Association between adherence to Eat-Lancet diet and incidence and mortality of lung cancer: A prospective cohort study
Yi Xiao and Linglong Peng contributed equally to this work.
Abstract
Previous research has shown that adhering to the Eat-Lancet diet (ELD) is associated with a lower risk of chronic diseases and mortality. However, the associations between ELD and lung cancer incidence and mortality are unclear. To address this gap, we conducted a prospective cohort study involving 101,755 adults from the Prostate, Lung, Colorectal, and Ovarian (PLCO) trial in the USA. The ELD score was utilized to assess compliance with the ELD, with higher scores indicating greater compliance. We employed Cox regression analyses to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) of ELD score with the incidence and mortality of lung cancer and its subtypes. In addition, sensitivity analyses were performed to ensure the robustness of our findings. In total, 1706 cases of lung cancer and 1217 lung cancer-associated deaths were recorded during the study period. Our analysis revealed that higher ELD scores were significantly associated with a reduced incidence (HRQuartile 4 vs. Quartile 1: 0.73; 95% CI: 0.60, 0.89; ptrend = 0.001) and mortality (HRQuartile 4 vs. Quartile 1: 0.74; 95% CI: 0.59, 0.93; ptrend = 0.005) of lung cancer in a dose–response manner (all pnonlinearity > 0.05). The reliability of these results was supported by sensitivity analyses. Notably, these associations were primarily observed in non–small-cell lung cancer. In conclusion, our findings suggest that adherence to the ELD may be associated with a reduced risk of lung cancer incidence and mortality.
Abbreviations
-
- BMI
-
- Body mass index
-
- BQ
-
- Baseline questionnaire
-
- CI
-
- Confidence interval
-
- DHQ
-
- Dietary history questionnaire
-
- ELD
-
- Eat-Lancet diet
-
- HR
-
- Hazard ratio
-
- NSCLC
-
- Non–small-cell lung cancer.
-
- PLCO
-
- Prostate, Lung, Colorectal, and Ovarian
-
- SCLC
-
- Small-cell lung cancer
1 INTRODUCTION
Lung cancer is one of the most prevalent types of cancer worldwide, accounting for approximately 11.6% of all cancer cases and is the leading cause of cancer-related deaths, with over 1.7 million fatalities reported globally in 2018.1 In the United States, lung cancer is estimated to cause 240,000 new cases and 130,000 deaths in 2023, and is the primary cause of cancer-related mortality in people aged 50 years or older.2 Although several risk factors, including smoking, exposure to secondhand smoke, radon gas, asbestos, air pollution, and genetic predisposition, have been identified for lung cancer,3, 4 the incidence, and mortality rates of lung cancer have not been well controlled.
Recently, there has been increasing focus on the effect of dietary quality in reducing the risk of both cancer incidence and mortality.5-7 A prospective study of over 250,000 individuals systematically examined the correlation between lung cancer risk and dietary quality, revealing that five distinct indices of dietary quality were related to reduced lung cancer incidence.7 In addition, a study based on the Health Professionals Follow-up Study (HPFS) and the Nurses' Health Study (NHS) demonstrated that four different healthy diet quality scores were correlated with a reduced risk of lung cancer mortality.8 However, traditional healthy dietary patterns have overlooked the importance of sustainability, which is a crucial factor in achieving the sustainable development goals outlined by the United Nations and ensuring environmental preservation within reasonable limits.9
In 2019, the EAT-Lancet Commission on Healthy Eating from Sustainable Food Systems developed the ELD as a specific sustainable dietary pattern recommended for promoting environmental sustainability and reducing chronic disease incidence and mortality associated with diet.9 This dietary pattern mainly prioritizes incorporating fruits, legumes, vegetables, nuts, and whole grains into the diet while limiting saturated fat, added sugar, and animal-source foods.9 In comparison with the preceding healthy plant-based diet, the ELD is a scientifically substantiated dietary pattern that considers various interrelations among health, nutrition, and the environment to estimate the health and environmental impacts of adopting an alternative diet. Importantly, the ELD provides comprehensive guidelines for sustaining the global population within planetary resource limitations and integrating sustainability criteria into national dietary recommendations for culturally diverse countries.
Previous studies have primarily focused on investigating the association between adherence to the ELD and chronic diseases such as diabetes and cardiovascular disease, with limited attention paid to cancer incidence and mortality.10-13 To our knowledge, only one study has analyzed the association between ELD and lung cancer incidence based on the NutriNet-Santé cohort, which mainly explored the relationship of following ELD with the risk of cardiovascular disease and overall cancer incidence, with a limited sample size for lung cancer patients.14 Additionally, no studies have explored the association between ELD and the mortality of lung cancer. To fill this gap, we comprehensively investigated the potential association of adherence to ELD with the incidence and mortality of lung cancer and its subtypes in a large US population based on the data obtained from the PLCO Screening Trial.
2 METHODS
2.1 Study design
The PLCO Cancer Screening Trial is a comprehensive and multicenter prospective study that seeks to ascertain if screening tests for ovarian, prostate, colorectal, and lung cancers can effectively lower the associated death rates of these malignancies.15 From November 1993 to September 2001, the PLCO trial successfully recruited a cohort of 154,887 adult individuals between the ages of 55 and 74 years from 10 strategically located screening centers across the USA.15 The participants were randomly assigned to either a screening group or a control group. The screening group received regular screening tests for the four types of cancer, while the control group received usual medical care. Participants were followed up until 2009 for cancer incidence and in 2018 for cancer-related mortality to evaluate the potential preventive and protective impact of screening tests on targeted cancers. The PLCO trial primarily collected demographic data through a BQ and dietary-related data through a DHQ. The PLCO Screening Trial project was granted approval by the Institutional Review Board of the National Cancer Institute (NCI), as well as each screening center involved in the study, with explicit, informed, and written consent obtained from all participants. Additionally, the NCI provided approval for our present study (Project ID: PLCO-1177).
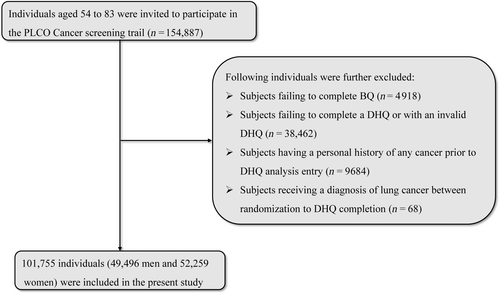
In the present study, our aim was to investigate whether adherence to ELD was associated with the incidence and mortality of lung cancer. Accordingly, 154,887 participants enrolled in the PLCO trial were further excluded based on the following criteria:
- Failure to complete the BQ (n = 4918).
- Failure to return DHQ responses (n = 33,241).
- Invalid DHQ responses, defined as those lacking a completion date, those completed after the death date, those with a high frequency of missing responses (≥8), or those with extremely high energy intake values (the first or last percentile) (n = 5221).
- Personal history of any type of cancer before the DHQ entry (n = 9684).
- Occurrence of outcome events during the period between DHQ entry and DHQ completion, which for this study, included the development of lung cancer, death, or lack of a follow-up analysis (n = 68).
Ultimately, in total, 101,755 participants, including 49,496 men and 52,259 women, were eligible for inclusion, as depicted in Figure 1.
2.2 Data collection and covariates assessment
The demographic data of the participants in our study were collected from a self-administered BQ used in the PLCO trial, including age, sex, race, occupation status, education level, trail arm, smoking status, cigarette consumption, BMI at baseline and age 20, weight fluctuations, aspirin use history, history of diabetes, chronic bronchitis, emphysema, hypertension, and family history of lung cancer. Especially, BMI was calculated by dividing the weight (in kilograms) by the height squared (in meters). Weight fluctuation was defined as the difference between the weight at age 20 and the weight at baseline, expressed in pounds. Cigarette consumption was assessed using two indicators: the number of cigarettes smoked per day during smoking (daily cigarette consumption, classified as 0, 1–20, >20) and the number of packs smoked per day multiplied by years smoked (pack-years, continuous). Dietary data, including drinking status, alcohol consumption, dietary energy intake, and nutritional profile, were collected via a DHQ in the PLCO trial over a 3-year period after participant enrollment. The DHQ is a self-administered food frequency questionnaire (FFQ) consisting of 137 items used to determine both portion size and frequency of dietary intake and supplement use over the previous 365 days. All 14 dietary indicators that constitute the ELT dietary pattern are encompassed in the DHQ. Notably, the DHQ was validated against 24-h recalls in the Eating at America's Table Study among 1640 randomly selected participants nationwide.16 Compared with two other widely used FFQs (Block and Willett), the DHQ demonstrated superior performance in assessing absolute nutrient intakes. This provides strong evidence for the validity of the DHQ in accurately capturing dietary intake for epidemiologic research. In addition, the physical activity in this study was considered as the total weekly time utilized for moderate-to-high intensity (exercise) activity and was evaluated through a self-reported supplemental questionnaire (SQX).
2.3 Assessment of Eat-Lancet diet
In the present investigation, the ELD score was employed, which is a validated dietary assessment tool developed by Knuppel et al. as per the recommendations established by the EAT-Lancet Commission.9, 17 Importantly, Knuppel et al. highlighted that the ELD framework defines the daily calorie intake as 2500 kcal.9, 17 As such, all participants' nutrient intakes were adjusted to grams per 2500 kcal prior to calculating the ELD scores. The ELD score encompasses 14 dietary components sourced from nine broad food classes, namely, tubers and starchy vegetables, whole grains, vegetables, dairy products, fruits, legumes, protein sources, as well as added fats and sugars. Each dietary constituent was allocated a reference value for recommended intake. The participants were assigned a score of 1 if they met or exceeded the reference value for intake of fruits, vegetables, nuts, and the ratio of unsaturated fat to saturated fat. Or, if they did not meet the recommended value, they were assigned a score of 0.17 For other dietary components, the opposite scoring method was used. The sum of all dietary constituent scores comprises the ELD score, with a possible range from 0 to 14. The degree of ELD adherence can be inferred from the magnitude of the ELD score, with higher scores representing higher compliance with the dietary guidelines. In the present study, the ELD score was segregated into quartiles, and the baseline characteristics of the participants were depicted by quartile of ELD score (quartile 1 to quartile 4). Notably, this study made minor adaptations to the individual constituents of the ELD, considering differences in dietary habits between the American and European populations based on dietary intakes of individuals as retrieved in DHQ. Additional details regarding the composition of the ELD and the proportion of individuals meeting the recommended intake values are provided in Table S1.
2.4 Outcome ascertainment
The PLCO Cancer Screening Trial primarily relied on yearly study update forms to identify cases of lung cancer. These forms were sent to surviving participants and provided questions regarding any cancer diagnoses, including the cancer type, date, and place of diagnosis, as well as the contact information of the study participant's physician. Reported cases of cancer were confirmed through medical records. Participant vital status was also verified via the yearly study update forms, with repeated attempts made to contact participants who failed to return the forms via phone or email. To ensure complete death information, the trial also routinely checked the national death index and used the International Classification of Diseases, Ninth Revision (ICD-9) to determine causes of death based on death certificates.
2.4.1 Statistical analysis
In cases in which variables had less than 5% missing data, missing values were imputed using mode and median imputation for classification and continuous variables, respectively.18 Multiple imputations methods were used to impute the continuous variable “physical activity level,” which had a missing rate of approximately 25%.19 Detailed imputation information on the types and proportions of missing data can be found in Table S2.
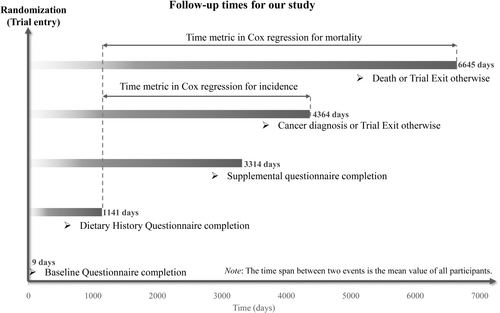
A Cox proportional hazards regression was applied to determine HR and 95% CI for the relationship between ELD score and the incidence and death rate of lung cancer, with the follow-up duration as the time metric. Here, for primary outcomes, namely lung cancer incidence, follow-up length was measured from the DHQ completion date to the date of lung cancer diagnosis, death, loss, or end of follow-up (the end date for the study was December 31, 2009), whatever happened first. In addition, for secondary outcomes, namely the mortality of lung cancer, follow-up duration was computed from the DHQ completion date to the date of death, loss to follow-up, or end of the study (2018), whichever occurred earlier (Figure 2). In order to test whether there was a trend between lung cancer incidence or mortality and the quartiles of ELD scores, each participant was designated a median quartile score within that quartile and subsequently treated like a continuous variable in the regression model, using the lowest quartile as the reference group. Additionally, the ELD score was directly considered a continuous variable for computing risk estimates per 1 score increment. A screening process was carried out to identify potential confounding variables through a comprehensive review of the relevant literature and clinical expertise of the researchers.3, 4, 20, 21 The selected covariates were subsequently incorporated into the Cox regression model to mitigate their potential influence on the outcomes. In detail, Model 1 was adjusted for demographic features, such as sex, age, education level, race, and occupational status. In Model 2, further adjustments were made for the trial arm, lifestyle factors (including smoking status, daily cigarette consumption, drinking status, alcohol consumption, energy intake from diet, BMI at baseline, BMI at age 20, and physical activity level), and health status (aspirin use history, history of diabetes, hypertension, emphysema, chronic bronchitis, and a family history of lung cancer). To depict lung cancer incidence and mortality trends across the entire range of ELD scores, a restricted cubic spline model with three knots at the 10th, 50th, and 90th percentiles was employed.22 The median of the first quartile of ELD scores (i.e., 9) was set as the reference value. The pnonlinearity was also determined by testing the null hypothesis, which implied that the regression coefficient of the second spline was zero. Furthermore, the above methodology was employed to assess the association between ELD score and the incidence and mortality rates of lung cancer subtypes, including NSCLC and SCLC. In addition to the primary analyses, we conducted exploratory analyses to assess the association between ELD scores and incidence and mortality of the two major lung cancer histological subtypes, adenocarcinoma and squamous cell carcinoma, with the same statistical methods.
Pre-specified subgroup analyses were carried out to explore the connection between ELD score and the incidence and mortality of lung cancer in terms of age (>65 years vs. ≤65 years), gender (male vs. female), BMI at baseline (≤30 vs.>30 kg/m2), family history of lung cancer (no vs. yes/possible), smoking status (never vs. current/former), history of emphysema (no vs. yes), aspirin use (no vs. yes), and trail arm (intervention vs. control). To prevent false subgroup effects, p-values for the interaction were estimated by comparison of models with and without multiplicative interaction terms prior to carrying out the above subgroup analyses. In addition, p-values for the trends between quartiles of ELD scores and lung cancer incidence or mortality were calculated separately for each individual subgroup using the previously described methods.
Various sensitivity analyses were applied to ensure the robustness of obtained results: (1) individuals with extreme energy intake (>4000 or <500 kcal/day) or extreme BMI (top 1% and bottom 1%) were excluded; (2) excluding outcome events of interest occurring within the first 2 or 4 years of follow-up to investigate the potential impacts of reverse causation; (3) individuals with diabetes or respiratory comorbidities (including emphysema and chronic bronchitis) were excluded due to their higher risk of developing lung cancer23-25; (4) adjustments were made to the number of cigarettes smoked for pack-years (continuous) instead of daily cigarette consumption (0, 1–20, or >20) to enhance the statistical power of the analysis; (5) repeated analysis in unimputed data cohort to ascertain whether obtained results were influenced by missing data imputation. All statistical analyses were carried out with the help of R software version 4.2.2, with a two-tailed test and p < 0.05 as a level of statistical significance.
3 RESULTS
3.1 Participant baseline features
In the present study population, the ELD scores actually ranged from 4 to 12 points and were divided into four groups according to the quartiles (Quartile 1: 4–9, Quartile 2: 10, Quartile 3: 11, Quartile 4: 12–13), which is consistent with the Knuppel et al.17 results. The mean value (standard deviation) for the ELD score and age of participants were 9.8 (1.3) points and 65.5 (5.7) years, respectively. Table 1 displays the baseline features of the study population categorized by quartiles of ELD scores. Participants from the highest ELD score quartile had higher education and physical activity levels compared with those in the lowest ELD quartile. Additionally, they had a higher chance of a lower BMI (at baseline or age 20) and weight change but were less likely to be smokers, or have a history of diabetes, hypertension, chronic bronchitis, and emphysema.
| Characteristics | Overall | Quartiles of overall Eat-Lancet diet score | |||
|---|---|---|---|---|---|
| Quartile 1 (4–9) | Quartile 2 (10) | Quartile 3 (11) | Quartile 4 (12–13) | ||
| Number of participants | 101,755 | 40,482 | 29,903 | 21,280 | 10,090 |
| Eat-Lancet Diet score | 9.8 ± 1.3 | 8.5 ± 0.7 | 10.0 ± 0.0 | 11.0 ± 0.0 | 12.1 ± 0.4 |
| Age | 65.5 ± 5.7 | 65.2 ± 5.6 | 65.6 ± 5.7 | 65.8 ± 5.8 | 66.0 ± 5.9 |
| Male | 49,496 (48.6%) | 22,027 (54.4%) | 14,479 (48.4%) | 9321 (43.8%) | 3669 (36.4%) |
| Race | |||||
| White | 94,066 (92.4%) | 37,648 (93.0%) | 27,693 (92.6%) | 19,589 (92.1%) | 9136 (90.5%) |
| Non-white | 7689 (7.6%) | 2834 (7.0%) | 2210 (7.4%) | 1691 (7.9%) | 954 (9.5%) |
| Occupation status | |||||
| Homemaker | 11,861 (11.7%) | 4363 (10.8%) | 3562 (11.9%) | 2581 (12.1%) | 1355 (13.4%) |
| Working | 40,723 (40.0%) | 16,446 (40.6%) | 11,872 (39.7%) | 8431 (39.6%) | 3974 (39.4%) |
| Retired/unemployed | 49,171 (48.3%) | 19,673 (48.6%) | 14,469 (48.4%) | 10,268 (48.3%) | 4761 (47.2%) |
| Education level | |||||
| College below | 64,953 (63.8%) | 27,823 (68.7%) | 18,958 (63.4%) | 12,676 (59.6%) | 5496 (54.5%) |
| College graduate | 17,848 (17.5%) | 6500 (16.1%) | 5348 (17.9%) | 3980 (18.7%) | 2020 (20.0%) |
| Postgraduate | 18,954 (18.6%) | 6159 (15.2%) | 5597 (18.7%) | 4624 (21.7%) | 2574 (25.5%) |
| Body mass index at baseline (kg/m2) | 27.2 ± 4.8 | 27.8 ± 4.9 | 27.2 ± 4.7 | 26.7 ± 4.6 | 25.9 ± 4.4 |
| Body mass index at age 20 (kg/m2) | 22.0 ± 3.0 | 22.2 ± 3.1 | 22.0 ± 3.0 | 21.9 ± 3.0 | 21.6 ± 2.8 |
| Weight change (pounds)a | 33.0 ± 27.2 | 36.0 ± 28.0 | 32.9 ± 26.9 | 30.2 ± 26.3 | 27.4 ± 25.1 |
| Intervention trail arm | 51,817 (50.9%) | 20,486 (50.6%) | 15,231 (50.9%) | 10,828 (50.9%) | 5272 (52.2%) |
| Smoking status | |||||
| Never | 48,580 (47.7%) | 18,095 (44.7%) | 14,713 (49.2%) | 10,602 (49.8%) | 5170 (51.2%) |
| Current/Former | 53,175 (52.3%) | 22,387 (55.3%) | 15,190 (50.8%) | 10,678 (50.2%) | 4920 (48.8%) |
| Daily cigarette consumption | |||||
| 0 | 48,685 (47.8%) | 18,138 (44.8%) | 14,743 (49.3%) | 10,626 (49.9%) | 5178 (51.3%) |
| 1–20 | 33,218 (32.6%) | 13,139 (32.5%) | 9589 (32.1%) | 7058 (33.2%) | 3432 (34.0%) |
| >20 | 19,852 (19.5%) | 9205 (22.7%) | 5571 (18.6%) | 3596 (16.9%) | 1480 (14.7%) |
| Smoking pack-years | 17.7 ± 26.6 | 20.4 ± 28.7 | 17.1 ± 26.3 | 15.3 ± 24.0 | 13.6 ± 22.6 |
| Drinker | 73,998 (72.7%) | 29,183 (72.1%) | 21,834 (73.0%) | 15,640 (73.5%) | 7341 (72.8%) |
| Alcohol consumption (g/day) | 12.3 ± 23.2 | 11.7 ± 22.2 | 12.6 ± 23.8 | 13.1 ± 24.4 | 12.4 ± 22.8 |
| Aspirin use history | 47,802 (47.0%) | 19,188 (47.4%) | 14,060 (47.0%) | 9939 (46.7%) | 4615 (45.7%) |
| Family history of lung cancer | |||||
| No | 88,738 (87.2%) | 35,139 (86.8%) | 26,048 (87.1%) | 18,671 (87.7%) | 8880 (88.0%) |
| Yes | 10,569 (10.4%) | 4230 (10.4%) | 3159 (10.6%) | 2158 (10.1%) | 1022 (10.1%) |
| Possibly | 2448 (2.4%) | 1113 (2.7%) | 696 (2.3%) | 451 (2.1%) | 188 (1.9%) |
| Diabetes history | 6806 (6.7%) | 3215 (7.9%) | 1941 (6.5%) | 1202 (5.6%) | 448 (4.4%) |
| Hypertension history | 33,048 (32.5%) | 13,925 (34.4%) | 9722 (32.5%) | 6469 (30.4%) | 2932 (29.1%) |
| Chronic bronchitis history | 4332 (4.3%) | 1790 (4.4%) | 1302 (4.4%) | 851 (4.0%) | 389 (3.9%) |
| Emphysema history | 2144 (2.1%) | 1009 (2.5%) | 593 (2.0%) | 377 (1.8%) | 165 (1.6%) |
| Physical activity level (min/week) | 122.0 ± 109.1 | 113.5 ± 105.6 | 122.8 ± 109.3 | 128.9 ± 110.5 | 139.1 ± 116.0 |
| Energy intake from diet (kcal/day) | 1738.6 ± 736.4 | 1746.8 ± 754.0 | 1730.3 ± 740.7 | 1737.7 ± 719.9 | 1732.6 ± 685.0 |
| ELD and other nutrients intakes | |||||
| Whole grains (g/day) | 288.6 ± 196.9 | 297.4 ± 212.7 | 288.2 ± 198.0 | 281.1 ± 183.1 | 269.9 ± 147.5 |
| Potatoes and cassava (g/day) | 75.5 ± 57.5 | 93.5 ± 66.2 | 71.8 ± 53.1 | 59.0 ± 42.2 | 48.6 ± 31.8 |
| All vegetables (g/day) | 455.6 ± 279.0 | 451.5 ± 278.3 | 451.7 ± 277.1 | 456.9 ± 279.3 | 481.4 ± 285.6 |
| All fruits (g/day) | 423.5 ± 310.8 | 351.5 ± 277.1 | 435.1 ± 299.8 | 484.1 ± 326.9 | 550.0 ± 359.3 |
| Dairy foods (g/day) | 346.4 ± 370.9 | 388.7 ± 403.4 | 344.0 ± 375.1 | 309.7 ± 331.7 | 261.4 ± 260.5 |
| Protein sources (g/day) | |||||
| Beef, lamb, pork | 65.9 ± 39.5 | 82.9 ± 40.2 | 66.1 ± 37.2 | 50.2 ± 30.5 | 30.2 ± 15.7 |
| Chicken, other poultry | 62.3 ± 37.3 | 78.4 ± 38.0 | 62.5 ± 35.1 | 47.5 ± 28.7 | 28.7 ± 14.8 |
| Eggs | 20.4 ± 25.4 | 28.2 ± 30.5 | 18.2 ± 22.7 | 13.6 ± 17.6 | 9.8 ± 11.5 |
| Fish | 38.7 ± 44.1 | 42.6 ± 50.3 | 37.4 ± 41.9 | 35.4 ± 37.7 | 33.5 ± 33.4 |
| Legumes (g/day) | |||||
| Dry beans, lentils, peas | 47.6 ± 45.9 | 53.2 ± 53.0 | 46.3 ± 43.8 | 42.8 ± 38.4 | 39.6 ± 31.2 |
| Soy foods | 2.5 ± 15.1 | 2.2 ± 14.3 | 2.4 ± 15.1 | 2.8 ± 16.1 | 3.4 ± 15.8 |
| Peanuts or tree nuts | 38.9 ± 52.4 | 23.0 ± 33.2 | 37.1 ± 47.4 | 53.0 ± 59.9 | 78.1 ± 78.1 |
| Added fats (unsaturated/saturated fat) | 2.0 ± 0.5 | 1.9 ± 0.4 | 2.0 ± 0.4 | 2.1 ± 0.5 | 2.2 ± 0.6 |
| Added sugars (g/day) | 151.4 ± 49.3 | 140.9 ± 46.9 | 152.9 ± 48.6 | 160.4 ± 49.7 | 170.7 ± 50.2 |
| Fiber (g/day) | 26.7 ± 8.8 | 24.9 ± 8.6 | 26.8 ± 8.5 | 28.2 ± 8.6 | 30.5 ± 8.6 |
| Dietary protein (g/day) | 96.4 ± 18.5 | 101.8 ± 18.2 | 95.9 ± 17.5 | 91.5 ± 17.5 | 86.1 ± 16.6 |
| Dietary carbohydrate (g/day) | 324.8 ± 59.1 | 312.5 ± 57.7 | 326.6 ± 58.0 | 335.2 ± 58.8 | 346.7 ± 56.8 |
| Dietary fat (g/day) | 88.1 ± 21.1 | 90.5 ± 20.8 | 87.4 ± 21.0 | 86.1 ± 21.2 | 85.2 ± 21.0 |
- Note: Descriptive statistics are presented as (mean ± standard deviation) and number (percentage) for continuous and categorical.
- a Weight change was defined as the participant's baseline weight minus weight at age 20.
3.2 Association between lung cancer incidence and ELD score
In total, 1706 newly diagnosed lung cancer cases, comprising 1464 NSCLC and 242 SCLC cases, were reported during a mean follow-up of 8.82 ± 1.95 years (897,809 person-years), with the overall incidence rate close to 0.19 cases per 100 person-years. Table 2 sums up the findings of multivariable and univariable Cox regression analyses on lung cancer and ELD score incidence. In comparison with participants in the lowest ELD score quartile, those in the highest quartile had a significantly reduced incidence of lung cancer post-adjustment for possible confounding variables (multivariable model: HRQuartile 4 vs. Quartile 1: 0.73; 95% CI: 0.60, 0.89; p = 0.001 for trend). When the ELD score was considered a continuous variable, lung cancer incidence was also found to be inversely associated (multivariable model: HR for 1-point ELD score increment: 0.93; 95% CI: 0.90, 0.97).
| Quartiles of ELD score | Cases | Person-years | Incidence rate per 100 person-years (95% confidence interval) | Hazard ratio (95% confidence interval) by Eat-Lancet diet score | ||
|---|---|---|---|---|---|---|
| Unadjusted | Model 1a | Model 2b | ||||
| Lung cancer | ||||||
| Quartile 1 | 782 | 355,526 | 0.22 (0.21, 0.24) | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) |
| Quartile 2 | 481 | 264,292 | 0.18 (0.17, 0.20) | 0.83 (0.74, 0.93) | 0.86 (0.76, 0.96) | 0.91 (0.81, 1.02) |
| Quartile 3 | 320 | 188,429 | 0.17 (0.15, 0.19) | 0.77 (0.68, 0.88) | 0.83 (0.72, 0.94) | 0.87 (0.76, 0.99) |
| Quartile 4 | 123 | 89,562 | 0.14 (0.12, 0.16) | 0.62 (0.52, 0.75) | 0.70 (0.58, 0.85) | 0.73 (0.60, 0.89) |
| p for trend | <0.001 | <0.001 | 0.001 | |||
| 1-point increment in ELD score | 1706 | 897,809 | 0.19 (0.18, 0.20) | 0.89 (0.86, 0.92) | 0.91 (0.88, 0.95) | 0.93 (0.90, 0.97) |
| Non–small-cell lung cancer | ||||||
| Quartile 1 | 672 | 355,526 | 0.19 (0.18, 0.20) | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) |
| Quartile 2 | 407 | 264,292 | 0.15 (0.14, 0.17) | 0.81 (0.72, 0.92) | 0.84 (0.74, 0.95) | 0.89 (0.79, 1.01) |
| Quartile 3 | 275 | 188,429 | 0.15 (0.13, 0.16) | 0.77 (0.67, 0.89) | 0.82 (0.71, 0.95) | 0.87 (0.75, 1.00) |
| Quartile 4 | 110 | 89,562 | 0.12 (0.10, 0.15) | 0.65 (0.53, 0.79) | 0.73 (0.59, 0.89) | 0.75 (0.61, 0.92) |
| p for trend | <0.001 | <0.001 | 0.002 | |||
| 1-point increment in ELD score | 1464 | 897,809 | 0.16 (0.15, 0.17) | 0.89 (0.86, 0.93) | 0.92 (0.88, 0.95) | 0.94 (0.90, 0.97) |
| Small-cell lung cancer | ||||||
| Quartile 1 | 110 | 355,526 | 0.03 (0.03, 0.04) | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) |
| Quartile 2 | 74 | 264,292 | 0.03 (0.02, 0.04) | 0.90 (0.67, 1.21) | 0.95 (0.71, 1.27) | 1.00 (0.74, 1.35) |
| Quartile 3 | 45 | 188,429 | 0.02 (0.02, 0.03) | 0.77 (0.54, 1.09) | 0.85 (0.60, 1.20) | 0.90 (0.63, 1.27) |
| Quartile 4 | 13 | 89,562 | 0.01 (0.01, 0.02) | 0.47 (0.26, 0.83) | 0.55 (0.31, 0.99) | 0.58 (0.32, 1.04) |
| p for trend | 0.007 | 0.054 | 0.115 | |||
| 1-point increment in ELD score | 242 | 897,809 | 0.03 (0.02, 0.03) | 0.86 (0.78, 0.95) | 0.89 (0.81, 0.98) | 0.91 (0.83, 1.01) |
- a Model 1: Model 1 was controlled with age (continuous), sex (male, female), race (white, non-white), education levels (college below, college graduate, postgraduate) and occupation situation (homemaker, working, retired/unemployed).
- b Model 2: Model 2 was additionally controlled with BMI at baseline (continuous), BMI at age 20 (continuous), weight change (continuous), trail arm (intervention, control), smoking status (never, current or former), daily cigarette consumption (0, 1–20, >20), history of drinking alcohol (no, yes), alcohol consumption (continuous), aspirin use (no, yes), family history of lung cancer (no, yes, possibly), history of hypertension (no, yes), history of diabetes (no, yes), history of chronic bronchitis (no, yes), history of emphysema (no, yes), total energy intake (continuous) and physical activity level (continuous).
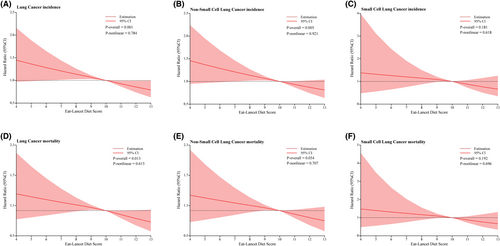
A similar inverse association was obtained when studying the relationship between ELD score and NSCLC incidence (multivariable model: HRQuartile 4 vs. Quartile 1: 0.75; 95% CI: 0.61, 0.92; p = 0.002 for trend, HR for 1-point ELD score increment: 0.94; 95% CI: 0.90, 0.97). Nevertheless, the correlation between ELD score and SCLC incidence in multivariable analyses was not significant (multivariable model: HRQuartile 4 vs. Quartile 1: 0.58; 95% CI: 0.32, 1.04; p = 0.115 for trend, HR for 1-point ELD score increment: 0.91; 95% CI: 0.83, 1.01). The restricted cubic spline model demonstrated a linear inverse dose–response association of ELD score with lung cancer and NSCLC incidence (all p > 0.05 for nonlinearity; Figure 3A,B).
In additional exploratory analyses, no significant associations were observed between ELD scores and the incidence of lung adenocarcinoma or squamous cell carcinoma (Table S3). However, the restricted cubic spline model revealed a negative linear trend between higher ELD scores and squamous cell carcinoma incidence, which was not seen for adenocarcinoma (Figure S1A,B).
The subgroup analyses demonstrated that the relationship between lung cancer incidence and ELD score was not influenced by age, sex, BMI at baseline, a family history of lung cancer, aspirin use, smoking status, history of emphysema, or trial arm (all p > 0.05 for interaction; Table S4). Moreover, in sensitivity analyses, the initial correlation between ELD score and lung cancer incidence remained materially unchanged (Table S5).
3.3 Association of ELD score with lung cancer mortality
Over a span of 15.07 ± 4.54 years, corresponding to 1,533,359 person-years of follow-up, it was ascertained that 1217 deaths were attributable to lung cancer, comprising 212 cases of SCLC and 1005 cases of NSCLC, yielding an overall death rate of 0.08 deaths per 100 person-years. The association between lung cancer mortality and ELD score was comparable with that observed between ELD score and lung cancer incidence. After adjusting for confounding factors in a multivariable model, individuals in the highest quartile of ELD score were found to have a decreased risk of mortality from lung cancer (HRQuartile 4 vs. Quartile 1: 0.74; 95% CI: 0.59, 0.93; p = 0.005 for trend), when compared with those in the lowest quartile (Table 3). In addition, the inverse association with lung cancer mortality persisted when the ELD score was regarded as a continuous variable (HR for 1-point ELD score increment: 0.94; 95% CI: 0.90, 0.98).
| Quartiles of ELD score | Cases | Person-years | Incidence rate per 100 person-years (95% confidence interval) | Hazard ratio (95% confidence interval) by Eat-Lancet diet score | ||
|---|---|---|---|---|---|---|
| Unadjusted | Model 1a | Model 2b | ||||
| Lung cancer | ||||||
| Quartile 1 | 557 | 601,634 | 0.09 (0.09, 0.10) | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) |
| Quartile 2 | 348 | 450,984 | 0.08 (0.07, 0.09) | 0.84 (0.73, 0.96) | 0.87 (0.76, 1.00) | 0.93 (0.81, 1.06) |
| Quartile 3 | 225 | 324,395 | 0.07 (0.06, 0.08) | 0.76 (0.65, 0.89) | 0.82 (0.70, 0.96) | 0.87 (0.74, 1.02) |
| Quartile 4 | 87 | 156,346 | 0.06 (0.05, 0.07) | 0.61 (0.49, 0.77) | 0.70 (0.56, 0.88) | 0.74 (0.59, 0.93) |
| p for trend | <0.001 | <0.001 | 0.005 | |||
| 1-point increment in ELD score | 1217 | 1,533,359 | 0.08 (0.08, 0.08) | 0.89 (0.86, 0.92) | 0.91 (0.88, 0.95) | 0.94 (0.90, 0.98) |
| Non–small-cell lung cancer | ||||||
| Quartile 1 | 461 | 601,634 | 0.08 (0.07, 0.08) | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) |
| Quartile 2 | 284 | 450,984 | 0.06 (0.06, 0.07) | 0.83 (0.71, 0.96) | 0.86 (0.74, 0.99) | 0.92 (0.79, 1.06) |
| Quartile 3 | 184 | 324,395 | 0.06 (0.05, 0.07) | 0.75 (0.63, 0.89) | 0.81 (0.68, 0.96) | 0.86 (0.72, 1.02) |
| Quartile 4 | 76 | 156,346 | 0.05 (0.04, 0.06) | 0.64 (0.51, 0.82) | 0.74 (0.58, 0.94) | 0.78 (0.61, 0.99) |
| p for trend | <0.001 | 0.002 | 0.016 | |||
| 1-point increment in ELD score | 1005 | 1,533,359 | 0.07 (0.06, 0.07) | 0.89 (0.85, 0.93) | 0.92 (0.88, 0.96) | 0.94 (0.90, 0.99) |
| Small-cell lung cancer | ||||||
| Quartile 1 | 96 | 601,634 | 0.02 (0.01, 0.02) | 1.000 (reference) | 1.000 (reference) | 1.000 (reference) |
| Quartile 2 | 64 | 450,984 | 0.01 (0.01, 0.02) | 0.90 (0.65, 1.23) | 0.93 (0.68, 1.28) | 0.99 (0.72, 1.36) |
| Quartile 3 | 41 | 324,395 | 0.01 (0.01, 0.02) | 0.80 (0.56, 1.16) | 0.87 (0.60, 1.26) | 0.92 (0.63, 1.33) |
| Quartile 4 | 11 | 156,346 | 0.01 (0.00, 0.01) | 0.45 (0.24, 0.84) | 0.52 (0.28, 0.98) | 0.54 (0.29, 1.02) |
| p for trend | 0.013 | 0.068 | 0.123 | |||
| 1-point increment in ELD score | 212 | 1,533,359 | 0.01 (0.01, 0.02) | 0.86 (0.78, 0.96) | 0.89 (0.80, 0.99) | 0.91 (0.82, 1.01) |
- a Model 1: Model 1 was controlled with age (continuous), sex (male, female), race (white, non-white), education levels (college below, college graduate, postgraduate) and occupation situation (homemaker, working, retired/unemployed).
- b Model 2: Model 2 was additionally controlled with BMI at baseline (continuous), BMI at age 20 (continuous), weight change (continuous), trail arm (intervention, control), smoking status (never, current or former), daily cigarette consumption (0, 1–20, >20), history of drinking alcohol (no, yes), alcohol consumption (continuous), aspirin use (no, yes), family history of lung cancer (no, yes, possibly), history of hypertension (no, yes), history of diabetes (no, yes), history of chronic bronchitis (no, yes), history of emphysema (no, yes), total energy intake (continuous) and physical activity level (continuous).
Similarly, ELD score was also inversely associated with NSCLC mortality in the fully adjusted multivariable model (multivariable model: HRQuartile 4 vs. Quartile 1: 0.78; 95% CI: 0.61, 0.99; p = 0.016 for trend, HR for 1-point ELD score increment: 0.94; 95% CI: 0.90, 0.99), but not with SCLC mortality (multivariable model: HRQuartile 4 vs. Quartile 1: 0.54; 95% CI: 0.29, 1.02; p = 0.123 for trend, HR for 1-point ELD score increment: 0.91; 95% CI: 0.82, 1.01). A linear inverse dose–response relationship with ELD score was demonstrated for lung cancer as well as NSCLC mortality (all p > 0.05 for nonlinearity Figure 3D,E).
In exploratory analyses of lung cancer mortality, no statistically significant associations were found between ELD scores and mortality from adenocarcinoma or squamous cell carcinoma (Table S6). However, the restricted cubic spline model indicated a negative linear trend between ELD score and squamous cell carcinoma mortality (Figure S1C,D).
In the subgroup analysis, the significant inverse relationship between ELD score and lung cancer mortality remained unaltered by pre-defined stratification factors (all p > 0.05 for interaction; Table S7). The significant inverse relationship between ELD score and lung cancer mortality also persisted in sensitivity analyses (Table S8).
4 DISCUSSION
This study sought to explore the relationship between the ELD score and lung cancer incidence and death rate as per data from the PLCO Cancer Screening Trial. The study participants were divided into quartiles based on ELD scores ranging from 4 to 13 points. The results indicated that higher ELD scores were associated with lower incidence and mortality rates of lung cancer, particularly for NSCLC. However, this association was not observed for SCLC. Furthermore, the relationship between lung cancer incidence and mortality with ELD score was not affected by lifestyle or demographic factors, indicating that the association is independent. An inverse linear trend was observed in the dose–response analysis, indicating a decrease in lung cancer incidence and mortality with increasing ELD score. Sensitivity analysis confirmed the robustness of these findings. Therefore, adherence to the ELD, as measured by the ELD score, may reduce the incidence and mortality of lung cancer, particularly for NSCLC.
So far, there has been only one study that investigated the link between ELD and lung cancer risk.14 In this study, Berthy et al. mainly investigated the correlation between ELD and cardiovascular disease (CVD) and overall cancer risk using a modified ELD score developed by Kesse-Guyot et al.14, 26 The study utilized a cohort of more than 60,000 participants in the NutriNet-Santé study. However, ELD and the risk of overall cancer and CVD were not found to be significant, neither combined nor separately. Of note, Berthy et al.14 demonstrated an inverse association between ELD score and lung cancer risk in the secondary outcome analysis of their study, but this association was found to be statistically insignificant after adjustment for energy intake and BMI. It should be noted that the representativeness of the Berthy et al.14 study population was limited, as NutriNet-Santé participants were typically younger, more educated, healthier, and predominantly female compared with the general population of France. Thus, the observed associations may have been underestimated. In our study, 101,755 American adults from the PLCO trial were chosen as the study population. The PLCO participants were included according to gender equality, and no significant differences with the general population of the USA have been reported. Our study demonstrated a significant linear association of ELD score with lung cancer risk, which was limited to NSCLC. These findings provide novel insights into the effect of ELD on the occurrence of cancers in the lungs.
Although the correlation of overall mortality risk with ELD score has been reported in previous literature, the potential relationship between ELD score and lung cancer mortality has not been explored. Knuppel et al.17 evaluated the connection between mortality risk and ELD score in a large prospective cohort of UK adults but failed to find a clear association. Conversely, Stubbendorff et al.13 conducted a study in Sweden and found that maximum adherence to ELD reduced the risk of all-cause and cancer-related mortality by 25% and 24%, respectively. Additionally, Zagmutt et al.27 questioned the efficacy of ELD as a recommended dietary guideline and emphasized the importance of considering BMI and energy intake when assessing the impact of dietary factors on mortality. Our present study comprehensively accounted for weight variables and energy intake in the analysis and incorporated them into the Cox regression model for adjustment. The obtained findings provide the first proof of a negative correlation between lung cancer mortality and ELD score, suggesting that adherence to ELD may be an effective means of reducing lung cancer mortality. Interestingly, the subtype analysis results demonstrated that the notable negative correlation of ELD score with lung cancer mortality is exclusive to NSCLC.
Actually, several previous different indices reflecting the recommendations for a healthy plant-based diet have partially support our research findings. For instance, in a multiethnic cohort study that involved 215,000 adults, the Healthy Eating Index-2015 (HEI-2015), Alternative Healthy Eating Index-2010 (AHEI-2010), and Alternative Mediterranean Diet (AMED) were significantly linked to a reduced risk of lung cancer.7 Moreover, the results from NHS and HPFS indicate that HEI-2015, AHEI, AMED, and the Healthful Plant-based Diet Index (HPDI) are all associated with a decreased risk of lung cancer-related death in both males and females.8 When comparing the composition of ELD with those of the aforementioned dietary patterns, it was found that vegetables, fruits, fiber, and red meat are the main overlapping components. These components may relate to mechanisms underlying the decreased lung cancer incidence and mortality seen. Accumulating evidence shows vegetables and fruits linked to reduced lung cancer risk,28-30 probably due to abundant bioactive compounds such as flavonoids that can scavenge free radicals, repair DNA damage, and inhibit carcinogenesis.31, 32 One prospective PLCO study found higher intake of dietary fiber was associated with a 38% lower lung cancer risk.33 The protective fiber effect may involve gut microbes producing short-chain fatty acids through fermentation, regulating insulin, anti-inflammatory pathways, cytokine production, and immune responses in anticancer defenses.34, 35 Additionally, limiting red meat intake decreases gut microbial carcinogen production and lowers animal fat-induced inflammation, inhibiting the release of tumorigenic factors, which could reduce lung cancer risk.36, 37
This study has several significant strengths. Firstly, it is based on a large prospective cohort of over 150,000 individuals from diverse occupations recruited from 10 screening centers all over the USA. They were also followed up for an extended period of time, thereby increasing the generalizability of these findings to similar populations. Secondly, it comprehensively adjusted for various possible confounding factors in the analyses. More importantly, this is by far the first study to systematically and robustly explore the relationship between the incidence and mortality of lung cancer and its subtypes with ELD scores. The obtained findings provide strong evidence that adhering to the ELD is effective in decreasing both the incidence and mortality of lung cancer and these results are robust even after conducting a series of sensitivity analyses.
The study also has certain limitations that should be considered. For instance, using a single dietary assessment at baseline can potentially introduce some bias because of the potential changes in dietary habits over time. However, published literature has demonstrated that the correlation between diet and disease incidence using only baseline data is generally weaker than when cumulative dietary intake is used,38 thereby decreasing the influence of this potential bias. Second, the self-reported FFQ may be subject to recall bias, thus affecting the accuracy of dietary information. Additionally, the study population consisted of older Americans with a mean age of 65.5 years, and it is unclear whether the observed association between ELD and risk of lung cancer incidence and mortality would hold in other regions or age groups. Therefore, future studies should explore this association in other populations to verify the generalizability of these findings.
In conclusion, this study utilized the ELD score developed by Knuppel et al. to investigate adherence to ELD among the United States population. Moreover, it revealed a linear dose–response association between ELD score and decreased risk of lung cancer incidence and mortality. Notably, this correlation was observed exclusively in NSCLC upon performing subtype analysis. Hence, these findings present a novel dietary approach to reducing both lung cancer incidence and mortality and establish an evidence-based foundation for the development of sustainable dietary guidelines and policies.
AUTHOR CONTRIBUTIONS
Yi Xiao, Ling Xiang, Yaxu Wang, and Linglong Peng contributed to the study design and data analysis. Yi Xiao and Linglong Peng contributed to the data interpretation and writing of the manuscript. Yi Xiao, Ling Xiang, Zhiquan Xu, Yunhao Tang, Hongmei He and Haitao Gu contributed to the data collection, and data curation of the present analysis. Linglong Peng, Yaxu Wang, and Haitao Gu assisted with statistical analysis and funding acquisition. All of the authors reviewed or revised the manuscript. All authors contributed to the article and approved the submitted manuscript. The work reported in the paper has been performed by the authors, unless clearly specified in the text.
ACKNOWLEDGMENTS
We sincerely acknowledge the PLCO study group and PLCO participants. This research was conducted using the PLCO resource (https://cdas.cancer.gov/plco/) under application number PLCO-1177.
FUNDING INFORMATION
This work was supported by The General Project of Chongqing Natural Science Foundation, Chongqing Science and Technology Commission, China [cstc2021jcyj-msxmX0153 (Linglong Peng)], [cstc2021jcyj-msxmX0112 (Yaxu Wang)], and [CSTB2022NSCQ-MSX1005 (Haitao Gu)], and Kuanren Talents Project of the Second Affiliated Hospital of Chongqing Medical University in China [kryc-yq-2110 (Haitao Gu)]. The funders had no role in the study design or implementation; data collection, management, analysis, or interpretation; manuscript preparation, review or approval; or the decision to submit the manuscript for publication.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
ETHICS STATEMENT
Approval of the research protocol by an Institutional Reviewer Board: N/A.
Informed Consent: N/A.
Registry and the Registration No. of the study/trial: N/A.
Animal Studies: N/A.
Open Research
DATA AVAILABILITY STATEMENT
The raw data used in this article are not available because of the National Cancer Institute's data policy. Access to the dataset should contact the National Cancer Institute by mail.




