Evaluating the effect of childhood sunburn on the risk of cutaneous melanoma through Mendelian randomization
Abstract
Despite numerous observational studies indicating an increased risk of cutaneous melanoma (CM) due to childhood sunburn, no studies have established a definitive cause-and-effect relationship. Therefore, our objective was to employ a Mendelian randomization (MR) design to explore a possible causal association between childhood sunburn and the risk of CM. To investigate the causal relationship between childhood sunburn and CM, we used large-scale genetic summary-level data from genome-wide association studies (GWAS), including childhood sunburn (n = 346,955) and CM (n = 262,288), building upon previous observational studies. In the analysis, we mainly used the inverse-variance weighted (IVW) method of the random effects model, supplemented by the weighted median method and MR-Egger method. The results of the IVW method demonstrated that genetically predicted childhood sunburn was significantly associated with higher odds of CM, with an odds ratio (OR) of 2.418 (95%CI, 1.426–4.099; p = .001). The weighted median method and MR-Egger regression also demonstrated directionally similar results (both p < .05). Furthermore, both the funnel plot and the MR-Egger intercepts showed the absence of directional pleiotropy between childhood sunburn and CM. Our study offers potential evidence linking genetically predicted childhood sunburn with CM, underscoring the need for individuals with a history of childhood sunburn to be extra vigilant regarding the occurrence of CM.
Abbreviations
-
- Cl
-
- confidence interval
-
- CM
-
- cutaneous melanoma
-
- GWAS
-
- genome-wide association studies
-
- IVs
-
- instrumental variables
-
- IVW
-
- inverse variance weighted
-
- LOO
-
- leave-one-out
-
- MR
-
- Mendelian randomization
-
- OR
-
- odds ratio
-
- SD
-
- standard deviation
-
- SNP
-
- single-nucleotide polymorphism
-
- UKBB
-
- UK Biobank
1 INTRODUCTION
Melanoma is a prevalent cancer worldwide, particularly in the form of cutaneous melanoma (CM), which originates from the basal layer of the skin's epidermis.1 This malignancy occurs when the melanocytes responsible for producing pigment in the skin undergo a malignant transformation in the epidermis.2 According to a recent global survey,3 there were 325,000 new cases of melanoma worldwide in 2020, with 174,000 cases in men and 151,000 cases in women. Additionally, the survey estimated 57,000 deaths, with 32,000 occurring in males and 25,000 in females. These estimates suggest a significant increase in melanoma cases by 2040, with an estimated 510,000 new cases (an increase of roughly 50%) and 96,000 deaths (an increase of 68%). These statistics demonstrate that melanoma is growing at a faster rate than any other type of cancer, making it a significant challenge for global cancer control and public health.
The development of melanoma is multifactorial and arises from a complex interaction between genetic susceptibility and environmental exposures. Despite advancements in research, sunlight exposure remains a major risk factor for melanoma.1 In 1987, a report4 emphasized the importance of increasing public awareness regarding the link between sunburn and CM. A recent cohort study5 conducted in Norway supports this recommendation by highlighting the association between frequent sunburns throughout one's lifetime and an increased risk of developing melanoma. While it is widely accepted that sunburn increases the risk of melanoma, most studies tend to focus on the association between general sunburn and melanoma, often neglecting the specific impact of childhood sunburn on the development of advanced melanoma in adulthood. Childhood sunburn is of particular interest, as it has been suggested to have a more noteworthy effect on melanoma risk compared with sunburn episodes in later life.
Further research is necessary to establish a causal link between childhood sunburn and CM. However, conducting large, long-term randomized studies is challenging due to the high costs and difficulties in maintaining randomization. Longitudinal studies with extended follow-up periods are needed to understand the time lag between sunburn exposure and the development of melanoma. However, these studies face practical obstacles such as the significant financial and logistical resources required to maintain contact with participants and achieve high follow-up rates. Instead, the epidemiological Mendelian randomization (MR)6 offers a viable alternative. By utilizing genetic variants as proxies for risk factors, we can leverage genetic variants associated with childhood sunburn as instrumental variables (IVs) to circumvent the need for prolonged follow-up, thereby making it a more feasible and cost-effective approach to assess the causal relationship between childhood sunburn and melanoma occurrence. MR analysis is an innovative epidemiological approach that is less susceptible to potential confounding and reverse causation. This is attributed to the use of genetic variants as IVs. By conducting a MR analysis, we can specifically examine the causal relationship between childhood sunburn and the development of melanoma, thereby providing a more comprehensive understanding of this specific risk factor.
Furthermore, observational studies have inherent limitations in their capacity to consider potential confounding factors and reverse causation, which can result in divergent conclusions. In accordance with Mendel's second law,7 genetic variations that are present from conception are randomly distributed and generally unaffected by environmental risk factors, developing before the onset of diseases and other factors. This concept is comparable to the design principle utilized in clinical randomized controlled trials. Consequently, MR can effectively address the issue of reverse causality, as allelic randomization occurs prior to the disease onset.
In light of the inconsistent results and potential methodological limitations of previous observational studies on childhood sunburn and CM, we conducted a two-sample MR study (Figure 1).8 This approach allowed us to comprehensively evaluate causation and increase statistical power by analyzing the causal association between response to childhood sunburn (exposure) and CM (outcomes) in individuals of European descent using multiple large-scale genome-wide association studies (GWAS) datasets.9
2 MATERIALS AND METHODS
2.1 Study design
Mendelian randomization analysis uses a group of single-nucleotide polymorphisms (SNPs) as IVs to infer the causal effect of an exposure on an interesting outcome. In general, MR analysis needs to meet three fundamental assumptions: (1) the IVs should be significantly associated with the exposure; (2) the IVs should be independent of potential confounders; and (3) the IVs should not affect the outcome except through the exposure. The overall design of this study is shown in Figure 2. We used a two-sided p-value, with statistical significance set at p < 0.05. All statistical tests were performed in R (version 4.2.2) using the TwoSampleMR package (version 0.5.0).10

2.2 Genome-wide association studies data for childhood sunburn
The GWAS data for childhood sunburn were derived from the UK Biobank (UKBB), which is a prospective cohort study that covered more than 500,000 individuals from the UK aged between 40 and 70 years. The UKBB study collected self-reports of individuals of European ethnicity about childhood sunburn occasions. In detail, the childhood sunburn occasions were retrieved based on the item: “Before the age of 15, how many times did you suffer sunburn that was painful for at least 2 days or caused blistering?”, and the answers were between 0 and 20. If it is >20, it needed to be confirmed with the participants. Specifically, we retrieved the GWAS dataset of childhood sunburn from the MRC integrative epidemiology unit (IEU) GWAS database (https://gwas.mrcieu.ac.uk/datasets/), which recruited 346,955 people from the UKBB cohort after the exclusion of individuals of non-European ancestry, closely related individuals (or at least one of a related pair of individuals), individuals with sex chromosome aneuploidies, and individuals who had withdrawn consent from the UKBB study. Based on the utilization of aggregated-level data in our research, which lack specific biological individual information, and in accordance with the data usage policy of the UKBB GWAS results, no further authorization was necessary for the utilization of UK biological database data in this study (https://www.nealelab.is/uk-biobank/faq).
2.3 Genome-wide association studies data for CM
The GWAS data for CM were derived from the FinnGen biobank (https://www.finngen.fi/fi), which had collected biological samples from 500,000 participants in Finland over 6 years. We used the data from the R8 release, where CM was defined based on the International Classification of Diseases 8th to 10th codes. After removing individuals with ambiguous gender, high genotype missingness (>5%), excess heterozygosity (4 SD), and non-Finnish ancestry, the dataset contained 2705 CM cases and 259,583 controls.
2.4 Instrumental variable selection
To ensure the validity of the IVs included in the MR analysis, we set the following screening criteria to extract eligible SNPs from the GWAS dataset (childhood sunburn). First of all, we selected SNPs that were significantly associated with our exposure (p < 5 × 10−8). Based on the European panel of the 1000 Genomes Project, we then pruned the significant IVs within a 10,000-kb window at linkage disequilibrium (LD) r2 < 0.001 to make the IVs relatively independent. For each identified exposure (childhood sunburn) SNP instrument, we extracted the per-allele beta coefficient for CM together with its standard error and p-value from the CM GWAS data. IVs that were identified as significantly associated with the outcome phenotype (CM) were eliminated (p < 5 × 10−8). Then, we unified the SNP instruments by coordinating the directions of the exposure SNPs and outcome SNPs alleles and excluded incompatible SNPs or palindromic SNPs whose direction could not be determined. Finally, to limit bias due to weak IVs, the F statistics had to be >10. Calculation of the F statistics has been described elsewhere.11
2.5 Mendelian randomization analyses
Single-sample MR analysis and dual-sample MR analysis are both methods used in observational studies to estimate the causal impact of exposure on outcome variables. In a one-sample MR analysis, data from a single population are used, which simplifies the analysis process. MR and traditional epidemiological results can be compared within the same individual. The disadvantage of this method is that the selection of IVs is relatively limited and susceptible to potential confounding factors. If the correlation between genetic variation and exposure is weak, it will cause the estimated values of IVs to shift toward the right confounding serious association direction, resulting in an increase in false positives (type 1 error rate). In two-sample MR studies, two independent GWAS data samples are selected from the same population, including association studies of genetic variation exposure factors and genetic variation outcome variables. The study population consists of two independent samples from the same population, such as GWAS studies on exposure factors and GWAS analyses on outcome variables. In other words, the dataset has two sources: the association between genetic variation and endogenous variables (variant–exposure associations) is estimated in the first dataset, while the association between genetic variation and outcome variables (variant–outcome associations) is estimated in another dataset. Two-sample MR improves statistical efficiency compared with single-sample MR and minimizes false-positive results as much as possible.12
To this end, we used a two-sample MR strategy in which the SNP exposure and SNP outcome associations were estimated using summary statistics from independent samples. In this study, we utilized three MR methods to estimate the causality between childhood sunburn and CM, including the inverse-variance weighted (IVW) method,13 the weighted median method,14 and the MR-Egger regression.15 The IVW method uses a weighted linear regression (Wald ratio of each SNP) of SNP-exposure coefficients and SNP-disease coefficients to estimate the effect of exposure on outcome. This method minimizes the mean variance and is often used in meta-analyses to integrate independent measurement results. The weighted median estimation provides accurate estimates if at least half of the IVs are valid. The MR-Egger regression assumes that all the IVs are invalid and is the least powerful in causality inference. It typically serves as the validation of the direction of the effects.
2.6 Sensitivity analyses
Notably, two-sample MR using multiple IVs for causality inference could be potentially biased by pleiotropy. Therefore, we conducted sensitivity analyses to evaluate the reliability of our results. The Cochran Q-test was applied to check SNPs' statistical heterogeneity using IVW estimates, with p < 0.05 deemed significantly heterogeneous. Funnel plots were used to judge heterogeneity visually. Besides, we evaluated the horizontal pleiotropy using the MR-Egger intercept term. MR-Egger is a weighted regression approach that provides the intercept to assess global pleiotropic effects. Horizontal pleiotropy was observed when the intercept term was significantly away from zero (intercept with p < 0.05).16 We then performed a leave-one-out (LOO) analysis by excluding each SNP in turn and repeated IVW estimation. If a certain SNP is eliminated, the result changes greatly, indicating that this SNP is an outlier and needs to be eliminated.
2.7 Power calculation
Power calculation for the IVW estimates was based on a web tool at https://shiny.cnsgenomics.com/mRnd/.17 Specifically, a sufficient statistical power is recommended to be over 80%.
2.8 Ethical approval
The data we used were obtained from published studies approved by the corresponding ethics committee; thus, no further ethical approval was required for this study.
3 RESULTS
3.1 The causal effect of childhood sunburn on CM risk
By screening and selecting SNPs based on the above criteria, we identified 65 eligible SNPs for MR analysis. The 65 selected index SNPs collectively explain 2.83% phenotypic variance of childhood sunburn, and the F statistics were above 10 (ranging from 29.7 to 3674.2, with an average of 151.9) (Table 1), largely ruling out the possibility of weak instrument bias.18
| SNP | effect_allele.exposure | other_allele.exposure | beta. exposure | se.exposure | pos.exposure | pval.exposure | F statistic | Variance explained |
|---|---|---|---|---|---|---|---|---|
| rs10202908 | T | C | −0.0126044 | 0.00227125 | 169,378,292 | 2.90E−08 | 30.7972409 | 8.88E−05 |
| rs10220751 | G | T | −0.0136896 | 0.00216389 | 47,923,520 | 2.50E−10 | 40.0228692 | 0.00011534 |
| rs10788627 | C | T | 0.0120186 | 0.00212884 | 82,203,069 | 1.60E−08 | 31.8727119 | 9.19E−05 |
| rs10810636 | G | A | 0.0275088 | 0.00249423 | 16,799,109 | 2.80E−28 | 121.637513 | 0.00035047 |
| rs10873552 | G | A | 0.0123545 | 0.00224357 | 105,433,129 | 3.70E−08 | 30.3227338 | 8.74E−05 |
| rs10896139 | T | C | −0.0136339 | 0.00240498 | 66,650,060 | 1.40E−08 | 32.1376941 | 9.26E−05 |
| rs11070811 | T | C | −0.0176101 | 0.00274862 | 31,394,082 | 1.50E−10 | 41.0480079 | 0.0001183 |
| rs11104733 | T | C | 0.0491816 | 0.00885213 | 88,490,119 | 2.80E−08 | 30.8679278 | 8.90E−05 |
| rs111391498 | G | A | −0.0473829 | 0.00497472 | 1,341,553 | 1.70E−21 | 90.7200048 | 0.00026141 |
| rs112089506 | T | C | −0.0580859 | 0.00400897 | 90,149,171 | 1.40E−47 | 209.929423 | 0.0006047 |
| rs11242899 | A | G | −0.0244788 | 0.0024111 | 460,302 | 3.20E−24 | 103.073572 | 0.00029699 |
| rs116125333 | G | T | −0.0397082 | 0.00719516 | 33,930,012 | 3.40E−08 | 30.4562994 | 8.78E−05 |
| rs11648436 | T | C | −0.0272738 | 0.00221874 | 14,008,674 | 9.90E−35 | 151.104135 | 0.00043533 |
| rs117132860 | A | G | 0.0593054 | 0.00672945 | 17,134,708 | 1.20E−18 | 77.6651158 | 0.0002238 |
| rs11739906 | C | A | 0.0130466 | 0.00226464 | 59,019,359 | 8.40E−09 | 33.1889861 | 9.56E−05 |
| rs117462393 | T | C | 0.0814822 | 0.00955355 | 14,277,804 | 1.50E−17 | 72.7435364 | 0.00020962 |
| rs12203592 | T | C | 0.153131 | 0.00252627 | 396,321 | 1.00E−200 | 3674.21204 | 0.01047897 |
| rs1233578 | G | A | −0.0205868 | 0.00278697 | 28,712,247 | 1.50E−13 | 54.5645105 | 0.00015724 |
| rs12350739 | A | G | 0.0354641 | 0.00219034 | 16,885,017 | 5.80E−59 | 262.151606 | 0.00075501 |
| rs1260326 | C | T | −0.0125281 | 0.00217252 | 27,730,940 | 8.10E−09 | 33.2537399 | 9.58E−05 |
| rs1267038 | C | A | −0.0178889 | 0.00299112 | 162,099,153 | 2.20E−09 | 35.7682962 | 0.00010308 |
| rs1278766 | C | T | 0.0191192 | 0.00213886 | 113,534,382 | 3.90E−19 | 79.9046733 | 0.00023025 |
| rs12913832 | G | A | 0.0605377 | 0.00253089 | 28,365,618 | 1.90E−126 | 572.140717 | 0.00164633 |
| rs1308048 | C | T | −0.0203501 | 0.00216433 | 66,888,542 | 5.30E−21 | 88.406369 | 0.00025474 |
| rs13332673 | T | G | −0.102103 | 0.0105348 | 89,940,386 | 3.30E−22 | 93.9338054 | 0.00027067 |
| rs139414522 | C | T | −0.0783884 | 0.00906851 | 89,821,155 | 5.40E−18 | 74.7185381 | 0.00021531 |
| rs141817469 | T | C | −0.0931955 | 0.00581987 | 89,927,151 | 1.00E−57 | 256.425271 | 0.00073853 |
| rs142314514 | G | A | −0.0815926 | 0.00702727 | 90,130,996 | 3.60E−31 | 134.810988 | 0.00038841 |
| rs1437635 | A | C | 0.0206986 | 0.00286686 | 16,358,722 | 5.20E−13 | 52.1274573 | 0.00015022 |
| rs150527451 | A | G | 0.0262396 | 0.00347045 | 68,817,897 | 4.00E−14 | 57.1664548 | 0.00016474 |
| rs1548714 | C | A | 0.0161579 | 0.00272296 | 26,280,204 | 3.00E−09 | 35.2116697 | 0.00010148 |
| rs17232484 | A | G | 0.0610238 | 0.00907405 | 89,861,650 | 1.80E−11 | 45.2267079 | 0.00013034 |
| rs1989483 | G | A | 0.0124961 | 0.00218968 | 16,942,661 | 1.20E−08 | 32.5675449 | 9.39E−05 |
| rs251468 | T | C | −0.0281983 | 0.00247434 | 149,194,485 | 4.40E−30 | 129.874594 | 0.00037419 |
| rs2737217 | G | A | −0.0254555 | 0.00215785 | 116,630,311 | 4.10E−32 | 139.161396 | 0.00040094 |
| rs3213737 | A | G | −0.0318659 | 0.00215925 | 96,379,806 | 2.70E−49 | 217.793084 | 0.00062734 |
| rs35563099 | T | C | −0.0310309 | 0.00291491 | 119,572,403 | 1.80E−26 | 113.327568 | 0.00032653 |
| rs3759579 | G | A | 0.0124491 | 0.00215924 | 103,851,272 | 8.10E−09 | 33.2407961 | 9.58E−05 |
| rs41563 | A | G | 0.0135055 | 0.00223217 | 104,852,654 | 1.40E−09 | 36.6070566 | 0.0001055 |
| rs4240559 | C | T | −0.0171048 | 0.00214258 | 98,437,775 | 1.40E−15 | 63.732399 | 0.00018366 |
| rs4272574 | T | C | 0.0152324 | 0.00212901 | 73,661,205 | 8.40E−13 | 51.189364 | 0.00014752 |
| rs4335021 | C | T | −0.0117908 | 0.00216154 | 32,386,619 | 4.90E−08 | 29.7548469 | 8.58E−05 |
| rs4578351 | C | T | −0.0204813 | 0.00257235 | 16,587,580 | 1.70E−15 | 63.3946901 | 0.00018269 |
| rs4670813 | A | G | −0.0126688 | 0.00214417 | 38,317,710 | 3.50E−09 | 34.9101066 | 0.00010061 |
| rs4840542 | T | G | 0.0185092 | 0.00213294 | 10,944,809 | 4.00E−18 | 75.3034067 | 0.000217 |
| rs511515 | G | A | −0.0203855 | 0.00232118 | 33,541,507 | 1.60E−18 | 77.1298756 | 0.00022226 |
| rs537894 | A | G | 0.0127256 | 0.00217696 | 138,348,595 | 5.00E−09 | 34.1706373 | 9.85E−05 |
| rs57994353 | C | T | 0.0127257 | 0.00232272 | 139,356,987 | 4.30E−08 | 30.0169803 | 8.65E−05 |
| rs6007506 | T | C | −0.0248133 | 0.00225726 | 45,622,014 | 4.10E−28 | 120.837922 | 0.00034816 |
| rs6059655 | G | A | −0.127339 | 0.00357982 | 32,665,748 | 1.00E−200 | 1265.31287 | 0.00363368 |
| rs61816766 | C | T | 0.0365779 | 0.00618793 | 152,319,572 | 3.40E−09 | 34.9416857 | 0.0001007 |
| rs61981034 | A | G | 0.0133257 | 0.00243789 | 97,377,089 | 4.60E−08 | 29.8778629 | 8.61E−05 |
| rs6689641 | G | A | 0.014934 | 0.00213425 | 110,720,400 | 2.60E−12 | 48.9619768 | 0.0001411 |
| rs6882046 | G | A | 0.01435 | 0.00242202 | 87,968,864 | 3.10E−09 | 35.1030514 | 0.00010117 |
| rs699780 | G | A | −0.0184485 | 0.00318541 | 120,455,441 | 7.00E−09 | 33.5420093 | 9.67E−05 |
| rs72821630 | T | C | −0.0150406 | 0.00245522 | 63,696,212 | 9.00E−10 | 37.5273725 | 0.00010815 |
| rs75300484 | T | C | −0.0342083 | 0.00580191 | 89,489,061 | 3.70E−09 | 34.762969 | 0.00010019 |
| rs784235 | G | A | −0.0163849 | 0.0027625 | 53,423,144 | 3.00E−09 | 35.1787025 | 0.00010138 |
| rs78444298 | A | G | −0.0466718 | 0.00771154 | 184,672,098 | 1.40E−09 | 36.6289192 | 0.00010556 |
| rs849138 | A | G | 0.0125585 | 0.00213095 | 28,177,338 | 3.80E−09 | 34.731737 | 0.0001001 |
| rs9328259 | A | C | 0.0267439 | 0.00237012 | 508,972 | 1.60E−29 | 127.322946 | 0.00036684 |
| rs9821675 | G | A | −0.0120372 | 0.00212501 | 49,902,544 | 1.50E−08 | 32.0868213 | 9.25E−05 |
| rs9832130 | A | G | −0.0127943 | 0.00216232 | 189,194,752 | 3.30E−09 | 35.0100492 | 0.0001009 |
| rs9858244 | A | G | 0.0189447 | 0.00259673 | 85,787,399 | 3.00E−13 | 53.2255499 | 0.00015339 |
| rs9867857 | T | C | 0.0136247 | 0.00213562 | 156,491,160 | 1.80E−10 | 40.7009731 | 0.0001173 |
| Average of the F statistics | 151.9 | |||||||
| Total variance explained | 2.83% | |||||||
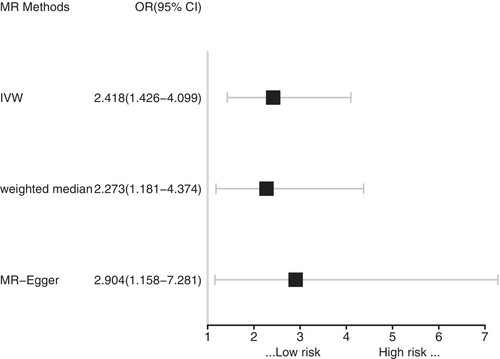
The primary analysis using the IVW method found that childhood sunburn was causally associated with an increased risk of CM (OR = 2.42, 95% CI = 1.43–4.10, p = 0.001). Specifically, standard deviation (SD) increase in childhood sunburn was causally associated with a 142% higher risk of CM. The power for the IVW estimate was determined to be 100% from our calculations. Drawing upon a prior systematic review and meta-analysis encompassing 57 studies on the relationship between sun exposure and melanoma,19 we observed that the minimum effect size of sunburn on melanoma incidence was 2.03 (95% CI = 1.73–2.37). Notably, the power value derived from this effect size was also determined to be 100%, indicating that the statistical power of the primary estimation was more than sufficient, surpassing the recommended threshold of 80%. Two other MR models, including the weighted median and MR-Egger regression, showed consistent results (weighted median: OR = 2.27, 95% CI = 1.18–4.37, p = 0.015; MR-Egger: OR = 2.90, 95% CI = 1.16–7.28, p = 0.026) (Table 2, Figures 3 and 4).
| MR Methods | 95%CL− | 95%CL+ | OR | Beta | SE | p |
|---|---|---|---|---|---|---|
| IVW | 1.426 | 4.099 | 2.418 | 0.883 | 0.269 | 0.001 |
| Weighted median | 1.181 | 4.374 | 2.273 | 0.821 | 0.336 | 0.015 |
| MR-Egger | 1.158 | 7.281 | 2.904 | 1.066 | 0.469 | 0.026 |
- Abbreviations: IVW, inverse-variance weighted; MR, Mendelian randomization.
3.2 Sensitivity analyses
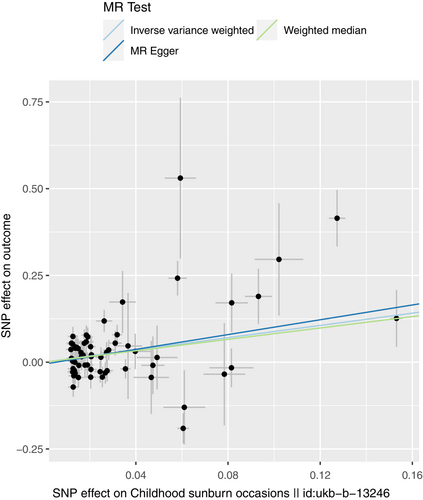
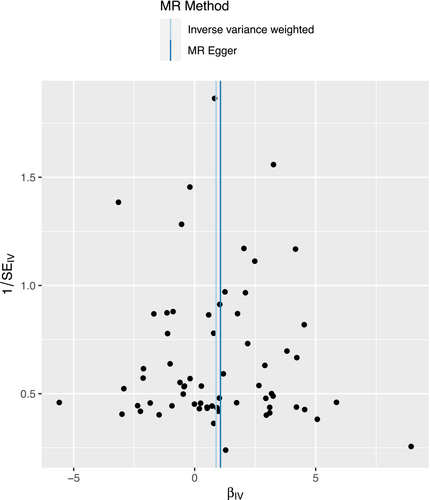
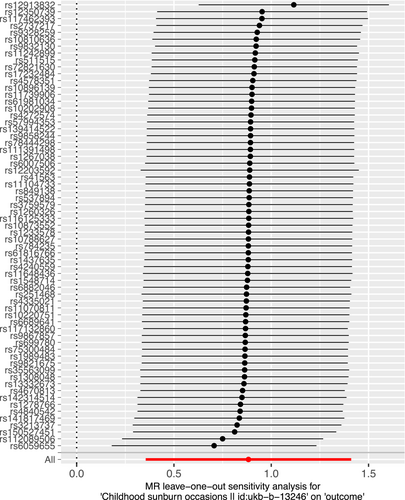
We performed sensitivity analyses to check whether the association might have arisen through violations of the MR assumptions. Although we found some heterogeneity in Cochran's Q test (p < 0.001, I2 = 60.72%), it did not affect our IVW results, as we used the random effect model in this study. We found no evidence that our risk estimates were influenced by directional pleiotropy, as the average pleiotropic effect of the MR-Egger regression intercept was close to null (MR-Egger intercept: −0.006, p = 0.63) (Figure 4). Visual assessment of directional pleiotropy using an MR funnel plot showed that variants were symmetrically distributed, indicating that our estimates were not driven by individual outliers (Figure 5). Finally, we performed a LOO sensitivity analysis to detect if the association was disproportionately influenced by a single SNP. The LOO results (see Figure 5) showed there was no bias caused by any single SNP (Figure 6). Taken together, our findings appeared to be robust, revealing that childhood sunburn had a causal association with CM.
4 DISCUSSION
Using a two-sample MR approach, our study has provided evidence of a causal relationship between childhood sunburn and an increased risk of CM. This suggests that the pathophysiological changes resulting from childhood sunburn may contribute to the development of skin malignancies. To our knowledge, this is the first study exploring the potential causal associations of childhood sunburn risk factors with melanoma on the basis of genetic data.
Our present MR study demonstrated significant positive associations between genetically predicted sunburn and the risk of CM within a combined sample of 2705 cases and 259,583 non-cases. These findings align with the majority, though not all, of previous studies conducted on this topic. A meta-analysis20 of 57 published observational studies on melanoma found that a history of sunburn was a significant risk factor. Additionally, a meta-analysis by the Centers for Disease Control and Prevention (CDC)21 showed that the relative risk of melanoma among people with a history of sunburn was 2.03 (95% CI = 1.73–2.37) compared with those without a history of sunburn. Our views were further supported by a subsequent meta-analysis22 that summarized the OR of CM risk associated with “ever” having experienced sunburn across 51 independent study populations. Among those who were “ever” sunburned, the risk of CM was highest in childhood (OR = 1.9), followed by adolescence (OR = 1.6), recent (OR = 1.6), and adulthood (OR = 1.4). This association was observed in cross-sectional studies of sunburn and skin melanoma by Cheng et al.,23 Holman et al.,24 and Wyant et al.25 However, it is important to consider that cross-sectional designs, susceptible to reverse causality, are not recommended for etiological research. Nonetheless, these studies consistently suggest that childhood sunburn may pose the greatest risk.
It is noteworthy that there is often a relatively low awareness of “sunburn in childhood” as a risk factor for melanoma.26 We speculate that there are several reasons why children experience sunburn, including excessive outdoor sun exposure activities, the overall lack of awareness of sunscreen and UV protection among children, and even the low coverage of green vegetation in childhood growth environments. Furthermore, parents may play a guiding role in outdoor sunlight exposure.27, 28 A cross-sectional study29 funded by the National Cancer Institute of the United States examined the relationship between sunscreen behavior among parents and adolescents. The study included 1859 parents and 1737 teens and found a moderate correlation between intentional outdoor tanning by parents and teens (OR = 0.45, 95% CI = 0.41–0.49). Studies from the Danish population indicated that children are more likely to experience sunburn than adults and that sunburn becomes less frequent with age (OR = 4.44; 15–19 vs. 50–59).30, 31 Several factors contribute to the increased risk of sunburn among individuals aged 12 to 15, including a lack of knowledge about the harmful effects of UV rays, the risk of developing melanoma due to UV exposure, and a perception barrier toward the use of sunscreen. Additionally, a general lack of awareness about the risks of sunburn and skin cancer in children contributes to this issue. A cross-sectional study32 from an English population containing 2173 adolescents (females: 50.7%, n = 1102, mean age of 12.4 [SD = 0.55]) also found that adolescents had poor sun protection practices and low skin cancer awareness. The results indicated that only 39% of people believed that getting sunburned more than once as a child increases the risk of cancer. Additionally, 42% and 26% of adolescents reported that their friends and family, respectively, held positive attitudes toward tanning.
However, a recent large-scale cross-sectional study33 by Ottwell et al. investigated the lifestyle of 437,436 American citizens diagnosed with skin cancer and those without a history of skin cancer. The research found no significant difference between the two groups in terms of sunburn frequency, use of sun protection, or indoor tanning habits. This contradicts the majority of research findings. One possible explanation for this discrepancy is that although the study had a sufficient sample size, the nature of data collection being in survey form makes the respondents' answers subject to recall bias. In our MR study, we selected specific IVs that were strongly related to childhood sunburn, conducted large sample studies, and provided sufficient statistical ability to assess the causal relationship between childhood sunburn and CM. As previously mentioned, the findings of numerous researchers align with the outcomes of our current study. However, it is imperative to acknowledge that melanoma behavior implicates risk factors beyond sun exposure and highlights the likelihood of intricate gene–environment interactions. Overall, in this situation, we believe it is reasonable to avoid excessive sunlight and sunburn as much as possible.
5 STRENGTHS AND LIMITATIONS
There are several strengths of this MR study. Firstly, MR research techniques use a combination of genetics and epidemiology to analyze causal issues, are designed to avoid the bias of reverse association, and generally reduce the confusion of other modifiable environmental exposures compared with observational studies. Compared with traditional observational studies, our research used a huge sample size to improve the accuracy of the estimation. Secondly, we explored the associations in two independent datasets to avoid overlapping sample populations as much as possible. Thirdly, we conducted our study merely among European populations. Consequently, the results are less susceptible to bias stemming from population stratification. Nonetheless, this restriction to a specific population may limit the generalizability of our findings to other populations.
Our study also has several limitations. The major issue for any MR study is horizontal pleiotropy that means selected genetic IVs influence the risk of outcome not via the exposure but other alternative pathways. We did not detect evidence of pleiotropy from the MR-Egger intercept test for this present MR analysis; thus, we think this pleiotropic effect should not bias our results.34 On the other hand, our research exhibits a certain degree of heterogeneity in some analyses, possibly attributed to differences in the population between Finland and Europe.
The Finnish population possesses several distinguishing characteristics compared with other European populations. Firstly, the Finnish population is known for its genetic homogeneity, which can be attributed to historical isolation and a relatively small population size. Consequently, Finns exhibit a higher degree of genetic similarity among themselves in comparison with genetically diverse populations. This characteristic results in fewer variations and differences in their genomes, facilitating easier analysis. Secondly, the Finnish population demonstrates reduced genetic variation, simplifying the interpretation of genomics data. This reduced variation enables researchers to efficiently identify genetic variants and their potential associations with diseases or traits. In contrast, genetically heterogeneous populations with numerous variations present challenges in determining meaningful patterns or associations. Additionally, the Finnish population has experienced founder effects, wherein a small group of individuals establish a new population. This phenomenon leads to a higher prevalence of specific genetic variants or mutations within the population. The concentration of these founder effects aids researchers in identifying genetic variations associated with diseases or traits, as they are more easily detectable.
Therefore, while the genetic uniqueness of the Finnish population offers advantages in certain genomic research contexts, it also contributes to the emergence of heterogeneity in this study. Even though there was heterogeneity among IVs in a few analyses, no pleiotropy in the MR-Egger suggested balanced pleiotropy, which is less likely to bias the results.16 In addition, the nonlinear association could not be estimated in the present MR analysis based on summary-level genetic statistics. Likewise, the gene–environment interaction could not be assessed in summary-level data. Overall, it should be noted that the physiological mechanisms of sunburn and CM are far more complex than these simplistic models suggest. Therefore, further investigations are required to uncover the underlying mechanisms that shed light on CM and aid in its prevention.
In conclusion, this two-sample MR study provides evidence that elevated number of sunburns in childhood increases the risk of CM. Therefore, we propose that individuals with a prior history of childhood sunburn should exercise heightened vigilance regarding the occurrence of CM. In conclusion, the persistent endeavor to diminish the occurrence of sunburns during childhood through the promotion of sun protection awareness among adolescents and their parents remains a significant objective in the realm of public health.
AUTHOR CONTRIBUTIONS
Shengdong Zhong: Conceptualization; data curation; formal analysis; investigation; methodology; project administration; resources; software; supervision; validation; visualization; writing – original draft; writing – review and editing. Liting Lan: Conceptualization; data curation; formal analysis; investigation; project administration; resources; supervision; validation; writing – review and editing. Yuqing Wen: Formal analysis; funding acquisition; project administration; supervision; writing – review and editing.
ACKNOWLEDGMENTS
The authors would like to thank the FinnGen consortium for making summary-level data from this consortium publicly available.
FUNDING INFORMATION
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
CONFLICT OF INTEREST STATEMENT
All authors declare no conflict of interest.
ETHICS STATEMENT
Approval of the research protocol by an Institutional Reviewer Board: N/A.
Informed Consent: N/A.
Registry and the Registration No. of the study/trial: N/A.
Animal Studies: N/A.




