Triple targeting of RSK, AKT, and S6K as pivotal downstream effectors of PDPK1 by TAS0612 in B-cell lymphomas
Abstract
B-cell lymphomas (BCLs) are the most common disease entity among hematological malignancies and have various genetically and molecularly distinct subtypes. In this study, we revealed that the blockade of phosphoinositide-dependent kinase-1 (PDPK1), the master kinase of AGC kinases, induces a growth inhibition via cell cycle arrest and the induction of apoptosis in all eight BCL-derived cell lines examined, including those from activated B-cell-like diffuse large B-cell lymphoma (DLBCL), double expressor DLBCL, Burkitt lymphoma, and follicular lymphoma. We also demonstrated that, in these cell lines, RSK2, AKT, and S6K, but not PLK1, SGK, or PKC, are the major downstream therapeutic target molecules of PDPK1 and that RSK2 plays a central role and AKT and S6K play subsidiary functional roles as the downstream effectors of PDPK1 in cell survival and proliferation. Following these results, we confirmed the antilymphoma efficacy of TAS0612, a triple inhibitor for total RSK, including RSK2, AKT, and S6K, not only in these cell lines, regardless of disease subtypes, but also in all 25 patient-derived B lymphoma cells of various disease subtypes. At the molecular level, TAS0612 caused significant downregulation of MYC and mTOR target genes while inducing the tumor suppressor TP53INP1 protein in these cell lines. These results prove that the simultaneous blockade of RSK2, AKT, and S6K, which are the pivotal downstream substrates of PDPK1, is a novel therapeutic target for the various disease subtypes of BCLs and line up TAS0612 as an attractive candidate agent for BCLs for future clinical development.
Abbreviations
-
- BCL
-
- B-cell lymphoma
-
- DLBCL
-
- diffuse large B-cell lymphoma
-
- mTOR
-
- mammalian target of rapamycin
-
- PDPK1
-
- 3-phosphoinositide-dependent kinase-1
-
- PKC
-
- protein kinase C
-
- PLK1
-
- polo-like kinase 1
-
- RSK2
-
- ribosomal S6 kinase 2
-
- S6K
-
- p70 S6 kinase
-
- SGK
-
- serum and glucocorticoid-regulated kinase 1
-
- TP53INP1
-
- tumor protein P53-inducible nuclear protein 1
1 INTRODUCTION
B-cell lymphoma (BCL) is one of the most common disease entities among hematological malignancies. It has both frequent and rare disease subgroups, such as aggressive lymphomas, including diffuse large B-cell lymphoma (DLBCL), Burkitt lymphoma (BL), mantle cell lymphoma (MCL), and lymphoblastic lymphoma, and indolent lymphomas, including follicular lymphoma (FL) and marginal-zone BCL.1, 2 The prognosis of most BCL subtypes has been dramatically improved by the advancements of disease subtype-oriented molecular-targeted therapeutics, including monoclonal antibodies (MoAbs) and small molecule compounds, which are adapted according to the immunological, genetic, and/or molecular phenotypes.3, 4 However, the changes and/or dysregulation of either expression pattern, function, or structure of targeted molecule/pathway in tumor cells directly cause the loss of response to target-specific agents,5, 6 and there remain patients incurable with currently available therapeutics. Thus, further development of effective new therapies, regardless of disease subtypes, is still awaited.
3-phosphoinositide dependent protein kinase 1 (PDPK1) is the master serine–threonine kinase of more than 20 AGC kinases, including ribosome S6 kinase (RSK) family proteins, AKT, p70 ribosomal S6 kinase (S6K), glucocorticoid-induced protein kinase (SGK), and protein kinase C (PKC), and several non-AGC kinases.7 By regulating a series of downstream molecules and mediating the RAS/ERK and PI3K/AKT pathways, PDPK1 plays a pivotal role in disease development and progression in various malignancies.8, 9 We previously demonstrated that PDPK1 is a potential novel therapeutic target in MCL, a treatment-difficult subtype of BCL, and multiple myeloma (MM), a mostly incurable mature B-cell malignancy.10, 11 Moreover, among various effector molecules of PDPK1, we demonstrated that RSK2, particularly at its N-terminal kinase domain (NTKD), plays a central role as a downstream signaling mediator of PDPK1, and AKT plays supportive and complementary roles in cell survival and proliferation of tumor cells of MCL and MM.12-14 Importantly, the PDPK1/RSK2 signaling axis is universally active in MCL and MM, and the blockade of this pathway has antitumor effects, regardless of cytogenetic/molecular manifestations in MM.10-14 These evoke the idea of further investigating the values of PDPK1 and its effector molecules, RSK2 and AKT, as universally active therapeutic targets in various types of mature B-cell malignancies in addition to MCL and MM. To answer this question, we first investigated the antitumor efficacy of the blockade of PDPK1 and its downstream effector molecules in various BCLs, such as DLBCL, FL, and BL. Then, we attempted to identify pathophysiologically relevant downstream effector molecules of PDPK1 in BCLs. Then, we tried to evaluate the antitumor efficacy of a novel compound that blocks the therapeutically relevant downstream target molecules of PDPK1 in BCLs, regardless of disease or molecular subtypes.
2 MATERIALS AND METHODS
2.1 Immunohistochemical (IHC) staining of phosphorylated (p-) PDPK1Ser241, p-RSK2Ser227, and p-AKTThr473 in biopsied specimens from patients with BCLs
According to the Declaration of Helsinki, biopsied tumor specimens and medical records of patients with DLBCL (n = 5) and FL (n = 5) were obtained with informed consent. Formalin-fixed and paraffin-embedded tissue samples were subjected to IHC staining with anti-p-PDPK1Ser241 MoAb, anti-p-RSK2Ser227 MoAb, and p-AKTSer473 MoAb (Cell Signaling Technology). These analyses were approved by our institution's Institutional Review Board (IRB) (RBMR-G-124-13).
2.2 Cells and reagents
Eight human BCL-derived cell lines, including two BL-derived cell lines (Daudi and Raji), two activated B-cell-like DLBCL-derived cell lines (HBL1 and DLBCL2), two FL-derived cell lines (FL518 and FL18), and two double-expressor DLBCL-derived cell lines (KPUM-UH1 and KPUM-MS3) were used.15 Cells were maintained in RPMI-1640 (Wako) containing 10% fetal calf serum (Life Technologies), 2-mM L-glutamate, and penicillin/streptomycin at 37°C in humidified 95% air and 5% CO2. The IRB approved the study using patient samples (RBMR-G-124-13) with written informed consent per the Declaration of Helsinki. Briefly, the biopsied tumor cells or bone marrow mononuclear cells were labeled with anti-CD19 MicroBeads and positively isolated using the Mini-MACS separator (Miltenyi Biotec) or EasySep Human CD19 Positive Selection Kit II (VERITAS Corporation). After confirming that more than 95% of the isolated cells were lymphoma cells by flow cytometric analysis, the isolated CD19-positive primary lymphoma cells were resuspended in conventional culture media supplemented with the CellXVivo Human B Cell Expansion Kit (R&D SYSTEMS). An RSK NTKD-specific inhibitor BI-D1870 (Cayman Chemical Company),16 an AKT inhibitor ipatasertib, a p70S6K (S6K) inhibitor PF-4708671 (Selleck Biotech Ltd), and a PDPK1 inhibitor BX-912 (Med Chem Express) were used. TAS0612 was provided by Taiho Pharmaceutical Co., Ltd.17
2.3 Quantitative reverse-transcription polymerase chain reaction (qRT-PCR)
Total RNA was extracted using an RNeasy Mini Kit (Qiagen). Primers were purchased from Hokkaido Systems Science Co., Ltd. (Table S1). qRT-PCR was performed using Fast SYBR Green Master Mix with a StepOnePlus instrument (Applied Biosystems). Transcriptional levels of target genes were adjusted based on that of β-actin (ACTB) using Taqman ACTB control reagents. Three independent experiments were performed for each target gene, and the results are expressed as means ± standard deviations (SDs).
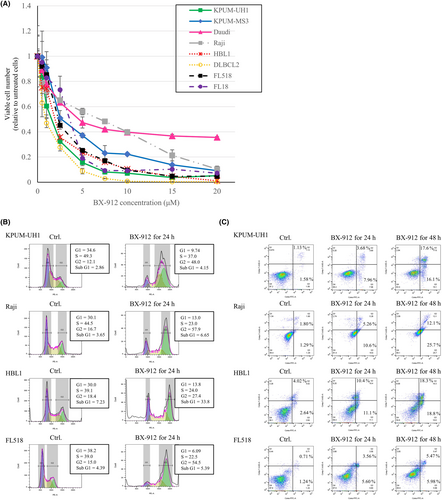
2.4 Analysis of cell proliferation, cell cycle, and apoptosis
Cells were seeded at 2.0 × 105 cells/mL and treated with various concentrations of BX-912, BI-D1870, ipatasertib, PF-4708671, or TAS0612 for various periods up to 72 h. Growth inhibition was analyzed by a modified 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay using a Cell Counting Kit-8 (Dojindo Molecular Technologies). For cell cycle distribution analysis, cells were treated with phosphate-buffered saline containing 0.1% Triton X-100, stained with propidium iodide (PI), and analyzed using flow cytometry. For apoptosis analysis, cells were counterstained with Annexin V–fluorescein isothiocyanate and PI using the Annexin V FITC Assay Kit (Cayman Chemical Company) and subjected to flow cytometric analysis. Data were analyzed using FlowJo (version X; Tomy Digital Biotechnology).
2.5 Western blotting
Western blotting was performed as described previously.10-14 The primary antibodies used were those against total RSK (RSK1/RSK2/RSK3), p-total RSKSer380, RSK2, p-RSK2Ser227, AKT, p-AKTSer473, p-AKTThr308, ERK, p-ERKThr202/Tyr204, PDPK1, p-PDPK1Ser241, PKCβII, p-PKCβIISer660, PLK1, p-PLK1Thr210, PRAS40, p-PRAS40Thr246, S6, p-S6Ser235/236, S6K, p-S6KThr389, SGK, S6, p-S6Ser235/236, TP53, β-Actin (ACTB) (Cell Signaling Technology), p-SGKSer422 (Assay Biotechnology Company, Inc.), TP53INP1 (Abcam), and p27Kip1 (Santa Cruz Biotechnology, Inc.).
2.6 RNA interference (RNAi)
RNA interference of TP53INP1 was performed by transfecting the shRNA plasmid into KPUM-UH1 cells. 1.0 × 106 cells were resuspended in 100 μL of transfection buffer and transfected with shRNA plasmid for TP53INP1 (#sc-76,715-SH) or with control shRNA plasmid (#sc-108060) (Santa Cruz Biotechnology, Inc.) using the Amaxa™ SF Cell Line 4D-Nucleofector™ X Kit (Lonza AG). The shRNA plasmid for TP53INP1 was a pool of three target-specific plasmids designed to knock down TP53INP1 gene expression.
2.7 Analysis of gene expression profile (GEP) and gene set enrichment analysis (GSEA)
Cells were treated with either BX-912 for 12 h or TAS0612 for 6 h at IC80 concentrations, and total RNA was isolated using an RNeasy Mini Kit. The GEP was analyzed using a Clarion S array (Affymetrix), a GeneChip WT Plus Reagent Kit (Thermo Fisher Scientific), and a GeneChip Scanner 7G (Affymetrix). Data were analyzed using the Affymetrix Transcriptome Analysis Console (TAC) software (ver. 4.0.0.25), and robust multiarray (RMA) normalization was performed with default parameters using TAC programming. GSEA was performed using the GSEA software (https://www.gsea-msigdb.org/gsea/index.jsp).18
2.8 Assay for the drug combinatory effect
Cells sensitive to the agents of interest were treated with eight concentrations (i.e., 0.25, 0.5, 0.75, 1.0, 1.25, 1.5, 1.75, and 2.0 × IC50) of either BI-D1870 or combinatory agent or with both agents for 48 h and were subjected to a modified MTT assay. Fractional effect concentrations (i.e., a fractional effect of 0.25 equals a 25% growth inhibitory effect) and the combination index (CI) were calculated using CalcuSyn (Biosoft). This method facilitates the quantification of synergism (CI <1) and antagonism (CI >1) at different doses and effect levels. CI calculations were performed assuming that drug mechanisms were not mutually exclusive.
2.9 Statistical analysis
Statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University) (The R Foundation for Statistical Computing).19 Student's t-test was used to compare continuous variables between groups, and p-values <0.05 denote statistical significance.
3 RESULTS
3.1 The activation status of PDPK1Ser241 and downstream effector molecules in various types of BCL
We first evaluated the activation status of PDPK1, RSK2, and AKT by investigating the phosphorylation status of PDPK1Ser241, RSK2Ser227, and AKTThr473 in patient-derived tumor tissues of DLBCL and FL. Of five specimens of DLBCL, p-PDPK1Ser241 was strongly positive in two (#1 and #2), moderately positive in one (#3), and weakly positive in two (#4 and #5). p-RSK2Ser227 at RSK2-NTKD was moderately to strongly positive in all specimens, and p-AKTThr473 was moderately positive in one (#1) and weakly positive in two (#2 and #5) but was negative in the remaining specimens (#3 and #4). In five FL specimens, p-PDPK1Ser241 was weakly to moderately positive in four (#6–9) and negative in one (duodenum FL) (#10). p-RSK2Ser227 was weakly to strongly positive in three specimens (#6, 8, and 9), and p-AKTThr473 was weakly to moderately positive in three specimens (#6, 7, and 9) but was negative in the remaining specimens. Thus, PDPK1Ser241, RSK2Ser227, and AKTThr473 are frequently active in DLBCL and FL. Furthermore, at least one of PDPK1Ser241, RSK2Ser227, and AKTThr473 was active in most patient-derived samples (Figure 1A).
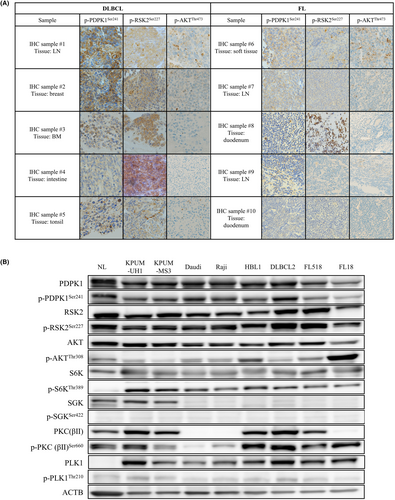
Then, we investigated the activation status of PDPK1 and its major downstream molecules, that is, RSK2, AKT, S6K, SGK, PKC, and PLK1, in eight cell lines derived from four subtypes of BCLs. As shown in Figure 1B, PDPK1, RSK2-NTKD, and S6K were commonly active in all eight cell lines examined, whereas the activation status of AKT and PKC varied among cell lines and was cellular context dependent. PLK was faintly active, and SGK was inactive in all cell lines examined.
3.2 Cellular and molecular effects of the inactivation of PDPK1 in BCL-derived cell lines
Next, we investigated the cellular effect of PDPK1 inactivation in eight BCL-derived cell lines using BX-912 as an inhibitor of PDPK1. BX-912 treatment dose-dependently induced growth inhibition in all eight cell lines examined (Figure 2A), accompanied by the blockade of G2/M and the induction of apoptosis, regardless of the disease subtype (Figures 2B,C).
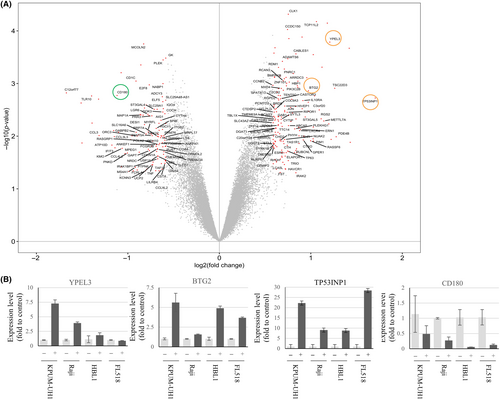
Then, we investigated the molecular effects of PDPK1 blockade on four cell lines of diverse disease subtypes, that is, KPUM-UH1, Raji, HBL1, and FL518 cells. At the gene expression level, GEP analysis with microarray revealed that 27 and 18 genes were commonly upregulated >1.5 folds (Table S2) and downregulated <0.67 folds (Table S3), respectively, by PDPK1 blockade at the transcription level. The commonly upregulated genes included several tumor suppressor genes, such as YPEL3, BTG2, and TP53INP1, which encode a bona fide stress-induced protein (Figure 3A). qRT-PCR validated the results of these microarray analyses, except for YPEL3 in FL518 cells (Figure 3B).
3.3 RSK2 is the central effector, and AKT and S6K are the subsidiary effectors of PDPK1 downstream in the proliferation of BCL-derived cell lines
Next, we attempted to identify functionally responsible downstream molecules of PDPK1 in the survival and proliferation of BCL-derived cell lines. For this purpose, we first examined the impact of PDPK1 inactivation on the activation status of its primary target molecules in BCL-derived cell lines of different subtypes. Treatment with BX-912 resulted in the dephosphorylation of RSK2 in all four cell lines examined. Moreover, treatment with BX-912 resulted in AKT and S6K dephosphorylation in cell lines with active AKT and/or S6K at the basal level. In contrast, BX-912 did not significantly or consistently reduce the phosphorylation status of SGK, PKC, or PLK1 (Figure 4A). These results reveal RSK2, AKT, and S6K as the relevant effector molecules of PDPK1 in BCLs. Moreover, the inactivation of RSK2-NTKD by its specific inhibitor BI-D1870 exerted universal and robust dose-dependent antiproliferative effects on all eight BCL-derived cell lines examined. In contrast, the growth inhibitory effects of exposure to ipatasertib, an inhibitor for AKT, and PF-4708671, an inhibitor for S6K, for 48 h were largely modest and varied among cell lines (Figure 4B).
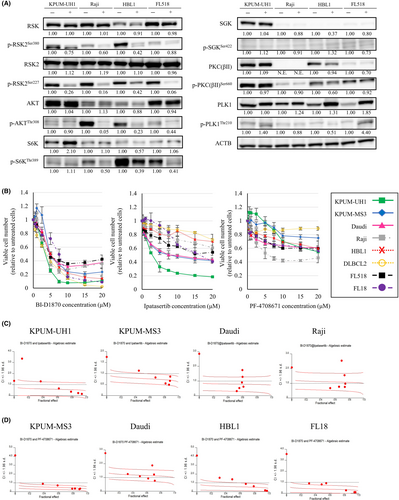
Based on these findings, we next examined the antiproliferative effects of the combinatory blockade RSK2-NTKD and either AKT or S6K on four cell lines with various sensitivities to ipatasertib and PF-4708671. As a result, the combinatory blockade of RSK2 and either AKT or S6K showed at least additive and synergistic effects in most settings on various types of BCL-derived cell lines (Figure 4C,D). These collectively indicate that RSK2, AKT, and S6K are the major downstream target molecules of PDPK1 and that RSK2-NTKD plays a central role and AKT and S6K play subsidiary functional roles as the downstream effectors of PDPK1 in the proliferation of BCL-derived cell lines.
3.4 Antitumor effect of TAS0612 in BCL-derived cell lines and patient-derived primary lymphoma cells
Based on the robust antilymphoma efficacy of the simultaneous blockade of RSK2 and AKT or S6K, we next examined the antitumor effect of TAS0612, the triple inhibitor against total RSK, including RSK2, AKT, and S6K.17 As shown in Figure 5A, TAS0612 treatment showed dose-dependent growth inhibitory effects on all eight BCL-derived cell lines, regardless of the disease subtype, with IC50 concentrations ranging from 0.41 μM to 6.73 μM. This growth inhibitory effect of TAS0612 was accompanied by the accumulation of its direct substrates p-total RSKSer380, p-AKTSer473, and p-S6KThr389 in most cell lines examined and the subsequent dephosphorylation of their respective target substrates in all cell lines examined. Furthermore, no feedback reactivation of ERK was observed following TAS0612 treatment (Figure 5B). In the process of growth inhibition, TAS0612 induced G1 cell cycle blockade in KPUM-UH1 cells accompanied by the accumulation of p-AKTSer473 and upregulation of p27Kip1; G2/M cell cycle blockade in Daudi, HBL1, and FL18 cell lines accompanied by the accumulation of phosphorylated of total RSKSer380; and the induction of apoptosis in all cell lines (Figure 5B–D, and Table S4).
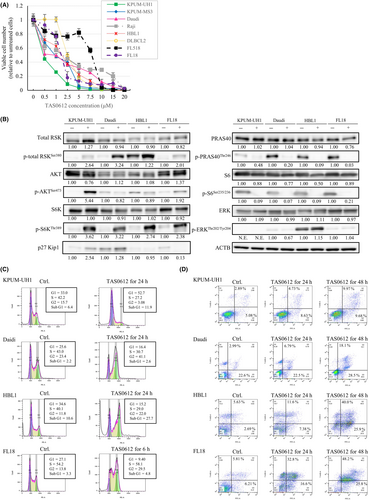
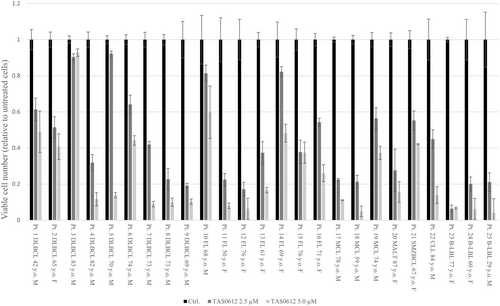
We also investigated the ex vivo antiproliferative effect of treatment with 2.5 and 5.0 μM TAS0612 for 72 h in patient-derived lymphoma cells of various disease subtypes. As a result, TAS0612 treatment showed an antitumor effect on all 25 patient-derived lymphoma cells examined, including nine DLBCLs, seven FLs, three MCLs, one mucosa-associated lymphoid tissue lymphoma, one splenic marginal-zone BCL, one chronic lymphocytic leukemia, and three B-lymphoblastic lymphomas (Figure 6).
3.5 Molecular effects induced by TAS0612 on BCL-derived cell lines
We examined the global molecular effects of TAS0612 using GEP on two cell lines derived from DEL, that is, KPUM-UH1 and KPUM-MS3 cells. TAS0612 treatment with IC80 concentrations for 6 h commonly resulted in a >2.0-fold increase in 222 genes (Table S5) and a <0.5-fold decrease in 296 genes (Table S6) between KPUM-UH1 and KPUM-MS3 cells (Figure 7A). Among several tumor suppressor genes upregulated by TAS0612 in both KPUM-UH1 and KPUM-MS3 cells, the increased expression of tumor protein 53-inducible nuclear protein 1 (TP53INP1) by treatment with TAS0612 was also confirmed at the transcriptional (Figure 7B) and protein (Figure 7C) levels in cell lines of other disease subtypes, that is, Daudi, HBL1, and FL18 cells. Furthermore, the upregulation of TP53INP1 was not accompanied by TP53 accumulation (Figure 7C).
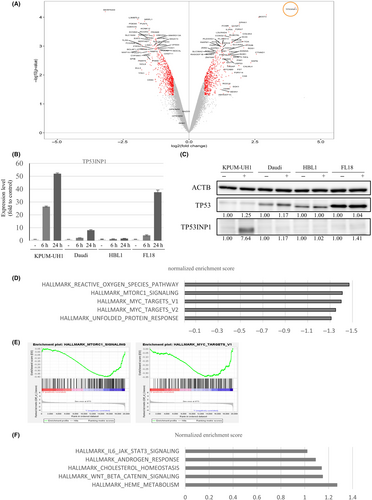
Next, we investigated functionally relevant changes in gene sets caused by the treatment of KPUM-UH1 and KPUM-MS3 cells using GSEA. As judged by the normalized enrichment score, TAS0612 significantly downregulated the gene sets driven by the upregulation of MYC and mTORC1, reactive oxygen species, and the unfolded protein response (Figure 7D,E). Simultaneously, significant gene sets upregulated by TAS0612 treatment included those for interleukin (IL)-6/JAK–STAT and WNT/βcatenin signaling (Figure 7F). We finally examined the functional role of TP53INP1 in the antitumor effect of TAS0612 using RNAi technique. As a result, the antitumor effect of TAS0612 was not significantly different between control cells and TP53INP1 knockdown KPUM-UH1 cells (Figure S1).
4 DISCUSSION
PDPK1 has been proposed as a therapeutic target molecule based on its functional involvement in disease development and aggressiveness, tumor cell dissemination, and therapeutic resistance in various solid cancers and sarcomas7, 8, 20-24; however, this is the first report to show the involvement of PDPK1 in the survival and proliferation of lymphoma cells. This study is also the first to show the universal antitumor effect of the blockade of PDPK1 on BCLs, regardless of disease subtypes, in addition to MCL.10 However, therapeutic targeting of PDPK1 may cause various unnecessary effects because PDPK1 regulates more than 20 downstream functional kinases associated with pleiotropic biological processes, such as immunity, bone formation, glucose metabolism, renal sodium transport, pH regulation, and mitochondrial activity.25-33
The on- and/or off-target-related adverse events remain almost unavoidable in cancer treatment using the molecular-targeted kinase inhibitors34-36; however, we attempted to select an effector molecule responsible for the oncogenic property of PDPK1 to preserve antilymphoma efficacy and to avoid unnecessary adverse events as much as possible. Thus, it was critically important to find that, among a series of downstream effector molecules of PDPK1, RSK2 plays a central role, and AKT and S6K play subsidiary functional roles in the proliferation of BCL-derived cells. While the operational involvement of AKT signaling has been repeatedly reported in association with lymphomagenesis and therapeutic resistance in various BCL types,37-40 information about the pathophysiological involvement of RSK2 in BCLs has been somewhat limited to our previous reports with MCL and MM until today,11, 12 and our study for the first time highlighted the universal functional importance of RSK2 in various BCL types. RSK2, as the downstream signaling mediator of the RAS/ERK pathway, has been shown to play critical roles in oncogenesis and tumor progression in various cancerous diseases, using its NTKD as an output hub to activate its series of oncogenic substrates.41-44 The involvement of S6K in lymphomagenesis has rarely been reported; however, S6K is also known to be involved in tumorigenesis in various cancers.45
Based on these findings, we examined and successfully demonstrated the antilymphoma efficacy of the RSK/AKT/S6K triple inhibitor TAS0612 by modulating cell cycle regulation and inducing apoptosis in BCL-derived cell lines. Although we did not perform an in vivo mice model study due to the lack of a reliable disease model with our cell lines, we showed the ex vivo efficacy of TA0612 in patient-derived B-lineage lymphoma cells of various disease subtypes, and the clinical activity and safety of TAS0612 are currently under evaluation in a clinical trial involving patients with locally advanced or metastatic solid tumors.46 Collectively, this study is the first to preclinically investigate its usage against hematological malignancies, suggesting TAS0612 as a novel therapeutic player for various disease subtypes of BCLs. The effect of TAS0612 on cell cycle regulation was diverse among cell lines; TAS0612 treatment caused G1/S blockade in two DEL cell lines and G2/M blockade in the other BCL cell lines examined in this study. Considering that the blockade of RSK2 by BI-1870 induces G2/M blockade,12, 13 while the blockade of AKT induces G1/S arrest in general via p27Kip1 activation,47-49 the cellular context-dependent different impacts on cell cycle redistribution caused by TAS0612 may depend on the various degrees of functional dependence on RSK, AKT, and S6K in each cell type. This bidirectional property of TAS0612 may be one of the underlying mechanisms for its broad efficacy against various subtypes of BCL-derived cells with diverse molecular/genetic features.
Our comprehensive molecular analyses indicated several mechanisms underlying the universal antitumor efficacy of TAS0612 against a broad range of BCL types with diverse genetic and molecular profiles. First, at the molecular level, RSK/AKT/S6K triple inhibition significantly upregulated the antioxidant tumor suppressor TP53INP1, which is also induced by PDPK1 inhibition. TP53INP1 is induced by various cellular stresses, such as chemotherapeutic toxicity, heat shock, alcohol, inflammation, high-fat diet, and, most remarkably, oxidative stress.50-52 In contrast, the direct interaction between TP53INP1 and either PDPK1, RSK, AKT, or S6K has remained unknown. Functionally, TP53INP1 enhances TP53 protein stability and transcriptional activity through the phosphorylation of TP53Ser46 via two protein kinases, HIPK2 and pPKC, and thereby induces G1/S cell cycle blockade, apoptotic cell death, and autophagy.50-52 The expression level of TP53INP1 has been reported to be decreased in most types of cancers, except for prostate and thyroid cancers.51, 53, 54 In our study, the knockdown of TP53INP1 gene was not associated with the growth inhibitory effect by TAS0612 in KPUM-UH1 cells, indicating the induction of TP53INP1 was not a necessary event but a secondary molecular event in the antitumor effect of TAS0612. By contrast, the functional involvement of TP53INP1 upregulation has been reported in cases with the antitumor effect by the blockade of EZH in B-cell malignancies, such as B-cell acute lymphoblastic leukemia and MCL.55, 56 Future research is needed to investigate the role of TP53INP1 modulation in improving treatment outcome of B-cell malignancies in the era of molecular-targeted therapy.
Furthermore, our study showed that treatment with TAS0612 significantly downregulated a series of MYC and mTOR target genes, consistent with previous findings in myeloma cells induced by the simultaneous blockade of RSK2 and AKT.14 Considering the pivotal roles of MYC and mTOR in lymphomagenesis, disease aggressiveness, and treatment resistance in BCLs,37, 57-59 the simultaneous downregulation of MYC- and mTOR-associated genes may be essential in inducing the antilymphoma effect of TAS0612 in BCLs, regardless of disease subtype. Furthermore, the downregulation of a series of MYC-regulated genes and genes involved in mTOR signaling and/or the blockade of the WNT signaling pathway presumably underlies the upregulation of TP53INP1.60-62 In conclusion, this study provides a rationale for the simultaneous blockade of RSK2, AKT, and S6K, which are the pivotal downstream substrates of PDPK1, as a novel therapeutic target for various disease subtypes of BCLs. In this regard, TAS0612 is an attractive candidate agent for BCLs, and future clinical development is expected.
AUTHOR CONTRIBUTIONS
Contribution: Y.K-T. performed experiments, analyzed the data, and drafted the manuscript. S.M. assisted and supervised the experiments. Y.K–K., A.M., R.I., H.O., T.F., K.M., and Y.S. supported the experiments and analyzed the data. A.O., A. M-H., and E.K. performed histopathological experiments. T.T. analyzed the data. M.T. handled the equipment. J.K. designed and supervised the research and drafted the manuscript.
ACKNOWLEDGMENTS
The authors greatly thank all participating patients and their families. We thank Ms. Mizushima K and Inada N for their excellent technical support.
FUNDING INFORMATION
This work was supported by JSPS KAKENHI Grant Number 22K08512, the Japanese Society Hematology Research Grant, the Japan Leukemia Research Fund Award, the Japanese Society for Myeloma Research Award (JK), and a grant-in-aid from Public Promoting Association Asano Foundation for Studies on Medicine (SM).
CONFLICT OF INTEREST STATEMENT
This work was partly supported by Taiho Pharmaceutical Co., Ltd. J.K. has received research funding from Kyowa Kirin, Chugai Pharmaceutical, Daiichi Sankyo Pharmaceutical, Eisai, Ono Pharmaceutical, Taiho Pharmaceutical, Sumitomo Pharma, Shionogi Pharmaceutical, and Bristol Myers Squibb (BMS), has received honoraria from Janssen Pharmaceutical, Chugai Pharmaceutical, Ono Pharmaceutical, Sanofi, and BMS, and is a consultant for Janssen Pharmaceutical, BMS, Asahikasei Pharma, and Pfizer. All other authors have no conflicts of interest.
ETHICS STATEMENT
Approval of the research protocol by an Institutional Reviewer Board: All of these research analyses were approved by our institute's Institutional Review Board (IRB) (RBMR-G-124-13). According to the Declaration of Helsinki, the biopsied tumor specimens and the patient-derived lymphoma cells used in this study were obtained with informed consent.
Informed Consent for Case Reports: N/A.
Registry of the Study/Trial: N/A.
Animal Studies: N/A.




