Novel Pentapeptide GLP-1 (32–36) Amide Inhibits β-Cell Apoptosis In Vitro and Improves Glucose Disposal in Streptozotocin-Induced Diabetic Mice
Abstract
We proposed that a pentapeptide, LVKGR amide, GLP-1 (32–36) amide, derived from the gluco-incretin hormone, glucagon-like peptide-1 (GLP-1), might possess favorable actions against diabetes. Therefore, GLP-1 (32–36) amide was synthesized and the effects of it were examined in INS-1 cell and streptozotocin-induced diabetic mice model. To determine the protective effects of GLP-1 (32–36) amide on INS-1 cell viability and apoptosis, cells were exposed to 1 μm streptozotocin (STZ) and GLP-1 (32–36) amide for 24 h. Results showed that GLP-1 (32–36) amide treatment decreased apoptosis rate and significantly retained cell viability compared with saline-treated controls. Then, GLP-1 (32–36) amide was administered intraperitoneally to streptozotocin-induced diabetic mice with normal mice used as control. Body weight, energy intake, plasma glucose, and histopathology of the pancreas were assessed. Results showed that GLP-1 (32–36) amide protected β-cell viability and apoptosis against STZ-induced toxicity, inhibited weight gain, and relieved symptoms of polydipsia. Moreover, GLP-1 pentapeptide-treated mice showed a slight trend toward reduced glucose excursions in intraperitoneal glucose tolerance test at the end of the experiment. GLP-1 (32–36) amide exerted favorable protective actions in streptozotocin-induced diabetic mice. The peptide curtailed weight gain and alleviates symptoms of polydipsia. These findings suggested the probable utility of GLP-1 (32–36) amide, a peptide mimetic derived there from GLP-1, for adjuvant treatment of diabetes.
Abbreviations
-
- DPP IV
-
- diaminopeptidyl peptidase IV
-
- GLP-1
-
- glucagon-like peptide-1
-
- HbA1c
-
- hemoglobin A1c
-
- IPGTT
-
- intraperitoneal glucose tolerance test
-
- MTT
-
- 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
-
- NEP
-
- neutral endopeptidase
-
- STZ
-
- streptozotocin
Type 2 diabetes is a multifactorial disorder with numerous causes and is usually a combination of genetic predisposition, lack of exercise, poor diet, and obesity. This is not surprising, as the pathogenesis of type 2 diabetes is complicated, the drug classes available have a range of effects and target organs, including promoting tissue glucose uptake and insulin secretion, enhancing satiety, decreasing appetite, and inhibiting carbohydrate digestion. All of these have been targeted and achieved success with varying degrees 1.
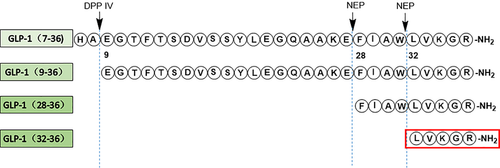
Glucagon-like peptide-1 (GLP-1) is an incretin hormone released by intestinal L cells, which has attracted much interest due to its multiple glucoregulatory functions 2. However, GLP-1 (7–36) amide is rapidly cleaved by the ubiquitous enzyme diaminopeptidyl peptidase IV resulting in the formation of GLP-1 (9–36) amide and another ubiquitous endopeptidase, the neutral endopeptidase NEP 24.11 forming a non-apeptide GLP-1 (28–36) amide and a pentapeptide GLP-1 (32–36) amide (LVKGR amide) 3, 4. GLP-1 (9–36) amide and GLP-1 (28–36) amide are generally considered as two inactive metabolites with little or no insulinotropic properties until they had been demonstrated more recently that the metabolites have several biological properties, such as curtailed weight gain and the development of diabetes in diet-induced obese mice 5-15. Moreover, it is increasingly recognized that these effects are more potent in states of ‘intolerance’ such as insulin resistance, obesity, and cerebrovascular and cardiovascular disease 16, 17. Lately, it has been shown that GLP-1 (28–36) amide administration improved glucose disposal, which is correlated with increased β-cell proliferation and pancreatic β-cell mass in diabetic model caused by β-cell toxin STZ 18.
In present study, we hypothesized that the pentapeptide GLP-1 (32–36) amide might be a bioactive peptide (Figure 1) and exerted cytoprotective actions on pancreatic β-cells which prompted an examination of the effects of the pentapeptide in INS-1 cell and STZ-induced diabetic mice.
Experimental procedures
Reagents
GLP-1 (32–36) amide (LVKGR amide), positive control exenatide (Ex-4), GLP-1 (9–36) amide and GLP-1 (28–36) amide were prepared by solid-phase synthesis and then purified by Shimadzu preparative reverse-phase high-performance liquid chromatography as previous reported 19. The molecular mass confirmation of the purified peptides was done by liquid chromatograph–mass spectrometer. STZ was purchased from Sigma Chemicals (St. Louis, MO, USA). Fmoc-protected amino acids and Fmoc Rink Amide-MBHA resin were obtained from GL biochem (Shanghai, China). N, N-dimethylformamide was purchased from SamSung fine chemicals (Ulsan, Korea). High-performance liquid chromatography-grade acetonitrile and methanol were provided by Merck (Darmstadt, Germany). Hemoglobin A1c (HbA1c) level was measured by Beckman Coulter Chemistry Analyzer (Tokyo, Japan). All other chemicals were of analytical grade.
Cell culture
Rat pancreatic INS-1 cells were maintained in RPMI 1640 medium (Gibco-BRL, Invitrogen, Paisley, UK) with 11.2 mm glucose supplemented with 10% heat-inactivated fetal calf serum, 10 mm N-2-hydroxyethylpiperazine-N-ethane-sulphonic acid, 2 mm l-glutamine, 1 mm sodium pyruvate, 50 μμ β-mercaptoethanol, 100 U/mL penicillin, and 100 mg/mL streptomycin at 37 °C in a humidified atmosphere (5% CO2 and 95% air).
Generation of STZ-induced diabetic mice
Male KM mice 20, 6–8 weeks old, weighing 18–22 g, were purchased from Comparative Medicine Centre of Yangzhou University, Jiangsu, China, and housed six per cage under the conditions of ambient temperature (24 ± 1 °C) under a reverse 12-h light/dark cycle, with free access to food and water. The mice were fasted for overnight before the injection of STZ intraperitoneally (40 mg/kg/day) for 5 consecutive days. Blood glucose was measured by glucometer to validate diabetic hyperglycemia. Five days after STZ injection, the mice with overnight fasting blood glucose level 11.1 mm or higher were assigned into groups with matched body weight and GLP-1 (32–36) amide, exendin-4, or vehicle was injected intraperitoneally once daily for 21 days. All animal experimental protocols were adhered to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health.
Cell viability assay
INS-1 cell were seeded in 96-well plates (approximately 5000 cells/well) for 24 h, then 1 μm STZ and exendin-4 (10 nm), GLP-1 (9–36) amide (1 μm), GLP-1 (28–36) amide (1 μm), or GLP-1 (32–36) amide (0.1, 1 or 10 μm) were added. After incubation for 24 h, cell viability was measured by adding 200 g/mL 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Dingguo, Beijing, China) to INS-1 cells and incubated for 3 h at 37 °C. The reaction was stopped and the purple formazan precipitate formed was dissolved in dimethyl sulfoxide, and the color intensity was measured at 550 nm with a multiwall spectrophotometer (Thermo Labsystems, Waltham, MA, USA).
Detection of apoptotic cells
For flow cytometric analysis (FACS), INS-1 cells were seeded in 96-well plates (5000 cells/well) for 24 h, then 1 μm STZ and exendin-4 (10 nm) or GLP-1 (32–36) amide (1 μm) were added. After incubation for 24 h, cells were collected by trypsinization, washed with cold phosphate-buffered saline, and incubated with Annexin V (Sigma, St. Louis, MO, USA) for 15 min, then stained with propidium iodide (Sigma). Cells in early apoptosis were Annexin V positive and PI negative. The analysis was performed with a FAC Scan flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) using the cellquest software (BD Biosciences).
Food and water intake of mice and body weight gain
Food and water intake of mice was monitored daily, and body weight gain was recorded (every other day during the experiment).
Fasting blood glucose and HbA1c levels
Overnight fasting blood samples were obtained from the tail vein, and blood glucose levels were tested on day 0, 5, 10, 15, and 20 with blood glucose test strips (SanNuo, Changsha, China). Intraperitoneal glucose tolerance test (IPGTT) was conducted at the beginning and 3 days after the completion of the study. On day 24, blood samples were collected and sera were separated subsequently for further analysis. The levels of HbA1c were determined by automatic biochemical analyzer.
Intraperitoneal glucose tolerance test
To assess the glucose tolerance of diabetic mice, IPGTT was conducted as previous literature 19. Briefly, mice were fasted overnight (12 h) prior to the glucose load (2.0 g/kg, 0 h). At 0, 0.25, 0.5, 1, and 2 h after oral administration of glucose, the blood was collected from the cut tip of the tail vein, and then the glucose levels were measured through a blood glucose monitoring system.
Histopathology of the pancreas of STZ diabetic mice
Pancreases (n = 3) were removed rapidly, fixed in 10% (v/v) formalin overnight after washed with ice-cold saline, dehydrated in alcohol, and then embedded in paraffin. To minimize sampling variation due to differences in cell size and anatomical location, the same region of pancreases was used for all animals. Tissue blocks were sectioned 5 mm thick and stained with hematoxylin and eosin. Two investigators were involved to view stained slides blindly and independently under an Olympus microscope at ×100 magnification, and images were captured by an Olympus C4000 zoom digital camera at ×400 magnification. The size was estimated using image-pro plus 6.0 software (Media Cybernetics, Bethesda, MD, USA). Five representative sections of each mouse were analyzed through this software. Representative photomicrographs of islet from each group were examined without statistical analysis.
Statistical analysis of the data
Statistical analyses were performed using prism version 5.0 software (GraphPad, San Diego, CA, USA). General effects were tested using 1-way anova. Pair-wise comparisons were performed using Dunnett's test for multiple comparisons, and t-tests for simple pairs. Data throughout were stated as mean ± SEM. Significance was set at p < 0.05.
Results
GLP-1 (32–36) amide retains cell viability and decreases apoptosis against STZ
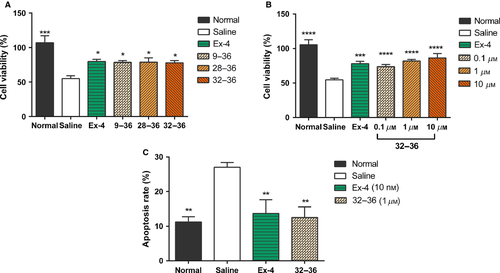
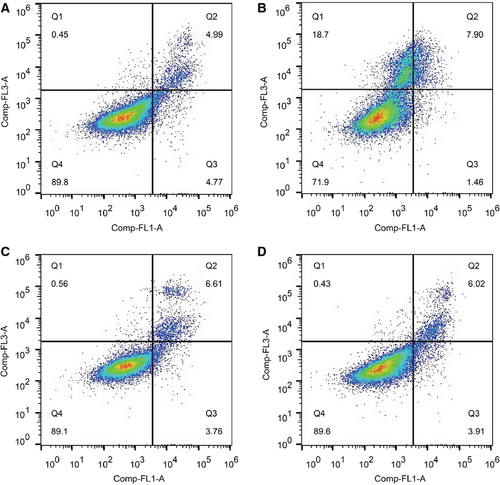
To determine whether GLP-1 (32–36) amide exhibited protective effects on INS-1 cells exposed to 1 μm STZ, GLP-1 (32–36) amide (1 μm) was added and incubated for 24 h in comparison with Ex-4 (10 nm), GLP-1 (9–36) amide (1 μm), and GLP-1 (28–36) amide (1 μm). As shown in Figure 2A, GLP-1 (32–36) amide possessed comparable effect with the selected positive control Ex-4 as well as other metabolites. Then, GLP-1 (32–36) amide at 0.1–10 μm was applied to further assess the protective effect on cell viability and apoptosis. Compared with saline-treated controls, GLP-1 (32–36) amide at 0.1–10 μm significantly retained cell viability. After 24-h incubation, cell viability of control INS-1 cells was significantly decreased by approximately 45%, while only approximately 30% in 0.1 μm and approximately 20% in ≥1 μm GLP-1 (32–36) amide-treated INS-1 cells (p < 0.001 versus saline; Figure 2B). FACS also showed similar results, 24-h treatment with 1 μm STZ caused a drastically increase in apoptosis in control INS-1 cells; however, less apoptosis was found in Ex-4 and GLP-1 (32–36) amide-treated INS-1 cells [13.7 ± 3.9% versus 27.0 ± 1.3% for Ex-4, 12.5 ± 3.0% versus 27.0 ± 1.3% for GLP-1 (32–36) amide, respectively, Figures 2C and 3], suggesting that GLP-1 (32–36) amide could protect β-cell viability and inhibit apoptosis against STZ-induced toxicity.
Intraperitoneal glucose tolerance test in normal mice 21
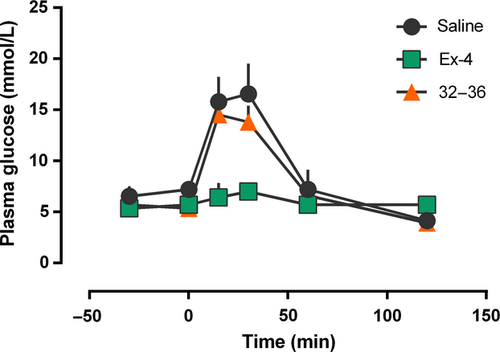
To assess the abilities of GLP-1 (32–36) amide to reduce glucose levels, IPGTT in normal mice was conducted and results (Figure 4) showed no remarkable difference of AUC0–120 min between GLP-1 (32–36) amide and saline. However, GLP-1 (32–36) amide could slightly reduce the mean glucose lever at 30 min after the challenge of glucose [13.8 ± 1.60 mmol/L versus 16.57 ± 2.95 mmol/L for GLP-1 (32–36) amide and saline].
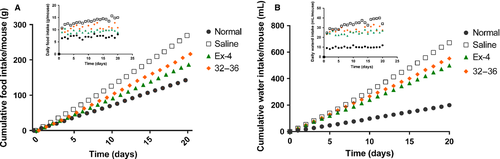
Food and water intake of STZ-induced diabetic mice in chronic study
To further probe in vivo activity and potential therapeutic utility of GLP-1 (32–36) amide, effects of chronic administration were tested in STZ-induced diabetic mice. GLP-1 (32–36) amide (1 μmol/kg) and Ex-4 (25 nmol/kg) were intraperitoneally injected once daily, in the meanwhile, saline served as control. The overall food and water intake of diabetic mice without treatment increased significantly compared with the normal control (Figures 4B and 5A), while Ex-4 and GLP-1 (32–36) amide therapy significantly lowers the cumulative values of food and water intake at the end of the experiment [approximately 30.5% and 25.6% for Ex-4; 19.45% and 17.8% for GLP-1 (32–36) amide] throughout the 20-day treatment course.
Prior to treatment, fasting concentrations in diabetic mice were 17.1 ± 1.9 mm, and in the vehicle-treated controls, worsened somewhat to 20.2 ± 2.8 mm over 20-day duration (Figure 5A). In contrast, at the end of the experiment, both Ex-4 and GLP-1 (32–36) amide treatment lowered fasting glucose toward the 12.7 ± 2.6 and 17.3 ± 2.5 mm levels, respectively. Moreover, GLP-1 (32–36) amide treatment prevented the deterioration of fasting glucose (p < 0.05), while fasting glucose in vehicle-treated mice increased by 3 mm at the end of the experiment.
As HbA1c was resulted from non-enzymatic irreversible glycation, compared with glucose concentration, it was a more sensitive index of glycemic control and recognized as a well-known indirect indicator of cumulative blood glucose concentrations. By design, HbA1c was initially well matched and significantly higher in all diabetic groups than in non-diabetic littermates in which HbA1c remained low throughout the experiment (Figure 6B). After the treatment, HbA1c still maintained at a high level in diabetic control group, while those daily treated with either Ex-4 or GLP-1 (32–36) amide exhibited a gradual reduction.
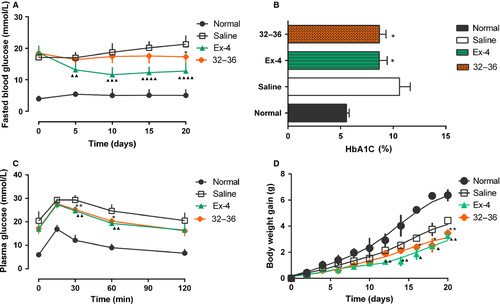
To further determine whether long-term GLP-1 (32–36) amide treatment improved glucose tolerance, IPGTT test was performed before and after treatment (day 0 and day 24). At the very beginning of treatment, all diabetic group showed no difference. However, after treatment, GLP-1 (32–36) amide (1 μmol/kg)-treated mice showed slight blood glucose reductions at 30 and 60 min (p < 0.01 for 30 min and p < 0.05 for 60 min) after the glucose challenge; meanwhile, saline-treated mice showed a typical diabetic pattern of slow reduction in the hyperglycemic state (Figure 6C).
Body weight gain was suppressed after administration of either Ex-4 or GLP-1 (32–36) amide, whereas body weight continually increased over the treatment period by approximately 4 g in diabetic control mice and approximately 6 g in normal mice (Figure 6D).
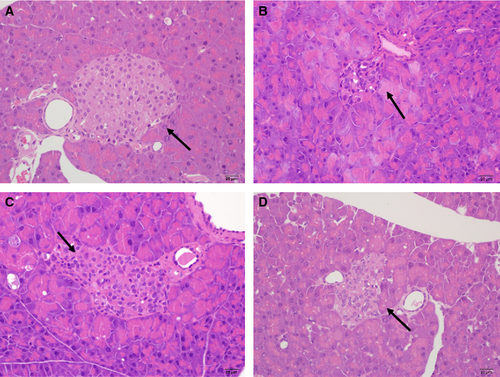
Histological changes in the pancreas of STZ-induced diabetic mice
Islets from each groups were counted and histologically assessed (Table 1). In contrast to the shrunken nuclear membrane and lytic cytoplasm in the islets of diabetic control mice treated with STZ alone, mice treated with GLP-1 (32–36) amide showed a significant improvement in β-cell area, β-cell mass, and the degree of degeneration. It was demonstrated that GLP-1 (32–36) amide could protect islet from damage and may alleviate islet loss in diabetic mice which were also consistent with the improved glycemic control observed above. Representative islet images were shown in Figure 7.
| Groups | Islet number | Islet area | Degree of β-cell degeneration |
|---|---|---|---|
| Normal control | 1.25 ± 0.31 | 1.90 ± 0.70 | 0.2 ± 0.16 |
| Saline | 0.50 ± 0.23 | 0.38 ± 0.31* | 1.95 ± 0.15*** |
| Ex-4 | 0.83 ± 0.28 | 0.81 ± 0.34 | 1.72 ± 0.25*** |
| 32–36 | 0.67 ± 0.39 | 0.83 ± 0.44 | 1.64 ± 0.27*** |
- The highest degree of β-cell degeneration was 3°. Values were shown as mean ± SEM, n = 3.
- *p < 0.05; ***p < 0.001, compared with normal mice.
Discussion
In this study, GLP-1 (32–36) amide, a known product of the cleavage of GLP-1 by the neutral endopeptidase NEP 24.11 22, was proved to function well in STZ-induced apoptosis of INS-1 cell and diabetic mouse model, exemplified by the improvement in glucose disposal and the stimulation of β-cell proliferation.
In plasma, GLP-1 (7–36) amide underwent the cleavage of enzyme dipeptidyl peptidase IV, resulting in a presumably inactive form, GLP-1 (9–36) amide. Both of GLP-1 (7–36) amide and GLP-1 (9–36) amide can be degradated by NEP 24.11 to produce GLP-1 (28–36) amide and NEP 24.11 was a widely distributed membrane-bound metalloenzyme which was involved in processing GLP-1, glucagon (39), and other hormonal and non-hormonal peptides 23. Recently, the major circulating form, GLP-1 (9–36) amide, being regarded as an inactive metabolite with no discernible insulinotropic effect previously, was found to exert beneficial effects. Tomas and Habener 24 described the insulin-like effect of the GLP-1 (9–36) amide in preclinical and clinical studies and further assessed the beneficial effect of GLP-1 (28–36) amide in high-fat diet-fed mice. They demonstrated that this non-apeptide GLP-1 (28–36) amide could inhibit weight gain, improve insulin sensitivity, and alleviate liver triglyceride accumulation through attenuating the development of hyperglycemia and hyperinsulinemia 15. Later, they reported that GLP-1 (28–36) amide suppressed oxidative stress and glucose production in primary mouse hepatocytes which might explain the improvement in glucose disposal observed in GLP-1 (32–36)-treated animals in our IPGTT studies 9. Liu et al. 12 reported that GLP-1 (28–36) amide protected β-cells in vitro which was independent of the GLP-1R activation, especially in conditions of glucolipotoxicity. This non-apeptide targeted mitochondria and improved impaired mitochondrial membrane potential, which is associated with reduced caspase activation, increased cellular ATP levels, and cell apoptosis 14. Our in vitro studies suggested that GLP-1 (32–36) amide protected INS-1 cell from apoptosis induced by STZ and probably share the mechanism with GLP-1 (28–36) amide. Taken studies of interest in this regard together, the GLP-1 (32–36) probably mediated effects in β-cells and hepatocytes via direct targeting of the mitochondria and activation of the cAMP/protein kinase A signaling network.
Tomas et al. 15 firstly investigated the effects of continuous delivery of the non-apeptide on weight gain in DIO mice and observed a significant reduction in weight gain compared with control animals. Subsequently, Ip et al. 11 intraperitoneally administered GLP-1 non-apeptide daily for 4 weeks and found obese mice receiving the non-apeptide showed lower body weight and weight gain (around 10% and 20%, respectively). In consistent with the previous finding, we found GLP-1 (32–36) amide significantly reduced the weight gain, whereas around 50% of that observed in normal mice and 30% in diabetic control animals. Moreover, the cumulative food and water intake in GLP-1 (32–36) amide group became obviously different from non-treatment group. IPGTT in normal mice revealed no obvious differences between the groups, although there was a slight trend toward reduced glucose excursions in the GLP-1 pentapeptide-treated mice. IPGTTs were conducted in STZ-induced diabetic mice after 20 days of treatment showed a clear reduction in glucose excursions in the treated mice at 30 and 60 min after the load of glucose compared with the control, suggesting improved regulation of hepatic glucose production.
Shao et al. 13 have also further investigated the effects of non-apeptide using a streptozotocin mouse model and found improved glucose tolerance upon treatment with GLP-1 non-apeptide. Consistent with this, in our study, fasting glucose levels were also significantly reduced in GLP-1 (32–36) amide- and exendin-4-treated mice and similar effects were observed histological changes in the pancreas of STZ-induced diabetic mice.
Conclusion and Future Directions
Although the findings reported here are of a preliminary nature, they are the first to describe the tentative identification of a small, active peptide (pentapeptide) derived from GLP-1 that has insulin-like actions on long-term administration to diabetic mice. GLP1 (32–36) amide might prove to be a therapeutic factor that could improve β-cell viability and function. To determine the actions of this GLP-1 pentapeptide in conditions of insulin resistance, further studies of metabolic syndrome in diet-induced obese mouse models are required. Moreover, the pentapeptide might be a cell-penetrating peptide which transported through the plasma membrane into cells, facilitating the epithelial permeation, while GLP-1 is poorly permeable across the intestinal epithelium due to suboptimal physicochemical properties.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No. 81172932 and 81273376), the Natural Science Foundation of Jiangsu Province (No. BK2012356), and the Project Program of State Key Laboratory of Natural Medicines, China Pharmaceutical University (No. JKGZ201103).
Conflict of Interest
All authors report no conflict of interest.




