The life cycle of a T cell after vaccination – where does immune ageing strike?
Summary
Vaccination is the optimal intervention to prevent the increased morbidity and mortality from infection in older individuals and to maintain immune health during ageing. To optimize benefits from vaccination, strategies have to be developed that overcome the defects in an adaptive immune response that occur with immune ageing. Most current approaches are concentrated on activating the innate immune system by adjuvants to improve the induction of a T cell response. This review will focus upon T cell-intrinsic mechanisms that control how a T cell is activated, expands rapidly to differentiate into short-lived effector cells and into memory precursor cells, with short-lived effector T cells then mainly undergoing apoptosis and memory precursor cells surviving as long-lived memory T cells. Insights into each step of this longitudinal course of a T cell response that takes place over a period of several weeks is beginning to allow identifying interventions that can improve this process of T cell memory generation and specifically target defects that occur with ageing.
OTHER ARTICLES PUBLISHED IN THIS REVIEW SERIES
Immunosenescence: the importance of considering age in health and disease. Clinical and Experimental Immunology 2017, 187: 1–3.
The convergence of senescence and nutrient sensing during lymphocyte ageing. Clinical and Experimental Immunology 2017, 187: 4–5.
Immune senescence: significance of the stromal microenvironment. Clinical and Experimental Immunology 2017, 187: 6–15.
Innate immune responses in the ageing lung. Clinical and Experimental Immunology 2017, 187: 16–25.
Age-related alterations in immune responses to West Nile virus infection. Clinical and Experimental Immunology 2017, 187: 26–34.
Intracellular signalling pathways: targets to reverse immunosenescence. Clinical and Experimental Immunology 2017, 187: 35–43.
Ageing and inflammation in patients with HIV infection. Clinical and Experimental Immunology 2017, 187: 44–52.
Considerations for successful cancer immunotherapy in aged hosts. Clinical and Experimental Immunology 2017, 187: 53–63.
Ageing and obesity similarly impair antibody responses. Clinical and Experimental Immunology 2017, 187: 64–70.
Herpes zoster and the search for an effective vaccine. Clinical and Experimental Immunology 2017, 187: 82–92.
Adult vaccination against tetanus and diphtheria: the European perspective. Clinical and Experimental Immunology 2017, 187: 93–99.
Introduction
Vaccination is one of the most successful and safe interventions in modern medicine and has allowed the nearly complete eradication of some devastating viruses, such as the poliomyelitis or smallpox viruses. Many diseases are now preventable, and childhood diseases such as measles, mumps or diphtheria are no longer a threat in vaccinated populations. Vaccination should therefore be the perfect intervention to reduce the increased morbidity and mortality from infections in an older population. Unfortunately, the results in this high at-risk population have been suboptimal 1. A prime example is the annual influenza vaccination 2-4. Although compliance in the western world has increased during the last two decades, the burden of mortality and morbidity linked to influenza infection in the older population remains high. A recent Cochrane Review has estimated the effectiveness of influenza vaccines, in the order of 70–90% in an immunocompetent population, to be as low as 30% 5, 6. Principally similar declining efficacies with age have been reported for vaccination with the attenuated varicella zoster virus (VZV). Protection from VZV reactivation manifesting as herpes zoster declines from 70% for 50–59-year-old adults to 38% for adults aged 70 years and older 7-10.
Approaches to improve vaccination outcome in an older population – empirical, based on system analysis or based on mechanistic models?
There is general consensus that we need to improve in producing better vaccines and developing better vaccination strategies 11. So far, strategies to overcome these defects associated with immune ageing have been empirical, with some, albeit limited, success. One strategy is to increase antigen doses to overcome presumptive low responsiveness of the T cell population. Higher vaccine doses are already employed for the VZV vaccine Zostavax 10. Trivalent and quadrivalent influenza vaccines are formulated for the total amount of haemagglutinin as the viral component presumably inducing immune protection 12, 13. Contrary to earlier findings that the current dose of haemagglutinin is ceilinged, a recent study showed the benefit of higher doses in an older population 14. Use of adjuvant is the most commonly proposed strategy to accomplish better vaccine responses 15, 16. While the use of alum as an adjuvant did not provide additional benefit for influenza vaccinations, oil-in-water emulsions such as MF59 and AS03 appear to reduce the frequency of confirmed infections compared to the non-adjuvanted influenza vaccine, although the difference at least for AS03 did not reach significance 17, 18. More impressively, VZV vaccination with an AS01B adjuvanted glycoprotein yielded 97% efficacy in adults without major decline with age 19.
How can we overcome the limitations of an empirical approach? One recent innovation is to use systems biology to interrogate the peripheral blood in humans after vaccination and identify signatures and pathways that are correlated with the immune response. This approach has been coined ‘systems vaccinology’, and is beginning to be applied to identify features that are characteristic for the immune response in older individuals 20, 21. In influenza vaccination trials of young, elderly and diabetic individuals, signatures of innate immunity and plasmablasts have been identified that predicted influenza antibody titres at 1 month after vaccination and were consistent over five seasons 22. The advantage of this approach is that it is unbiased and is directly applicable to human populations, circumventing the need for mouse models that may differ substantially from humans in the immune ageing process. Whether and how age-specific signatures, if identified, can be translated into specific interventions to improve the vaccine response in the older population is currently without precedence.
The alternative is the more conventional, hypothesis-driven approach. Such an approach can now build upon the vast amount of knowledge that has been collected in mouse models on the generation of immune memory and the beginning of molecular understanding of what immune ageing entails in humans. Importantly, we need to take into consideration that memory cell generation is a process of several weeks that begins with, but is not only determined by, the initial antigenic stimulation and can fail for many reasons along the way.
Most studies on human vaccine responses have identified antigen-specific antibodies as a surrogate marker of vaccination-induced protection. Considerable progress has been made in mouse models in understanding the generation and function of follicular T helper cells that are crucial for B cell differentiation and plasma cell generation 23; these studies provide an important foundation for human studies in an older population. Equally important is the progress in understanding the generation of short-lived T effector and long-lived T memory cells. Assessment of cellular immunity is ignored frequently in human vaccine studies, as the necessary logistics and the experimental standardization to determine the frequency and function of long-lived memory T cells are difficult to accomplish. However, in at least some viral diseases, T cells are the ultimate agent of protection. Reactivation of VZV is related clearly to decreasing frequencies of VZV-specific CD4 T cells and not to the titre of VZV-specific antibodies 24-26. Even in the case of influenza vaccination, McElhaney and other investigators have stressed the importance of virus-specific T cells for reduced morbidity from infection in a setting where complete protection by antibodies cannot be accomplished 27-30. Indeed, influenza split vaccines that include matrix proteins and nucleoproteins inducing T cell memory may be more potent in an older population than the subunit vaccine 31. Of note, the water-in-oil adjuvanted vaccines only improve antibody generation, but do not induce CD8 T cell memory 15, 32. Improving the T cell response after vaccination is therefore not limited to the generation of the T follicular helper cells, but also needs to enhance T cell immunity by increasing the frequencies of effector memory and long-lived memory cells.
Generation of T cell memory – insights from murine infectious disease models
T cell responses encompass a clearly defined sequence of events (Fig. 1). T cells encounter antigens, are activated and divide rapidly more than 10 times to undergo massive expansion while they differentiate into effector cells that are mainly short-lived. Depending on the vaccine or infection, antigen-specific T cell frequencies peak between days 7 and 14 to then contract, with only a small percentage of cells surviving as long-lived memory cells 33. In quantitative terms – ignoring possible differences in memory cell functional polarization – the T cell responses are therefore determined by the frequencies of antigen-specific cells in the pre-existing repertoire, the breadth of how many of these antigen-specific T cells are activated and start to divide, the number of divisions within the constrained time-frame and the frequencies of cells that survive the post-peak contraction phase.
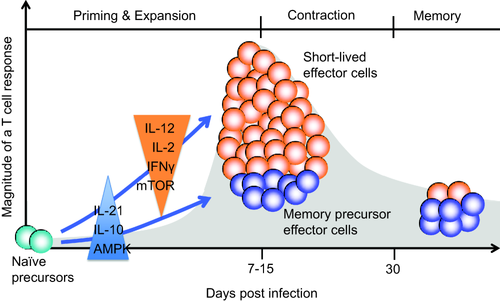
Kinetics of a T cell response. Upon activation, antigen-specific T cells divide rapidly and expand into short-lived effector cells and memory precursor cells. Cytokines present at the early stage bias the differentiation towards effector or memory cells. After peak responses, effector T cells mostly die while memory precursor cells develop into long-lived memory cells under the influence of adenosine monophosphate kinase (AMPK) activation and mammalian target of rapamycin complex (mTORC) inhibition.
The question of how complete recruitment of antigen-specific cells into the memory cell compartment is and how far the naive pre-existing repertoire influences the richness and diversity of the memory repertoire has been addressed in a number of important studies. Schumacher et al. have shown that nearly all naive CD8 T cells with the appropriate specificity are recruited and that therefore the size of the initial repertoire and the cell division rate but not the completeness of activation are limiting the peak response 34. Jenkins and other investigators have shown that the frequencies of antigenic epitope-specific T cells in the naive repertoire vary and determine their frequencies in an effector or memory cell population 35, 36. This observation appears to hold for humans, as shown by the high correlation of epitope-specific T cells in the naive and memory cell compartments for the anthrax antigen 37. Also, decreasing frequencies of naive antigen-specific CD8 T cells with age translated into a reduced number of antigen-specific effector CD8 T cells in an in-vitro system 38. Shrinkage of the naive T cell compartment in size and diversity with age will therefore have a negative impact on the generation of memory cells; i.e. it is important to determine whether and how T cell homeostatic mechanisms maintain repertoire complexity. Unfortunately, murine studies are here of limited value, because the contributions of thymic T cell generation and peripheral proliferation to T cell homeostasis differ substantially between mice and men 39. As will be discussed below, the human TCR repertoire remains very diverse in older healthy individuals, although it loses in richness and, perhaps more importantly, displays shifts in clonal size distributions with increasing clonality and increasing autoreactivity 40-42.
While the naive antigenic peptide-specific T cell repertoire can be relatively large, and all peptide-specific T cells enter expansion, clonal selection occurs throughout the primary response and during the subsequent secondary response 43. After antigen stimulation, clonal selection results in the dominance of few clonotypes in the effector pool for each peptide 44, 45. As a consequence, the effector T cell repertoire is usually narrower and of higher affinity than the naive T cell repertoire. In addition to or perhaps even more than clonal expansion, TCR repertoire selection occurs at the level of expanded T cells transitioning into long-lived memory cells 46-48. There is also evidence that recall responses underlie similar selective forces with the primary selection being reproducible during the secondary response 49, 50.
Studies into memory cell development have been facilitated by the definition of phenotypical markers in the mouse distinguishing short-lived effector and memory precursor CD8 T cells at the time of the peak response, while the absence of such markers have hampered similar studies for CD4 T cells 51-53. Memory precursor CD8 T cells can be identified based on the increased expression of the interleukin (IL)-7 receptor alpha chain, while short-lived effector CD8 T cells express the natural killer cell receptor KLRG1 during anti-viral immune responses. Clearly, it is of great relevance for the understanding of vaccine responses to identify the conditions that favour the generation of memory precursor cells that survive and differentiate into long-lived memory cells. Indeed, early exposure to the inflammatory environment influences the composition of the effector cell populations and their memory potential (Fig. 1). In general, inflammatory cytokines and in particular IL-12 favour the generation of short-lived effector T cells that express T-bet highly and are dependent upon IL-15 for short-term survival 51. Also, stimulation with IL-2 or interferon (IFN)-γ or high expression of the IL-2 receptor CD25 promotes effector cell generation at the expense of memory precursor CD8 T cells 54-56. Conversely, IL-10 and IL-21 improve the generation of memory precursor cells 57. How these observations translate into humans, where phenotypical markers are lacking and how they influence the rational choice of adjuvants, remains to be examined.
An important decision point in lineage commitment has been related to activation of the mammalian target of rapamycin complex (mTORC) pathway. Activation of mTORC1 is important for the adaptation of the metabolic pathways that support the initial T cell expansion through rapid cell division and effector cell differentiation. However, a switch to adenosine monophosphate kinase (AMPK) activation or pharmacological inhibition of mTORC1 is conducive of memory cell generation 58. More recent studies have also assigned a role to mTORC2 through nuclear destabilization of forkhead box protein O1 (FOXO1) in curtailing memory development 59. Clearly, more human studies are needed. However, the emerging understanding of the signalling pathways that control the generation of memory precursor cells and long-lived memory T cells identifies targets for pharmacological interventions beyond the use of adjuvants, in particular if these pathways are dysregulated in immune ageing.
Age-associated defects in T cell responses – the influence of T cell repertoire diversity
Thymic involution is one of the most prominent features of ageing 60, 61. During the last decades it has become increasingly clear that throughout adult life, T cell homeostasis is maintained mainly by peripheral proliferation of naive and memory T cells and not by the de-novo production of new T cells 62, 63. One of the central questions of immune ageing is therefore whether this process of self-renewal is efficient to maintain compartment size and sufficiently unbiased to maintain diversity of the T cell population, both needed to generate an immune response. Clearly, the naive T cell compartments shrink in older age – much more, however, for CD8 than for CD4 T cells, the latter being affected only moderately 64, 65. A recent vaccination study with the yellow fever virus representing a primary response showed lower peak frequencies of CD8, but not of CD4 virus-specific T cells in older individuals at the time of the peak response, which appeared to be explained at least in part by the higher loss in naive CD8 T cells with age 66.
While TCR repertoire diversity in peripheral blood has been described to decline with age, this decline is explained at least in part by a shift in the ratio between naive and memory T cells 67. When T cells subsets are analysed separately, a three- to fivefold reduction is found for naive T cells in healthy older individuals 40. In spite of this contraction, the richness of the repertoire, i.e. the number of total different receptors available remains extremely high, suggesting that compromised immunity is not caused by a hole in the repertoire 68. More strikingly than richness, older individuals have an increasing variance in clonal sizes even in naive compartments. Using in-silico modelling, we had predicted increased outgrowth of larger clones as a consequence of uneven homeostatic proliferation and peripheral selection 69. Such unevenness may prevent smaller antigen-specific clones to be recruited into the memory population, contrary to the murine studies discussed above, where most of the antigen-specific T cells were recruited in an immune response. Prolonged or repeated antigen exposure may therefore be necessary to generate a broad antigen-specific memory repertoire.
The global TCR repertoire in CD8 memory cells is much smaller than that of CD4 memory cells; however, both of them do not change with age 40. When studied at the level of CD4 T cells specific for the VZV, the number of different T cell receptors in the total virus-specific repertoires differed by a factor of up to 10 between individuals 70. This extraordinary variability did not appear to be correlated with age, and therefore does not appear to be the reason for the age-associated increase in reactivation of the latent virus. Vaccination with the live attenuated zoster virus resulted in the expansion of only 20–80% of all virus-specific CD4 T cells. Unexpectedly, pre-existing larger clones were relatively unresponsive, while smaller virus-specific T cell clones, including even previously naive CD4 T cells, were stimulated and expanded 70. Vaccination therefore resulted in a desirable broadening of the repertoire; however, the overall gain in frequencies with a single immunization was limited because large clones did not respond.
Age-associated defects in T cell activation and expansion
As shown for VZV, vaccination can recruit naive CD4 T cells into the memory compartment even in a presumptive recall response, emphasizing that even in the case of chronic infection the naive repertoire is not depleted completely of antigen-specific T cells and, moreover, that these naive T cells are responsive. Indeed, age-related alterations in the responsiveness of human naive CD4 T cells are very subtle, and are therefore only apparent with suboptimal anti-CD3/CD28 stimulation or more physiological stimulation systems 39. Again, CD8 naive T cells appear to be more prone to develop age-associated defects and exhibit impaired activation, proliferation and differentiation in response to dendritic cells and a model antigen 38.
Frequently, the age-associated deficiencies involve physiological mechanisms that are functional in T cell receptor threshold calibration. The classical example is the expression of miR-181a, which is one of the most abundant microRNA species expressed in the thymus, in particular, in double-negative thymocytes 71. The high level of miR-181a inhibits the expression of several phosphatases including DUSP6 and lowers the threshold of the T cell receptor to respond to stimulation, which is necessary for the positive selection on self-antigen. Expression levels drop sharply with differentiation into double-positive thymocytes and single-positive T cells to restrict responses to high-affinity stimulation with an exogenous antigen. Indeed, over-expression of miR-181a by transfecting the appropriate miRNA precursor lowers the TCR activation threshold and restores the ability of a mature T cell to respond to autoantigens. The decline in miR-181a expression with differentiation continues throughout life. Memory T cells have lower expression than naive T cells, and both naive and memory T cells lose expression with age, due presumably to partial differentiation with homeostatic proliferation 72. The resulting increased expression of DUSP6 attenuates T cell receptor-induced extracellular-regulated kinase (ERK) phosphorylation and lowers the responsiveness to low-affinity peptides. Thus, the loss of miR-181a in T cells, initially necessary to avoid autoimmunity, is eventually counterproductive and interferes with normal responsiveness to exogenous antigen in an older population. Increasing miR181a expression or inhibiting miR181a-regulated molecules could be explored as interventions to improve vaccine responses.
Memory T cells show only a minor decline in miR-181a expression with age; moreover, they already have lower miR-181a expression levels and they are less dependent upon ERK signalling compared to naive T cells, suggesting that DUSP6-mediated defects in proximal T cell receptor signalling are less important 72. However, as discussed above for VZV vaccination, activation of memory cells is incomplete, and in particular large expanded clones fail to appropriately expand 70.
Failure to be activated and to expand has been attributed to the expression of negative regulatory receptors (Fig. 2). There is a multitude of such receptors, including programmed death 1 (PD1), T cell immunoglobulin and mucin-domain containing-3 (Tim-3), various killer inhibitory receptors (KIRs) and immunoglobulin (Ig)-like transcript 2 (ILT-2)/leucocyte Ig-like receptor 1 (CD85j), that are expressed increasingly on T cells dependent upon their clonal sizes 73-75. It is a matter of ongoing debate whether the expression of any of these receptors with age indicates an exhaustive state or cellular senescence or terminal differentiation of effector cells that are no longer required to proliferate 76, 77. PD1, and also in the mouse Tim-3, both markers of cellular exhaustion, have been described to be expressed increasingly with age 76, 78. KIRs and CD85j are found frequently on T cells that also express CD57, a marker that has been associated with senescence or end-differentiation rather than exhaustion 65, 79. Remarkably, some of these negative regulatory receptors interfere preferentially with proliferation and to a much lesser extent with effector functions such as cytotoxic activity or cytokine production (unpublished observation) 80. PD1 expression has been described on VZV-specific T cells; however, whether the expression of any of the negative regulatory receptor accounts for the differential response of VZV-specific T cell clones to vaccination is undetermined 81. Whether checkpoint inhibition will gain a role outside cancer therapy, possibly given for a short time after vaccination, or whether the risk for autoimmunity is too high, remains to be determined.
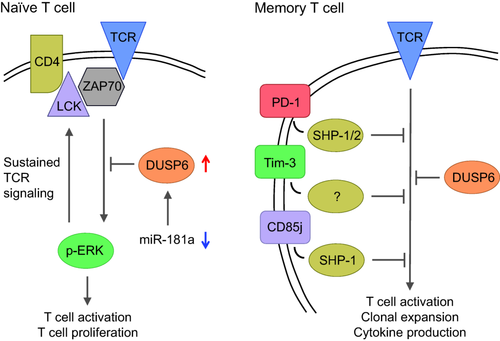
Age-associated defects in T cell activation. A loss of miR-181a expression with age results into increased expression of dual specificity phosphatase 6 (DUSP6) in naive T cells (left). DUSP6 attenuates extracellular signal-regulated kinase (ERK) activation and thereby interferes with a positive feedback loop that is needed for sustained T cell receptor (TCR) signalling and cell activation. Memory cells with age, in addition to the decline in miR-181a, gain the expression of a multitude of negative regulatory receptors that attenuate activation-induced phosphorylation events.
During the expansion phase, T cells are under replicative stress and telomere maintenance and regulation of cell cycle progression is therefore essential. Telomeric attrition with age in naive as well as memory cells is a characteristic finding, due possibly to cumulative homeostatic proliferation 82, 83. Increasing expression of the cell cycle inhibitor p16, a hallmark of cellular senescence, has also been described in lymphocytes with age 84. In the yellow fever vaccination study described above, an inverse correlation of the frequencies of T cell receptor excision circles (TREC) – an indicator of cumulative homeostatic proliferation – with the vaccine response was observed 66. However, the TREC data were not corrected for age, and it therefore remains unclear whether or not replicative history influences proliferative potential. In summary, naive CD4 T cells from older individuals appear to have a surprisingly unabated proliferative capacity, in particular after correcting for the lack of miR-181a expression, while naive CD8 T cells are more compromised.
In contrast to naive cells, expression of negative regulatory receptors affects TCR-induced proliferation in memory or effector cells preferentially; additional mechanisms in telomeric elongation appear to constrain clonal expansion. Inhibition of p38 activity in terminally differentiated effector cells has been shown to up-regulate telomerase expression, and to restore proliferative responses 85-87. p38 inhibition may therefore be an option to improve expansion. It will be interesting to see in in-vivo studies whether age affects primarily the expansion phase between the induction of an immune response and the time-point when frequencies of antigen-specific T cells are at their peak.
Age-associated defects in memory T cell survival – the role of purinergic signalling
Most vaccine studies concentrate upon the initial stages of T cell activation and determine the vaccination outcome 4 weeks and later in the form of titres of antigen-specific antibodies, while they treat the interim period essentially as a black box. More recently, the finding of short-lived plasmablasts in the peripheral blood at approximately day 7 permitted a more detailed examination of the B cell response 88, 89. With the distinction between short-lived effector cells and memory precursor cells in murine studies, and the finding that mTORC1 inhibition can improve generation of T memory cells, the later stages of the T cell effector response have gained increasing attention. Clearly, this stage is of importance for at least two reasons; T cells need to function as follicular helper cells to initiate and support B cell differentiation; and T cells need to survive to differentiate into long-lived memory cells. Age has been shown to affect follicular helper cell activity. Wherry and colleagues found frequencies and function of circulating follicular helper cells to be lower in older individuals after influenza vaccination and, in contrast to younger adults, not correlated with the vaccination-induced increase in antibody titres 90.
We have started to profile CD4 T cells several days after activation to identify age-related differences that may influence their function or their ability to survive (Fig. 3). In our initial studies, we found increased transcription of DUSP4 with age 2–4 days after superantigen-mediated activation in vitro as well as 8 days after influenza vaccination in vivo 91. Dual specificity phosphatase 4 (DUSP4) is a nuclear phosphatase that dephosphorylates nuclear Janus kinase (JNK) and ERK. Over-expression of DUSP4 has been associated recently with cellular senescence 92, 93. In our studies, the increased expression of DUSP4 was related to the inability of activated CD4 T cells to function as helper cells for B cells. Reducing DUSP4 expression by gene silencing in CD4 T cells from older individuals restored their ability to express CD40L and ICOS and to help B cells to differentiate into plasmablasts 91.
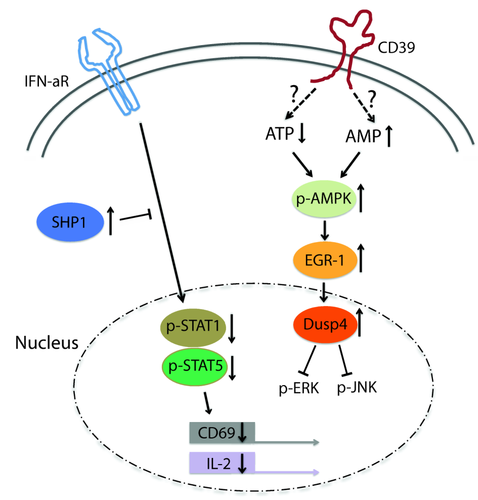
Age-associated defects interfering with function and survival of activated T cells. T cells that have differentiated into effector/memory precursor cells gain responsiveness to type I interferon (IFN) that is required for the sustained expression of interleukin (IL)-2 and CD69 that retains activated T cells in lymph nodes. With age, this pathway loses efficacy due to increased recruitment of Src homology region 2 domain-containing phosphatase-1 (SHP1). Also with increasing age, activated T cells preferentially differentiate into CD39-expressing short-lived effector cells. CD39 expression is associated with a decrease in cytoplasmic adenosine triphosphatase (ATP) and increased adenosine monophosphate kinase (AMPK) activation and the expression of nuclear dual specificity phosphatase (DUSP4) that terminates Jun kinase (JNK) and extracellular signal-regulated kinase (ERK) signalling and interferes with T helper function and proliferation.
We have also identified several pathways that appear to be involved in the decision whether to differentiate into a short-lived effector cell or a memory precursor cell. Upon activation, naive T cells gain in sensitivity to type I IFN stimulation, due in part to the increased expression of signal transducer and activator of transcription (STAT)-1 molecules. Type I IFN stimulation of activated T cells maintains the ability to produce IL-2 and sustains CD69 expression important for T cell retention in the lymph node and survival 94. Naive CD4 T cells from older individuals have a lesser up-regulation of type I IFN responsiveness, due mainly to increased recruitment of Src homology region 2 domain-containing phosphatase-1 (SHP-1) to the IFN receptor signalling complex, and therefore lose the ability to produce IL-2 and express CD69 95.
A second pathway is related to purinergic signalling. We have identified ectonucleoside triphosphate diphosphohydrolase (ENTPD) 1, a cell membrane adenosine triphosphatase (ATPase) also referred to as CD39 as a cell surface marker for short-lived effector T cells 96. Upon activation, CD39 expression is induced, peaking at 4–5 days after activation in central memory and effector memory cells in vitro and at a later time-point in stimulated naive cells. The subset of CD39+ cells after activation is increased in older individuals, suggesting preferential production of short-lived effector T cells. CD39+ cells are highly susceptible to undergo apoptosis. Interestingly, the ATPase activity of CD39 is involved directly in effector cell differentiation and apoptosis, suggesting a role for purinergic signalling. As shown in Fig. 4, ATP is released through the Pannexin channel; extracellular ATP induces calcium fluxes by stimulating P2X channels to maintain calcium signalling in activated T cells. CD39 cleaves ATP to adenosine biphosphate (ADP) and adenosine monophosphate (AMP), which can be further cleaved to adenosine by nucleotidases such as CD73. ADP as well as adenosine stimulates various G-protein coupled receptors (P2Y and A2AR, respectively). Adenosine can also be taken up through the nucleoside transporter equilibrative nucleoside transporter 1 (ENT1). The CD39-mediated modified purinergic signalling activates the pAMPK–p53 pathway as well as stimulates the A2AR receptor by adenosine, both of which lead to cell death. Increased expression of CD39 with age will lead to an increased cell loss and reduced generation of long-lived memory cells in a vaccine response. Prevention of CD39 expression is therefore predicted to be associated with improved immune responses to vaccination. Indeed, this is the case; older individuals carrying a SNP in the CD39 promoter that prevents transcription have improved generation of antigen-specific T cells after VZV as well as influenza vaccination 96.
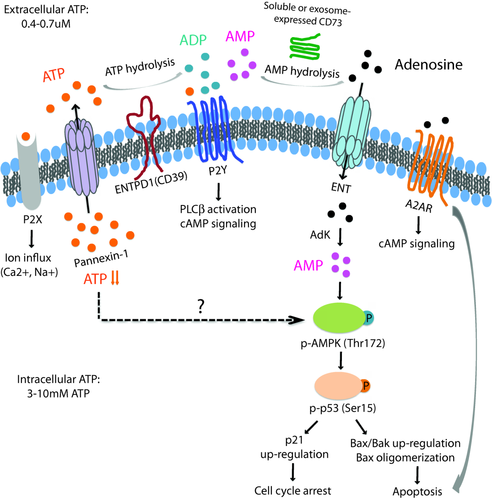
Schematic diagram of purinergic signalling. With age, T cells upon activation and proliferation gain the expression of the ecto-adenosine triphosphatase (ATPase) CD39 that induces the apoptosis of short-lived effector cells by regulating purinergic signalling. ATP is released through the Pannexin channel for autocrine stimulation. Extracellular ATP stimulates P2X ion channels or selected G-protein-coupled P2Y receptors. CD39 cleaves ATP to adenylate kinase (ADP) that also stimulates selected P2Y receptors, and further to adenosine monophosphate (AMP). AMP is cleaved to adenosine by nucleotidases such as CD73. Adenosine is either taken up by the equilibrative nucleoside transporter equilibrative nucleoside transporter 1 (ENT1) or stimulates the G-protein receptor A2AR, both of which are expressed on CD39 T cells.
Conclusion
T cell responses to vaccinations are a staged process starting with activation of antigen-specific T cells followed by expansion and differentiation into short-lived effector and memory precursor cells, and then attrition with preferentially memory precursor cells surviving as long-lived memory T cells. The repertoire of antigen-specific memory T cells is determined by the pre-existing repertoire as well as selection mechanisms during expansion and attrition. The obvious objective of vaccination is to generate a large and diverse antigen-specific T cell memory repertoire. Each of these stages is regulated by T cell-extrinsic as well as T cell-intrinsic mechanisms, several of which have been shown to be affected by immune ageing, and therefore represent promising targets for immune intervention to enhance the benefit from vaccination in an older population.
Acknowledgements
This work was supported by the National Institutes of Health (R01 AR042527, R01 AI044142, HL 117913, R01 AI108906 and P01 HL058000 to C. M. W. and R01 AI108891, R01 AG045779, U19 AI057229, U19 AI057266, and I01 BX001669 to J. J. G.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure
The authors declare no disclosures.