ventilation, while OCR was associated with hospitalization and ICU admission. Smith et al66 examined 1439 patients with MS on DMDs, of whom 230 had COVID-19. They reported that, whereas no mAbs (NTZ, OCR, and RTX) were found to be significantly associated with an increased risk for the onset of infection, RTX was associated with increased severity.66 Salter et al64 also reported that RTX, but not OCR, was associated with hospitalization. As of September 25, 2021, the Novartis Safety Database recorded 90 confirmed COVID-19 cases receiving OFAB. Most cases (80 patients) were nonserious. From an analysis of the ALITHIOS (open-label, ongoing extension phase 3b trial of OFAB) study, 245 out of 1703 patients (14.3%) receiving OFAB developed COVID-19.67 Most cases were of mild (44.1%) or moderate (46.5%) severity, and 9% had a severe/life-threatening course (9.8%). These data did not suggest any evidence of an increased risk of severe or fatal infection compared to hospitalization and fatality rates, as reported in the literature for the general and MS populations.68
Recently, the immune response to SARS-CoV-2 in patients with MS treated with anti-CD20 mAb has been reported.69, 70 Thornton et al71 reported two OCR-treated patients with MS and mild COVID-19 who were negative for IgG against SARS-CoV-2 virus even at 9–12 wk postinfection. Conte et al69 analyzed 24 patients with MS infected with COVID-19; only four out of 15 patients using OCR developed antibodies against SAR2-CoV-2, while eight out of nine on other DMDs developed antibodies. Similar case reports and cohort studies demonstrating the attenuation of antibody responses following COVID-19 infection during OCR treatment have been published.72, 73 Florez-Gonzalez et al74 reported an OFAB-treated woman with MS with asymptomatic COVID-19 who was fully depleted of CD-19-positive B cells in the blood but mounted a successful humoral response to the virus through IgG and IgM antibodies. Adamic et al75 analyzed four OFAB-administrated MS cases with mild COVID-19. Three out of four patients showed fully depleted CD-19-positive B cells. The total serum IgG and IgM levels were normal in all four patients. However, three out of four patients were negative for serum SARS-CoV-2 IgG at the onset of COVID-19, and two patients were still negative 6 mo after the onset, indicating a diminished humoral response to the virus. In contrast, T-cell immunity against SARS-CoV-2 was observed in three out of four patients in the interferon-γEPISpot assay. Recovery from COVID-19 infection without the development of a humoral immune response to the virus suggests that T-cell-mediated responses may adequately contribute to protection against SARS-CoV-2.76
Interestingly, one reported by Florez-Gonzalez et al,74 who showed the presence of CD19-positive B cells and SARS-CoV-2 IgG after COVID-19 onset, was administered OFAB 24 d prior to COVID-19, but had a gap of 28 wk of OFAB application beforehand due to low IgM levels. The link between the time to last infusion or the number of doses of B-cell-depleting mAbs and COVID-19 risk is a problem that needed to be investigated. Reducing the frequency of dosing or adjusting it according to the monitoring of B-cell repopulation kinetics may allow maintenance of efficacy while limiting the risk of infection.77 However, the safety and efficacy of EID of B-cell-depleting mAbs have not been established yet. If people with MS take DMDs, especially B-cell-depleting mAbs and test positive for COVID-19, they should immediately contact their healthcare provider to discuss potential treatment options.57
The use of ATZ, which depletes both B cells and T cells, did not seem to cause a severe COVID-19 course.63, 78 However, the number of patients with MS treated with ATZ who developed COVID-19 is too low to draw meaningful conclusions. NTZ does not increase the risk of developing severe COVID-19 and stopping NTZ treatment or delaying its induction is not recommended.79
Emerging research has been published demonstrating attenuation of the vaccine response following COVID-19 vaccination in various patients with MS/NMOSD treated with DMDs, especially CD-20 depleting agents. As of March 2022, mainly mRNA (Moderna, Pfizer-BionNTech, New York, NY, USA)80, 81 and adenovirus-based (Johnson & Johnson, New Brunswick, NJ, USA)82 COVID-19 vaccines have been used worldwide. Some reports have demonstrated an attenuated immune response against SARS-COV-2 in patients treated with OCR following exposure to both vaccines. Bigaut et al83 reported that in vaccinated patients with MS, the median IgG index was lower in patients treated with CD-20-depleting agents and S1PR modulators than in patients receiving other DMDs, with exposure to both Pfizer and Moderna vaccines. Achiron et al84 demonstrated attenuated IgG production only in 22.7% of all Pfizer-vaccinated patients with MS treated with OCR. Guerrieri et al85 also found that the serological response was recognized in only 37.5% of all vaccinated patients with MS under OCR treatment. The response was greater (62.5%) in patients treated with fingolimod.
The Multiple Sclerosis International Federation (MSIF) published an expert consensus recommendation for vaccines.57 The MSIF has prompted global COVID-19 advice for people with MS, including a description of the timing of COVID-19 vaccination in relation to DMDs (Table 4).
| Drugs | For the patients about to start treatment | For the patients already taking treatment |
|---|---|---|
| Natalizumab | Do not delay starting for COVID-19 vaccine injection |
No adjustments to the drug are needed |
| Alemtuzumab | Consider getting fully vaccinated at least 4 wk before starting |
Consider getting vaccinated at least 24 wk after the last ATZ dose When possible, resume ATZ at least 4 wk after getting full vaccinateda |
Ocrelizumab Rituximab |
Consider getting fully vaccinated two to 4 wk before starting |
Consider getting vaccinated at least 12 wk after the last OCR/RTX dose When possible, resume OCR/RTX at least 4 wk after getting fully vaccinateda |
| Ofatumumab | Consider getting fully vaccinated at least 2 wk before starting |
There is no data to currently guide timing of the vaccine in relation to the last OFAB dose Consider getting vaccinated 4 wk after your last dose of OFAB When possible, resume OFAB injections 4 wk after getting fully vaccinateda |
- DMTs, disease-modifying therapies; MS, multiple sclerosis; COVUD-19, severe acute respiratory syndrome coronavirus 2 infection; ATZ, alemtuzumab; OCR, ocrelizumab; RTX, rituximab; OFAB, ofatumumab.
- a Vaccinated the single dose of the J&J or the second dose of other types of vaccines.
The relationship between mAbs used for seropositive NMOSD and the risk of COVID-19 is unclear. Interestingly, a survey of 192 neurologists who saw patients with NMO from the USA and Canada found that 52% of these patients had been treated with RTX and 11% with eculizumab (ECZ) before the COVID-19 pandemic. Among them, 74% of neurologists differed in the dose for RTX, and 90% of neurologists changed the dosing interval for RTX.86 Although INEB has not been reported to increase the risk of COVID-19,87 the risk of COVID-19 could be inferred from its similar mechanism of action to other B-cell-depleting agents, such as RTX and OCR.
Tocilizumab (TOC) (not approved for NMOSD) and satralizumab (SAT) bind to membrane-bound and soluble IL-6 receptors and inhibit the IL-6 signaling pathway that involved inflammation.88, 89 To date, the relationship between SAT and COVID-19 remains unknown. In COVID-19, IL-6 is an important proinflammatory cytokine and is a biomarker for the COVID-19 severity.90 IL-6R mAbs have been used for COVID-19 treatment in the early acute phase, and its efficacy for mitigating the severity and mortality has been reported.91 However, impairment of IL-6 signaling may result in normalization of C-reactive protein levels and body temperature, even in the presence of systemic infections, and may delay diagnosis. The effect of the long-term use of an IL-6R mAbs on the risk of COVID-19 is an important problem to be resolved.
Regarding ECZ, an anti-C5 mAb approved for seropositive NMOSD, there has been one case report of a patient with NMOSD who developed COVID-19 during a year-long treatment with ECZ.85 The patient showed a mild clinical course without suspension of ECZ during infection.92 It has been suggested that C5a activation plays a role in the inflammatory mechanism of acute respiratory distress syndrome (ARDS) development in the early and late phases of COVID-19,93 and blockade of terminal complement C5 may prevent inflammation after infection. However, the effect of chronic ECZ use on the risk of COVID-19 remains unknown.
In 2020, Hamdy et al94 described the management strategies for patients with NMOSD during the COVID-19 pandemic era. Treatment decisions regarding NMOSD during the COVID-19 pandemic era should be individualized according to the risk of disease relapse, age, and individual comorbidities.
2.2 Hepatitis B and C infections
Chronic and resolved hepatitis B virus (HBV) is associated with potentially fatal viral reactivation during the use of immunosuppressants, particularly, anti-CD20 mAbs. When the drugs are administered, lymphocyte function is suppressed, and many effector pathways, including the production of viral inhibitory cytokines, are inhibited.95 This permits increased viral replication and viral protein expression on the hepatocyte surface. After the drugs are discontinued, immune system reconstitution occurs, and cytotoxic T cells recognize viral peptide-expressing hepatocytes.96 HBV infection is diagnosed by the presence of hepatitis B surface antigen (HBsAg), and in most infected patients, detectable serum HBV-DNA can be as low as 10 IU/mL or as high as several billion IU/mL.96 The antibody to hepatitis core antigen (anti-HBc Ab) is an accurate and reliable marker of current and previous infections.
HBV reactivation has been reported in patients with cancer treated with RTX-containing immunosuppressants.97 In contrast, in rheumatological patients, although the risk of HBV reactivation under RTX has been less studied, it is probably lower.98, 99 Data on OCR and the risk of HBV reactivation are limited. In phase III trials of HBsAg-negative/anti-HBc Ab-positive patients with MS with undetectable HBV DNA, no cases of HBV infection were reported.100 A single case report of HBV reactivation has been described in patients with MS who were HBsAg-negative/anti-HBc Ab-positive and undergoing OCR treatment.101 To date, there have been no cases of HBV infection during OFAB in MS. But the Food and Drug Administration (FDA) has warned of the risk of HBV infection during OFAB treatment.102
Regarding ATZ, no cases of HBV reactivation during treatment for MS have been reported, while hematological patients have a high risk of reactivation.103 At present, NTZ is not considered to be associated with a high risk of HBV reactivation, although a fatal case of reactivation from the carrier state has been reported.104
The relationship between anti-IL6R agents and the risk of HBV reactivation is yet to be elucidated. However, reactivation of resolved HBV may occur in patients with treated with TOC.105, 106 The Japan Society of Hepatology has listed both SAT and TOC as drugs that could cause HBV reactivation.107
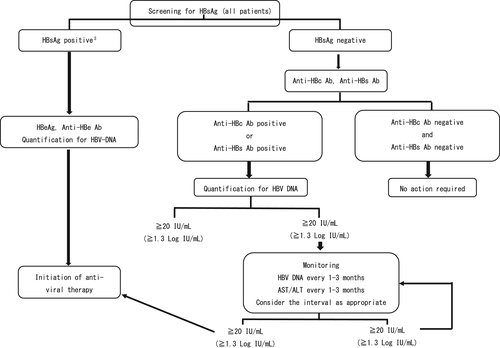
Screening for HBsAg and anti-HBc Ab is mandatory before starting mAbs. The Japan Society of Hepatology has published guidelines for strategies to combat HBV infection following treatment with immunosuppressive agents and chemotherapy (Figure 1).98 In principle, when HBsAg is positive, patients and doctors should consult specialized physicians.
HCV reactivation is uncommon. However, Grebely et al108 reported that HCV-RNA recurrence was observed in 19% patients after adequate treatment for hepatitis. RTX is the most commonly used agent to precede reactivation. The authors reported a female patient with RRMS who developed HCV reactivation during treatment with fingolimod.109 She had been previously treated for hepatitis C and remained an undetectable HCV-RNA state for more than 4 y. The clinical manifestations of HCV reactivation may vary from asymptomatic to markedly increase aminotransferase levels, and severe hepatic failure may occur.110, 111 If patients have a previous history of HCV treatment, HCV-RNA should be regularly evaluated after starting immunosuppressive treatments.
2.3 Herpes viruses
Herpes simplex (HSV) 1 and 2, varicella-zoster virus (VZV), and cytomegalovirus (CMV) are the most common herpes viruses that require treatment. Disseminated VZV infections can be life-threatening in immunocompromised individuals. Among the mAbs used for MS and NMOSD, ATZ is associated with an increased incidence of severe VZV infection. In a randomized controlled phase III trial, the incidence of HSV and herpes zoster infections was more than 4-fold in patients with ATZ compared with that in patients with interferonβ-treated patients.112, 113 Consequently, the FDA product label recommended prophylaxis with acyclovir from the start of ATZ treatment until CD4-positive lymphocytes are recovered to at least 200 cells/μL, with a minimum duration of prophylaxis of 2 mo, even if CD4-positive lymphocytes resolve earlier. While CMV frequently occurs in patients with ATZ with hematologic diseases, it rarely occurs in patients with MS.114
Although herpetic infections during NTZ treatment are rare, ~14 cases have been reported of VZV-associated CNS infections or acute retinal necrosis (ARN) during NTZ treatment.115-122 Among the 14 cases of ARN, nine resulted in visual impairment or blindness. Herpes simplex encephalitis has also been reported.116 Herpetic infection might occur in patients both with and without prior immunosuppressive drug use, irrespective of the length of NTZ treatment.116 Temporary suspension or discontinuation of NTZ should be considered when patients develop a severe herpetic infection.
The association between anti-CD20 mAbs and herpetic infections remains unclear. In clinical trials, OCR was associated with increased herpetic infections compared to no treatment (4.7% vs. 3.3%)123 and IFNβ (5.9% vs. 3.4%).124 Herpetic infection during RTX therapy has been reportedly lower than that during NTZ and fingolimod therapy.125
Before starting mAb treatment, VZV-IgG and HSV-IgG should be evaluated in all patients. Vaccination is recommended in some cases. Patients who are VZV-IgG seronegative and do not have a history of prior varicella infection or vaccination should receive a varicella live-attenuated vaccine (BIKEN in Japan) before starting mAbs. Although the Shingrix (not a live recombinant vaccine) is not indicated to prevent primary varicella infection, it is more effective than the live vaccine in preventing VZV reactivation.126 In Japan, the Shingrix vaccination of patients with MS before and during mAbs is not generally used. However, it may be recommended for patients who receive low-dose immunosuppressive therapy in addition to mAbs.
3 BACTERIAL INFECTION
3.1 Mycobacterium tuberculosis
Mycobacterium tuberculosis (TB) infection in adults usually occurs due to the reactivation of the latent foci of bacteria. TB remains among the top 10 causes of death worldwide. In Japan, both mortality and morbidity are gradually decreasing, but morbidity trends in younger age groups are increasing slightly. Strategies for latent TB infection (LTBI) are becoming important in the era of widespread biological agents. A medical history, physical examination, and chest radiography are needed to diagnose LTBI. In addition, an interferon-γrelease assay (IGRA) should be performed. QuantiFERON-TB Gold (QFT) and T-SPOT.TB (T-SPOT) are the main assays used. The T-SPOT assay, performed with sorted lymphocytes from the blood, can more accurately distinguish between positive and negative results than QFT.127
ATZ can prompt TB infection because it selectively targets cell surface CD52 to deplete circulating T and B cells, leading to a distinctive pattern of cellular repopulation. TB reactivation occurred in only two of more than 900 cases in an ATZ randomized large-scale phase III trials for MS.112, 113 However, ATZ is associated with high rates of TB infection in patients with hematological malignancies.128, 129
Anti-B-cell agents, including OCR and RTX, are not considered to increase the risk of TB infections.123, 124, 130 Even in TB-endemic countries, anti-B-cell-depleting agents may be safely chosen.131
NTZ may have an influence on the immune control over TB, because it blocks α4-integrin and prevents lymphocyte migration to organs. Nevertheless, TB infection was not observed in randomized control trials,12, 13 and TB reactivation among latent infections did not occur in the postmarketing experience.132 However, notably, Dahdaleh et al133 reported two female Irish and Turkish patients who developed apparent TB infection during NTZ treatment.
Anti-IL6R mAbs might be associated with TB infection, as the blockade of IL-6 signaling may suppress protective immunity against mycobacteria.134 In some studies106, 135 screening before TOC initiation and prophylactic treatment for LTBI under the use of TOC has been emphasized. Naturally, in the package inserts of TOC and SAT, careful medical history, evaluation of chest radiographs, and IGRA or tuberculin reaction tests are strongly recommended.
3.2 Other bacterial infections
ECZ inhibits the terminal complement protein C5, prevents its cleavage into C5a and C5b, and increases the risk of encapsulated bacterial infection, particularly meningococcal infection caused by Neisseria meningitis. Meningococcal disease develops rapidly and causes meningoencephalitis, bacteremia, and pneumonia. In fulminant cases, disseminated intravascular coagulation and adrenal insufficiency occur, and mortality is 10%–15%, even with appropriate antibiotic therapy. In Japan, meningococcal vaccination with Menactra (MenACWY), which is effective against serotypes A, C, W, and Y, is highly recommended before the induction of ECZ. The Advising Committee on Immunization Practice recommends serogroup B meningococcal vaccine (MenB)136 in addition to MenACWY, because serogroup B meningococcal infection is more common in the USA than in Japan. MenB is not produced or approved in Japan. In ECZ-treated NMOSD, meningococcal infection has not been reported in either large clinical trials137, 138 or postmarketing studies. However, from 2008 to 2016, 16 cases of meningococcal infection were reported after MenACWY (88%) and MenB (75%) in the USA.139 Even after vaccination prior to ECZ induction, strict observation is indispensable to avoid overlooking infection during treatment. If patients treated with ECZ present with a high fever, they must immediately consult medical doctors. Prompt examination, including blood culture test, and immediate initiation of antibiotic therapy are essential.
ATZ is associated with listeriosis caused by Listeria monocytogenes. The estimated prevalence of listeriosis after ATZ treatment is ~0.26%. Mazzitelli et al140 reported one case and reviewed eight previous case reports; in some cases, the symptoms developed within a few days after ATZ infusion.140 Therefore, maintaining dietary precautions for listeria from 2 wk before the start to at least 1 mo after ATZ therapy is recommended.
Chronic use of anti-IL-6R mAbs can result in bacterial infections, such as bacterial pneumonia.106 Evaluation of chest radiography is recommended when patients develop minor symptoms, even if white blood cells or C-reactive protein levels are not elevated in blood test results.
4 CONCLUSION
Newer mAbs for MS and NMOSD are quite effective in attenuating relapse rates and preventing disability progression, and many patients worldwide benefit from these drugs. However, each drug has a characteristic mode of action that can modulate and interfere with patient's protective immune responses. As mentioned above, each mAb may increase the risk of different infections. As NTZ increases the risk of PML, regular monitoring of MRI and serum anti-JCV titers is essential. COVID-19 has been pandemic for about three-and-a-half years. Its infectious risks in relation to differential mAbs have been actively discussed worldwide, and strategies for mitigating the infectious risk under mAb therapy are currently being published.
Although I mentioned particularly important infectious complications, each mAb can cause several infections by other pathogens. Before starting mAbs, a profound understanding of the risks and benefits of each drug, taking into consideration the personal condition of each patient, is needed.
DISCLOSURE OF ETHICAL STATEMENTS
Approval of the Research Protocol: N/A.
Informed Consent: N/A.
Registry and the Registration No. of the study/trial: N/A.
Animal Studies: N/A.
CONFLICT OF INTEREST
None declared.




