Risk Factors for Fatal and Near-Fatal Food Anaphylaxis: Analysis of the Allergy-Vigilance Network Database
Funding: The authors received no specific funding for this work.
ABSTRACT
Background
Gaining a better understanding of the risk factors for severe anaphylaxis represents a crucial challenge for physicians. This survey aimed to analyse cases of severe food anaphylaxis and assess potential risk factors for severity.
Methods
We retrospectively analysed food anaphylaxis cases recorded by the French-speaking Allergy-Vigilance Network (2002–2021) and compared the main characteristics of grade 3 (Ring classification) and grade 4 cases using univariate and multivariate statistical analyses.
Results
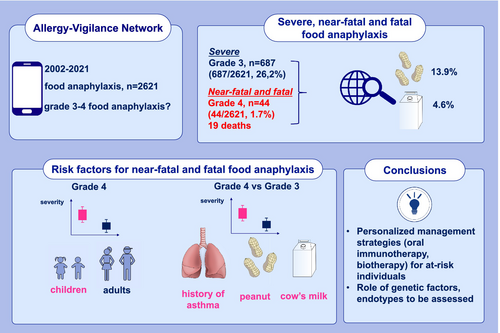
Of the 2621 food anaphylaxis cases reported, 731 (27.9%) were considered severe (grade 3, n = 687 [94%] and grade 4, n = 44 [6%]; 19 deaths). Overall, 56.1% of cases were adults (mean age: 28.3 years) and 53.7% were male. The most frequent triggers were peanut (13.9%), wheat (9.4%), cashew (5.8%), shrimp (5.3%), and cow's milk (4.6%). More grade 4 anaphylaxis cases occurred in children than in adults (26 vs. 18; p = 0.01). In univariate analysis, individuals with grade 4 anaphylaxis were more likely to have a history of allergy to the culprit food (71.1% vs. 42.1%; p < 0.001), asthma diagnosis (59.5% vs. 30.4%; p < 0.001), and peanut as the culprit food (34.1% vs. 12.6%; p < 0.001). In multivariate analysis, factors predictive of grade 4 anaphylaxis were asthma diagnosis (OR [95% CI]: 3.41 [1.56–7.44]; p = 0.002) and peanut as the culprit trigger (OR [95% CI]: 3.46 [1.28–9.34]; p = 0.014).
Conclusions
Our data highlight the risk factors for severe food anaphylaxis, notably a history of asthma and peanut as the culprit food. These individuals should benefit from personalised management strategies.
Graphical Abstract
- Of the 2621 food anaphylaxis cases, 731 (27.9%) were considered as severe (grade 3, [94%] and grade 4 [6%]; 19 deaths).
- There were more grade 4 anaphylaxis cases in children than in adults (p = 0.01).
- In the multivariate analysis, individuals with a grade 4 anaphylaxis were more likely to have a history of asthma (OR [95% CI]: 5.46 [2.02–14.73], p < 0.001), peanut and cow's milk as culprit food triggers (OR: 4.94 [1.50–16.23], p = 0.009; OR: 6.44 [1.20–34.56], p = 0.03; respectively), compared to individuals with grade 3 anaphylaxis.
Abbreviations
-
- ACE
-
- angiotensin-converting enzyme
-
- AVN
-
- Allergy-Vigilance Network
-
- BB
-
- beta-blockers
-
- IQR
-
- interquartile range
-
- NORA
-
- network for Online Registration of Anaphylaxis
-
- NSAID
-
- non-steroidal anti-inflammatory medications
-
- OR
-
- odds ratio
-
- SD
-
- standard deviation
Summary
- Near-fatal and fatal anaphylaxis accounted for 1.7% of all food-induced anaphylaxis cases in this study.
- Severe food-induced anaphylaxis is more frequent in children than in adults.
- Asthma diagnosis and specific food triggers (peanut, milk) are associated with higher anaphylaxis severity.
1 Introduction
Gaining a better understanding of the risk factors for severe anaphylaxis represents a crucial challenge for physicians. Various risk factors and cofactors associated with fatal anaphylaxis have been reported and may depend on the elicitors [1-4]. The literature mentions several risk factors associated with severe food anaphylaxis, namely asthma, specific allergen-related features (persistent cow's milk allergy, lipid transfer protein monosensitisation), previous anaphylaxis, adolescence and young adulthood, exercise, concomitant medication (non-steroidal anti-inflammatory medications [NSAID], beta-blockers [BB], angiotensin-converting enzyme [ACE]), upright posture, and inadequate management of anaphylaxis [1, 3-6]. However, descriptive data on severe food anaphylaxis and associated fatalities are limited and do not provide insights into the risk factors for severe allergic reactions.
In a meta-analysis of 88 studies investigating the risk factors for severe food anaphylaxis, prior anaphylaxis, asthma, biomarkers of IgE sensitisation, and basophil activation test results were not good predictors of severe allergic reactions [5]. By contrast, adolescence and young adulthood were associated with a higher risk of severe outcomes, although the contribution of risk-taking behaviour remains uncertain. This meta-analysis lacked evidence about the impact of cofactors such as concomitant medications and exercise on severity. It thus highlighted the knowledge gaps in this field as well as our current inability to accurately stratify the risk of food-allergic individuals.
We previously reported data on 18 patients who died from food anaphylaxis, as recorded by the French-speaking Allergy-Vigilance Network (AVN), along with a series of 70 fatal and near-fatal anaphylaxis cases of all causes [7, 8]. In this series, the main allergens involved in 60% of cases were foods, mainly peanut (20%) and milk (11%). When comparing individuals with near-fatal food anaphylaxis to fatal cases, we did not identify any characteristics associated with survival [8]. However, we found that severe food anaphylaxis had specific features compared to other causes, such as young age, asthma history, and exercise.
This study aimed to compare the main characteristics of fatal and near-fatal food anaphylaxis cases to those of severe (but not near-fatal or fatal) cases in order to identify potential risk factors for higher severity.
2 Methods
2.1 General methods
The AVN, a French-speaking network comprising approximately 300 allergists, has been documenting cases of anaphylaxis (grade ≥ 2 according to the Ring classification) of any cause since 2002 based on voluntary declarations. A team of expert allergists analyses, validates, and comments on the collected data, sharing information about the participants and collaborating with the French health authorities. The diagnosis of anaphylaxis is based on the definition of the National Institute of Allergy and Infectious Diseases and the Food Allergy and Anaphylaxis Network [9].
The Ring classification (secondarily modified by Behrendt) has been used since the creation of the AVN in 2002 to assess anaphylaxis severity [9].
We defined severe anaphylaxis cases as grade 3 cases according to the Ring classification, whereas near-fatal and fatal anaphylaxis corresponded to grade 4 (characterised by cardiac and/or respiratory arrest). We retrospectively analysed food anaphylaxis cases recorded by the AVN from 2002 to 2021 and compared near-fatal and fatal anaphylaxis to severe cases in order to identify risk factors for higher severity.
2.2 Statistical Analysis
Qualitative variables were presented as frequencies and percentages and then compared using the Chi-square or Fisher's exact test. Continuous variables with normal distribution were expressed as mean and standard deviation and then compared using Student's t-test. Non-normally distributed continuous variables were presented as medians.
Multivariable logistic regression analysis was performed to identify risk factors for severe anaphylaxis. Variables with p-values < 0.2 in univariable analyses were included. For the analysis of risk factors for near-fatal and fatal anaphylaxis, we included age, history of food allergy to the culprit food or to another food allergen, asthma diagnosis at any time (but no asthma symptoms at the time or just before the anaphylactic event) and allergic rhinitis in addition to exercise as a cofactor and the food elicitor. Clinical manifestations were not included in the multivariable logistic regression analysis, as these findings form part of the criteria to determine the grade of severity.
Statistical analyses were conducted using R++ software, an interface for R 3.6.3, with a significance threshold of p < 0.05.
2.3 Regulatory aspects
This study is regulated by the European General Data Protection Regulation. The French data protection authority has transposed this regulation by publishing the national repository (MR-004) to which the data controller has declared itself compliant (declaration number 2213246 v 0 of 26 April 2019). This study is declared compliant with this repository and is registered under number 23617416 in the Health Data Hub (HDH) public directory. It is approved by the Clinical Research Unit of Roubaix Hospital. Data were anonymised in accordance with the current legislation of the French Commission for Data Protection. Consent was obtained from participants or their relatives after providing oral information about AVN studies.
3 Results
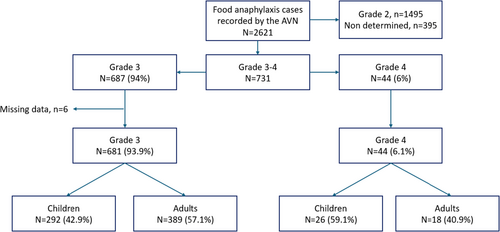
Of the 2621 food anaphylaxis cases collected by the AVN, 731 (27.9%) were severe (grades 3 and 4), and 687 (94%) were classified as grade 3 (Figure 1); 6 cases were excluded due to missing data. A total of 725 food anaphylaxis cases (grade 3, n = 681 [93.9%]; grade 4, n = 44 [6.1%]) were finally analysed. Nineteen (0.7%) deaths were reported. The distribution of the 725 grade 3–4 food anaphylaxis cases by time period (2002–2021) is presented in Figure S1.
3.1 Grade 3–4 Food Anaphylaxis Cases and Patient Characteristics
The main characteristics of the patients with grade 3–4 food anaphylaxis, along with their food elicitors and clinical symptoms during anaphylaxis, are summarised in Tables 1 and 2. Of the 725 individuals with grade 3–4 food anaphylaxis, 53.7% were male and 46.9% were children (≤ 18 years). The mean age was 28.3 years (SD: 21.8). According to allergy history, an allergy to the culprit food involved in the anaphylaxis was reported in 43.7% of patients (n = 313/717), and a diagnosis of asthma in 31.9% (n = 229/718). The most frequent non-atopic comorbidities were cardiovascular disease (n = 39, 5.4%), while one individual reported mastocytosis (Table 1).
| Total N = 725 (%) | Grade 3 anaphylaxis N = 681 (%) | Grade 4 anaphylaxis N = 44 (%) | p | |
|---|---|---|---|---|
| Male sex | 389/725 (53.7) | 364/681 (53.5) | 25/44 (56.8) | 0.78 |
| Mean age, years (SD) | 28.3 (21.8) | 28.6 (21.8) | 22.8 (20.5) | 0.07 |
| Median age, years (IQR) | 23 (9–46) | 24 (9–46) | 15 (8.8–33.8) | 0.11 |
| Children (< 18 years) | 318/725 (46.9) | 292/681 (42.9) | 26/44 (59.1) | 0.05 |
| Allergy history | ||||
| Allergy to the culprit food | 313/717 (43.7) | 286/679 (42.1) | 27/38 (71.1) | < 0.001 |
| Allergy to another food allergen | 228/721 (31.6) | 211/681 (31.0) | 17/40 (42.5) | 0.18 |
| Asthma | 229/718 (31.9) | 207/681 (30.4) | 22/37 (59.5) | < 0.001 |
| Atopic dermatitis | 123/724 (17.0) | 116/681 (17.0) | 7/43 (16.3) | 1.00 |
| Allergic rhinitis | 189/725 (26.1) | 186/681 (27.3) | 3/44 (6.8) | 0.005 |
| Other comorbidities | ||||
| Cardiovascular disease | 39 (5.4) | 38 (5.6) | 1 (2.3) | 0.5 |
| Mastocytosis | 1 (0.1) | 1 (0.1) | 0 | 0.99 |
| Diabetes | 8 (1.1) | 7 (1.0) | 1 (2.3) | 0.4 |
| Malignant disease | 7 (1.0) | 7 (1.0) | 0 | 0.99 |
| Food elicitors | ||||
| Peanut | 101/725 (13.9) | 86/681 (12.6) | 15/44 (34.1) | < 0.001 |
| Wheat | 68/725 (9.4) | 66/681 (9.7) | 2/44 (4.6) | 0.42 |
| Cashew | 42/725 (5.8) | 40/681 (5.9) | 2/44 (4.6) | 1 |
| Shrimp | 39/725 (5.4) | 36/681 (5.3) | 3/44 (6.8) | 0.72 |
| Cow's milk | 35/725 (4.8) | 31/681 (4.6) | 4/44 (9.1) | 0.16 |
| Goat's or sheep's milk | 25/725 (3.4) | 21/681 (3.1) | 4/44 (9.1) | 0.06 |
| Walnut | 21/725 (2.9) | 20/681 (2.9) | 1/44 (2.3) | 1 |
| Hazelnut | 20/725 (2.8) | 19/681 (2.8) | 1/44 (2.3) | 1 |
| Egg | 13/725 (1.8) | 12/681 (1.8) | 1/44 (2.3) | 0.56 |
| Fish | 11/725 (1.5) | 11/681 (1.6) | 0/44 | 1 |
| Apple | 11/725 (1.5) | 11/681 (1.6) | 0/44 | 1 |
| Pistachio | 9/725 (1.2) | 9/681 (1.3) | 0/44 | 1 |
| Almond | 8/725 (1.1) | 8/681 (1.2) | 0/44 | 1 |
| Soy | 8/725 (1.1) | 7/681 (1.0) | 1/44 (2.3) | 0.40 |
| Orange | 3/725 (0.4) | 3/681 (0.4) | 0/44 | 1 |
| Pear | 3/725 (0.4) | 3/681 (0.4) | 0/44 | 1 |
| Other food identified | 273/725 (37.7) | 272/681 (39.9) | 1/44 (2.3) | < 0.001 |
| Non-identified or suspected food | 28/735 (3.9) | 19/681 (2.8) | 9 (20.5) | < 0.001 |
| Place of the reaction | NC | |||
| Home | 217 (45.3) | 205 (45.8) | 12 (38.7) | |
| Restaurant | 58 (12.1) | 55 (12.3) | 3 (9.7) | |
| School | 32 (6.7) | 28 (6.3) | 4 (12.9) | |
| With family, outside the home | 29 (6.1) | 28 (6.3) | 1 (3.2) | |
| Friend's home | 22 (4.6) | 21 (4.7) | 1 (3.2) | |
| Workplace | 22 (4.6) | 22 (4.9) | 0 | |
| Hospital | 11 (2.3) | 8 (1.8) | 3 (9.7) | |
| Outdoors (hiking/jogging) | 9 (1.9) | 7 (1.6) | 2 (6.5) | |
| Miscellaneous | 79 (16.5) | 74 (16.5) | 5 (16.1) | |
| Undetermined | 246 (33.9) | 233 (34.2) | 13 (29.5) | |
- Abbreviations: IQR, interquartile range; NC, non-calculable; SD, standard deviation.
| Total N = 725 (%) | Grade 3 anaphylaxis N = 681 (%) | Grade 4 anaphylaxis N = 44 (%) | p | |
|---|---|---|---|---|
| Clinical manifestations | ||||
| Convulsions | 5/723 (0.7) | 3/681 (0.4) | 2/42 (4.8) | 0.03 |
| Vomiting | 169/724 (23.3) | 159/681 (23.4) | 10/43 (23.3) | 1.00 |
| Abdominal pain | 119/723 (16.5) | 115/681 (16.9) | 4/42 (9.5) | 0.30 |
| Rhinitis | 76/725 (10.5) | 74/681 (10.9) | 2/44 (4.6) | 0.30 |
| Dyspnoea | 242/724 (33.4) | 225/681 (33.0) | 17/43 (39.5) | 0.48 |
| Bronchospasm | 207/724 (28.6) | 194/681 (28.5) | 13/43 (30.2) | 0.94 |
| Facial swelling | 222/725 (30.6) | 213/681 (31.3) | 9/44 (20.5) | 0.18 |
| Laryngeal angioedema | 193/725 (26.6) | 181/681 (26.6) | 12/44 (27.3) | 1.00 |
| Generalised hives | 363/724 (50.1) | 353/681 (51.8) | 10/43 (23.3) | < 0.001 |
| Bradycardia | 7/725 (1.0) | 5/681 (0.7) | 2/44 (4.6) | 0.06 |
| Tachycardia | 90/725 (12.4) | 85/681 (12.5) | 5/44 (11.4) | 1 |
| Cofactors | ||||
| Exercise | 151/683 (22.1) | 147/639 (23.0) | 4/44 (9.1) | 0.049 |
| Alcohol | 81/682 (11.9) | 75/659 (11.7) | 6/43 (14.0) | 0.85 |
| Concomitant medication | ||||
| Beta-blockers | 40/683 (5.9) | 39/639 (6.1) | 1/44 (2.3) | 0.50 |
| Non-steroidal anti-inflammatory drug | 50/683 (7.3) | 47/639 (7.4) | 3/44 (6.8) | 1.00 |
| Proton pump inhibitor | 21/683 (3.1) | 21/639 (3.3) | 0/44 (0) | 0.39 |
| Angiotensin-converting enzyme | 23/683 (3.4) | 22/639 (3.4) | 1/44 (2.3) | 1.00 |
| Statins | 5/683 (0.7) | 4/639 (0.6) | 1/44 (2.3) | 0.28 |
| Adrenaline administration | ||||
| Adrenaline injection | 282 (46.1) | 254 (43.9) | 28 (82.4) | < 0.001 |
| Adrenaline autoinjector use | 74 (12.1) | 64 (11.1) | 10 (29.4) | 0.001 |
| Undetermined | 113 (15.6) | 103 (15.1) | 10 (22.7) | |
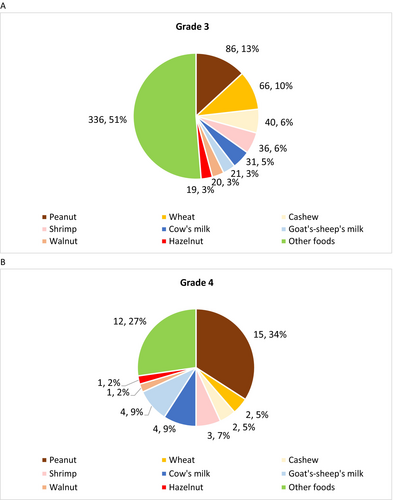
The most frequent food elicitors were peanut (n = 101/725, 13.9%), wheat (n = 68/725, 9.4%), cashew (n = 42/725, 5.8%), shrimp (n = 39/725, 5.4%) and cow's milk (n = 35/725, 4.8%) (Table 1, Figure 2A,B). The distribution of the main food elicitors according to severity is presented in Figure S2.
As clinical manifestations, the most frequent symptoms were generalised hives (n = 363/724, 50.1%), dyspnoea (n = 242/724, 33.4%), facial swelling (n = 222/725, 30.6%), bronchospasm (n = 207/724, 28.6%) and laryngeal angioedema (n = 193/725, 26.6%). The two most frequent cofactors were exercise (n = 151/683, 22.1%) and alcohol (n = 81/682, 11.9%).
The most frequent places of reaction were the home (n = 217, 45.3%), restaurant (n = 58, 12.1%), and school (n = 32, 6.7%), while 11 (2.3%) reactions occurred at the hospital.
3.2 Comparison Between Fatal and Near-Fatal Food Anaphylaxis (Grade 4) and Severe Anaphylaxis Cases (Grade 3)
Univariate statistical analysis (Tables 1 and 2) identified the following factors as more prevalent in grade 4 than in grade 3 anaphylaxis: being a child (59.1% vs. 42.9%, respectively; p = 0.05), previous diagnosis of allergy to the culprit food (71.1% vs. 42.1%; p < 0.001), diagnosis of asthma (59.5% vs. 30.4%; p < 0.001), peanut as the culprit food (34.1% vs. 12.6%; p < 0.001) and convulsions as a clinical manifestation of anaphylaxis (4.8% vs. 0.4%; p = 0.03). By contrast, the following factors were less prevalent in grade 4 anaphylaxis compared to grade 3 cases: history of allergic rhinitis (6.8% vs. 27.3%, respectively; p = 0.005), generalised hives as a clinical manifestation of anaphylaxis (23.3% vs. 51.8%; p < 0.001) and exercise as a cofactor of anaphylaxis (9.1% vs. 23.0%; p = 0.049).
In multivariate analysis (Table 3, Figure S3), independent factors predictive of grade 4 anaphylaxis were a diagnosis of asthma (OR [95% CI]: 3.41 [1.56–7.44]; p = 0.002) and peanut as the culprit food trigger (OR [95% CI]: 3.46 [1.28–9.34]; p = 0.014). By contrast, a history of allergic rhinitis was an independent factor predictive of not having grade 4 anaphylaxis (OR [95% CI]: 0.16 [0.05–0.56]; p = 0.004).
| Odds ratio | 95% CI | p | |
|---|---|---|---|
| Age, mean (y) | 1.01 | 0.98–1.03 | 0.35 |
| Sex (male) | 0.9 | 0.44–1.86 | 0.78 |
| Allergy history | |||
| Allergy to the culprit food | 2.1 | 0.95–4.65 | 0.066 |
| Allergy to another food allergen | 1.06 | 0.48–2.34 | 0.89 |
| Asthma | 3.41 | 1.56–7.44 | 0.002 |
| Allergic rhinitis | 0.16 | 0.05–0.56 | 0.004 |
| Cofactors | |||
| Exercise | 0.61 | 0.20–1.88 | 0.39 |
| Food elicitor (compared to other food elicitors) | |||
| Peanut | 3.46 | 1.28–9.34 | 0.014 |
| Cow's milk | 3.25 | 0.83–12.74 | 0.091 |
| Goat's or sheep's milk | 1.9 | 0.36–10.14 | 0.45 |
3.3 Comparison of Grade 3–4 Food Anaphylaxis According to Age Groups (Children vs. Adults)
We compared children (age < 18 years) and adults (Table S1). The following variables were more frequent in the paediatric population (n = 318) than in the adult population (n = 407): grade 4 anaphylaxis (8.2% vs. 4.4%; p = 0.01), a history of food allergy to another food than the culprit food (44.4% vs. 21.47%; p < 0.001) and atopic dermatitis (31.9% vs. 5.4%; p < 0.001). Peanut, cashew, and cow's milk as the culprit food were more frequent in children compared to adults (p < 0.001 for each), whereas wheat and shrimp were more frequent in adults (p < 0.001 and p = 0.004, respectively).
In terms of clinical manifestations, children more frequently experienced vomiting, abdominal pain, rhinitis, dyspnoea, and bronchospasm (p < 0.001 for each), whereas adults more frequently presented laryngeal angioedema (p = 0.05) and cofactors such as alcohol and exercise (p < 0.001 and p = 0.01, respectively).
We also compared the main characteristics of the anaphylaxis reactions in children according to age groups (preschool children, school-age children, and adolescents) (Table S2). We found that older children were more likely to have a known allergy to the culprit food, a history of asthma, and allergic rhinitis (p < 0.001 for each). More fatalities occurred in older children compared to preschool-age children (p = 0.015). The distribution of the culprit foods differed according to these age groups, with peanut being more frequently involved in school-age children and adolescents and cow's milk in preschool-age children (p = 0.034).
4 Discussion
Our study, performed on 2621 food-induced anaphylaxis cases collected by the AVN, reveals that near-fatal and fatal (grade 4) anaphylaxis are not so rare, being involved in 1.7% of all food anaphylaxis cases. Multivariate analysis found that any diagnosis of asthma (but not asthma symptoms at the time or just before the anaphylactic event) and peanut as the food elicitor were independent predictors of grade 4 anaphylaxis, although in univariate analysis, age < 18 years and previous allergy to the culprit food were associated with grade 4 anaphylaxis. By contrast, exercise was less likely to be reported in grade 4 anaphylaxis compared to grade 3 in the univariate model, but not in the multivariate model. Concomitant medication and alcohol had no impact on severity.
Our data highlight that a prior history of asthma was associated with an increased risk of near-fatal and fatal anaphylaxis compared to severe anaphylaxis. On the contrary, in the meta-analysis of Turner et al. regarding the risk factors for severe food anaphylaxis, evidence of the link between asthma diagnosis and anaphylaxis severity was ultimately found to be weak or non-existent [5]. However, according to the 32 primary research studies and one systematic review assessed in the meta-analysis, the impact of asthma diagnosis on anaphylaxis severity was contradictory, even within the same dataset, depending on the definition of severity. Using a random effects model to address the heterogeneity of these studies, the authors did not find any consistent evidence that a diagnosis of asthma was associated with increased anaphylaxis severity or the need for intensive care unit admission and/or intubation and mechanical ventilation. However, the impact of asthma on severity should not be considered by diagnosis alone. The key points to assess are asthma severity, exacerbation or insufficient asthma control prior to anaphylaxis, bronchial inflammation, treatment required for asthma control, and treatment compliance. These data have not been collected systematically by the AVN since its creation. In addition, the impact of other atopic comorbidities (e.g., atopic dermatitis, allergic rhinitis, pollen food syndrome, type of IgE sensitisation) and seasonality on anaphylaxis severity remains unclear and must be assessed more closely.
The second most striking finding of our study was that peanut was associated with increased anaphylaxis severity in the multivariate analysis. The food allergens involved in near-fatal and fatal anaphylaxis vary according to age as well as culinary and cultural traditions, but certain foods may cause more severe anaphylaxis reactions than others, even when correcting for prevalence [5, 6, 10]. Peanut is one of the major food allergens from infancy to adulthood, triggering nearly a third of food anaphylaxis in children in the European NORA database [11]. Peanut and milk are also reported as the main elicitors of near-fatal and fatal food anaphylaxis in most countries (i.e., United States, Australia, United Kingdom, France) [7, 8, 10-15]. In a cohort of 1989 children admitted to North American intensive care units with anaphylaxis, the main food allergens were peanut (39%), tree nuts and seeds (16%), followed by milk (8%) [14]. In the United Kingdom, 46% of the 152 fatal cases (1992–2018) were triggered by peanut or tree nuts, although cow's milk was responsible for 26% of deaths in children and 5% in adults [12]. In France, among the 166 anaphylaxis cases observed in paediatric intensive care, food was involved in 62 cases (37%), and the main food allergens were milk (30%) and peanut (27%) [15]. Of the 25 fatal food anaphylaxis cases reported by the AVN, deaths were linked to peanut in eight individuals and milk in three [7, 8]. However, it remains unclear why peanut is associated with increased severity. Indeed, the causal link between these foods and anaphylaxis severity is unknown.
Other potential risk factors for severity related to allergen presentation have been investigated, such as food matrix and processing or dose/level of allergen exposure. However, data are limited, and the relationship between the eliciting dose and severity is complex and unclear. We did not assess these issues in our study.
Whereas older age is associated with more severe outcomes for anaphylaxis of all causes, epidemiological data suggest an increased risk in adolescence and young adulthood for food anaphylaxis [3, 5, 7, 8, 16]. These findings are in accordance with our results. We found that near-fatal and fatal anaphylaxis was more frequent in children, particularly in school-age children and adolescents than in adults; individuals with grade 3 anaphylaxis were also older than those with near-fatal and fatal anaphylaxis, even if this finding was not statistically significant (mean age: 28.6 vs. 22.8 years; p = 0.07). In a previous study based on the AVN, we also demonstrated that individuals with food-induced grade 4 anaphylaxis were younger (22.2 vs. 55 years; p < 0.001) than individuals with grade 4 anaphylaxis induced by other triggers [8]. However, this higher risk for fatal and near-fatal food anaphylaxis persists into the fourth decade of life according to data from the national fatal anaphylaxis registry in the United Kingdom [12]. The reasons for this age-related susceptibility remain unclear, potentially due to risk-taking behaviours in younger people and a potential age-specific vulnerability [5, 17].
In our data, we did not find any association between food anaphylaxis severity and previously reported cofactors: history of food allergy to the culprit food, exercise, alcohol, and concomitant medications. Studies have identified periods of exercise in around 10%–20% of severe anaphylaxis cases, suggesting that it may be a cofactor of severity [5]. However, the link between exercise (type and intensity) and anaphylaxis severity remains under investigation. There is increasing evidence that concomitant medications (ACE and/or BB and/or non-NSAID) play a role in anaphylaxis severity, but mainly in drug-related anaphylaxis [5]. In the literature, the consumption of alcohol and recreational drugs has not yet been reported as a potential risk factor for severe food anaphylaxis. Both may increase the likelihood of accidental allergen exposure through disinhibition, mask the early warning signs of anaphylaxis, or suppress physiological responses to hypotension; alcohol may also increase the absorption of food allergens through increased intestinal permeability that can activate, in vitro, effector cells [18]. Alcohol consumption was reported in over 10% of our adult population. However, we did not find any association between alcohol consumption and the severity of the reaction.
In addition, our study did not assess other conditions reported in the literature as potential factors that may increase severity, such as psychological burden, baseline mast cell tryptase, and systemic mastocytosis, hereditary alpha tryptasemia, IgE sensitisation, and basophil activation test results [16, 19]. Until now, these data have not been collected routinely in the AVN database. Regarding systemic mastocytosis and hereditary alpha tryptasemia, these conditions have been reported as factors increasing anaphylaxis severity, particularly for hymenoptera venom-triggered anaphylaxis, although data are lacking regarding food elicitors [20-23].
5 Limitations
Our study has several limitations. The AVN data are retrospective, and although collected using a standardised form, it was not always possible to obtain a precise description of the circumstances or a detailed history for all patients. Some factors possibly associated with severity could not be analysed with sufficient precision in all patients due to missing data: the interval between first symptoms and injection by the medical emergency team, dosage of adrenaline autoinjector, and number of adrenaline doses injected. The detailed circumstances in terms of asthma control and/or exacerbation were not recorded at the time of the reaction. Henceforth, data about the circumstances at the time of the reaction will assess asthma control, treatment useful to control asthma, and any information about asthma exacerbation during the previous year. In addition, we did not assess tryptase level as a potential risk factor because these data were only available for a few patients. Finally, the AVN does not routinely collect blood samples that may be useful as biomarkers.
Based on voluntary declarations, the AVN data do not constitute an exhaustive analysis of all cases of anaphylaxis in France, similar to the NORA and most anaphylaxis registries across the world, which may induce bias and affect the validity of our results [24]. However, one major strength of the AVN is based on the quality of its assessments and the declarations made by allergists and validated by anaphylaxis experts.
6 Conclusions
Addressing knowledge gaps in food anaphylaxis, including more severe or refractory anaphylaxis, is a priority. Our data highlight risk factors for fatal and near-fatal food anaphylaxis compared to severe anaphylaxis, notably a history of asthma and peanut as the anaphylaxis elicitor. These high-risk individuals should benefit from personalised management strategies such as oral immunotherapy and biotherapy.
With the goal of improving clinical care and treatment, we need to go further in identifying biomarkers and analysing endotypes, pathophysiologic pathways, and genetic factors that may be associated with an increased risk of near-fatal anaphylaxis. This objective requires further clarification of the real impact of comorbidities, intrinsic and extrinsic cofactors in terms of severity, through larger cohort studies and international collaboration between clinicians and researchers.
Author Contributions
Guillaume Pouessel analysed, interpreted the data, and wrote the manuscript draft. Rémy Diesnis performed the statistical analysis, interpreted the data, and reviewed the draft. Claire Egea, Amandine Divaret-Chauveau, Pascale Beaumont, Eléna Bradatan, Pascale Dumond, Xavier Van der Brempt, Camille Braun, and Dominique Sabouraud-Leclerc were major contributors in reviewing the draft and improving the paper with critical analysis. Sélina Tscheiller collected data from the Allergy-Vigilance Network. All authors read and approved the final manuscript.
Acknowledgements
We acknowledge all members of the Allergy-Vigilance Network who participated in this study and collected data.
Conflicts of Interest
Guillaume Pouessel has provided consultation and speaker services to Bioprojet, Viatris, Stallergenes, Novartis, DVB technology, and ALK-Abello, and currently works as a medical consultant/advisor for Bioprojet, Viatris, and Theravia. Amandine Divaret-Chauveau, outside of the submitted work, reports grants from Don du Souffle, Novartis, ARAIRLOR, and CICBAA, consulting fees from Sanofi, Stallergenes, ALK, and AImmune Therapeutics, payment for presentations for AImmune Therapeutics, Novartis, ALK, and DBV, support for attending meetings from Mead Johnson, Nutricia, AImmune Therapeutics, Novartis, and ALK, and stocks from Essilor Luxottica. The other authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.