Development of novel tracers for sentinel node identification in cervical cancer
Abstract
Indocyanine green (ICG) with near-infrared (NIR) fluorescence imaging is used for lymphatic mapping. However, binding of ICG to blood proteins like serum albumin can shorten its retention time in sentinel lymph nodes (SLNs). Here, we investigated the efficacy and safety of a new fluorescence tracer comprising phytate and liposome (LP)-encapsulated ICG. Coadministration of phytate with LP containing phosphatidic acid promotes chelation mediated by Ca2+ in bodily fluids to enhance SLN retention. Uniformly sized LPs (100 nm) encapsulating ICG under conditions that minimized fluorescence self-quenching during storage were produced. We analyzed the behavior of the new tracer (ICG-phytate-LP) and control tracers (ICG and ICG-LP) in the lymphatic flow of mice in terms of lymph node retention time. We also tested lymphatic flow and safety in pigs that have a more human-like lymphatic system. LPs encapsulating stabilized ICG were successfully prepared. Mixing LP with phytate in the presence of Ca2+ increased both the particle size and negative surface charge. In mice, ICG-phytate-LP had the best lymph node retention, with a fluorescence intensity ratio that increased over 6 h and then decreased slowly over the next 24 h. In pigs, administration of ICG and ICG-phytate-LP resulted in no death or weight loss. There were no obvious differences between blood test results for the ICG and ICG-phytate-LP groups, and the overall safety was good. ICG-phytate-LP may be a useful new tracer for gynecological cancers that require time for lymph node identification due to a retroperitoneal approach.
Abbreviations
-
- 99mTc
-
- technetium-99m
-
- DLS
-
- dynamic light scattering
-
- DMPA
-
- dimyristoyl-sn-glycero-3-phosphatidic acid
-
- HSPC
-
- hydrogenated soybean phosphatidylcholine
-
- ICG
-
- indocyanine green
-
- LP
-
- liposome
-
- NIR
-
- near-infrared
-
- PB
-
- phosphate buffer
-
- PdI
-
- polydispersity index
-
- PLN
-
- pelvic lymph node
-
- PLND
-
- pelvic lymph node dissection
-
- RI
-
- radioisotope
-
- SLN
-
- sentinel lymph node
-
- SLNB
-
- sentinel lymph node biopsy
-
- SN
-
- secondary lymph node
1 INTRODUCTION
Sentinel lymph nodes (SLNs) are the first lymph nodes to receive lymphatic inflow from a primary tumor, and sentinel lymph node biopsy (SLNB) is used to determine whether lymph nodes associated with solid tumors are metastatic. Since SLNs were first reported in relation to malignant melanoma,1 they have been clinically applied in gynecology, general surgery, urology, and otolaryngology for monitoring of several types of solid tumors.2-6
In cervical cancer, pelvic lymph node dissection (PLND) is an important procedure for determining the stage of progression, and the presence or absence of lymph node metastasis is an important indicator of the need for adjuvant therapy.7, 8 However, since the rate of lymph node metastasis in early-stage cervical cancer is approximately 10%–20%,9-11 many patients may be receiving unnecessary PLND. In addition to increasing operative time and blood loss, PLND can also cause postoperative complications such as lymphedema and lymphangitis that may decrease quality of life.12, 13 Such complications can be avoided by omitting PLND for cases without lymph node metastasis that are diagnosed intraoperatively by SLNB.13
Dye, radioisotope (RI), and fluorescent tracers are currently used to monitor SLN.14 Technetium-99m (99mTc)-labeled phytic acid used with RI methods has a high molecular weight and is retained for long periods in the first node of lymphatic flow from a tumor, thus making this method suitable for SLNB. In fact, we have reported a high detection rate for SLN metastases in cervical cancer patients using the 99mTc-based RI method alone.13, 15, 16 However, RI methods require specialized equipment and expertise, which limits their availability. To avoid these restrictions, clinical application of a simpler fluorescent method involving indocyanine green (ICG) alone is increasing not only for cervical cancer,14, 17, 18 but also for breast cancer,19 malignant melanoma,20 and vulvar cancer.21 However, ICG aggregates in solution and its fluorescence intensity diminishes rapidly if injection does not occur within a few hours of preparation.22 In addition, ICG is a small molecule, and thus is quickly extravasated through collecting lymphatics or taken up by venous capillaries at the injection site or high endothelial veins in the lymph nodes.23
Liposomes (LPs) having a wide range of compositions and surface properties as well as good safety can be generated. They can also be miniaturized or diluted without loss of stability.24 Based on these characteristics, we hypothesized that LPs could be promising agents for imaging the lymphatic system as part of cancer therapy. Here, we describe the development of a new tracer, which is a complex between phytate and LPs carrying the fluorescent probe ICG that forms via chelation mediated by Ca2+ in bodily fluids. We investigated the efficacy and safety of this tracer in mice and pigs. The new tracer is expected to improve the stability of ICG in biological fluids while increasing its retention time in SLN.
2 MATERIALS AND METHODS
2.1 Tracer experimental section
2.1.1 Materials
Cholesterol was purchased from Tokyo Chemical Industry. Hydrogenated soybean phosphatidylcholine (HSPC) was purchased from NOF Corporation. Chloroform, 1,2-dimyristoyl-sn-glycero-3-phosphatidic acid (DMPA) and methanol were purchased from Wako Pure Chemical Industries. Di-sodium hydrogen phosphate, sodium dihydrogen phosphate, and anhydrous were purchased from Nacalai Tesque. ICG was purchased from Daiichi Sankyo. Phytate was purchased from FUJIFILM Toyama Chemical. Filter supports and 50× polycarbonate membranes (200 nm) were purchased from Avanti Polar Lipids and AVESTIN, respectively.
2.1.2 Stability of ICG in buffer solutions
Indocyanine green powder was dissolved in deionized water (final concentration, 320 mM [250 mg/mL]), and the solution was then diluted to 20 μM with various buffer solutions (10 mM phosphate buffer [PB], pH 7.4), saline solution (150 mM NaCl), Dulbecco's phosphate-buffered saline (DPBS), and deionized water. The stock solutions were stored at 4°C and the fluorescence intensity of the solutions was measured weekly using an Infinite 200 PRO (TECAN) instrument with λex 774 nm and λem 810 nm. PB solutions having an ICG concentration ranging from 0 to 100 μM were prepared to examine concentration dependence. The fluorescence intensity was also measured weekly as described above.
2.1.3 Preparation of ICG-LPs
To prepare ICG-LPs, lipids were first dissolved in a chloroform/methanol (9:1 vol:vol) solution at a molar ratio of 55:40:5 HSPC–cholesterol–DMPA (100 μM total lipid). LPs were formed using a thin-film hydration method. The lipid solutions were transferred to a pear-shaped flask that was placed in a water bath and attached to a rotary evaporation system. The chloroform/methanol solution was evaporated at 60°C, while the pressure was gradually reduced. The resulting lipid films were stored overnight in a vacuum desiccator. Then, 20 μM ICG solution (10 mM PB, pH 7.4) was added to the lipid film. The solutions were heated intermittently at 60°C for 30 min and then vortexed. The LPs were extruded 11 times at 60°C through a polycarbonate membrane (pore size: 200 nm) using a syringe extrusion device (Avanti Polar Lipids). After extrusion, the sample was quickly cooled and stored at 4°C. Phytate solution (1.76 mM in 10 mM PB [pH 7.4] was added 1:1 to LP to make LP-phytate.
2.1.4 Measurement of LP size and ζ-potential
The size and ζ-potential of the LPs was analyzed with a Zetasizer Nano ZS ZEN3600 (Malvern Instruments) and reported as particle size distributions and polydispersity index. ICG was diluted in saline solution. LPs were prepared with a total lipid concentration of 25 μM.
2.1.5 Tracers for testing
For the experiments described in this study, ICG alone, ICG-LP, and ICG-phytate-LP were prepared. The retention time in lymph nodes, safety, and efficacy of the three preparations were tested in mice. In experiments involving pigs, ICG alone and ICG-phytate-LP were used based on the results of experiments involving mice.
2.1.6 Animals
All procedures involving mice were conducted under the guidelines approved by the Animal Care and Use Committee of Kyushu University (approval reference A19-265-0, A21-127-0), and all experiments involving pigs were approved and performed by the Intervention Technical Center Animal Welfare Committee, Kobe Japan (IVT20-142, IVT-21-15, IVT21-144). The study was conducted according to the Animal Research Reporting In Vivo Experiments (ARRIVE) guidelines.
2.1.7 Mice
BALB/c nu/nu nude mice (CLEA Japan) that were 5–8 weeks old and weighed 20–25 g were used. ICG, ICG-LP, or ICG-phytate-LP (20 μL of a 20 μM solution) were administered subcutaneously in the right inguinal region of the mice. A median incision was made in the abdomen, and tracer was delivered to the lymph node area in the inguinal region under induction of anesthesia with 4% isoflurane, maintained at 2% or less. Mice were euthanized by cervical dislocation at the end of the observation period. Fluorescence of ICG in near-infrared (NIR) imaging was evaluated using an in vivo imaging system (IVIS) imaging system (Perkin Elmer).
Lymphatic flow in mice proceeds from the inguinal to the axillary region based on the SLN theory of lymphatic flow. If the inguinal lymph nodes are SLN, the axilla can be assumed to be secondary or later lymph nodes (Figure S1). All mice underwent a laparotomy in which the bilateral inguinal and bilateral axillary lymph nodes were removed, and fluorescence intensity was checked with the IVIS fluorescence system. To measure fluorescence intensity of lymph nodes over time, three groups of abdominal lymph nodes were collected 1, 3, 6, and 24 h after probe administration, and the fluorescence was measured with the IVIS system.
2.1.8 Pigs
Six female conventional specific-pathogen-free domestic pigs, strain LWD (Landrace-White-Duroc pig, crossbreeds) weighing 20–25 kg were used.
The control (ICG alone) and test substance (ICG, phytate acid, and a three-drug LP combination) were administered laparoscopically to the uterine cervix. The flow to the lymph vessels and lymph nodes after administration was observed hourly until 3 h after administration. After the observation period, the laparoscope was reinserted to check the administration site and surrounding tissues. The pelvic lymph node (PLN) near the cervix in the left PLN region was designated as the proximal lymph node, and those upstream of it were designated as the distal lymph node. To evaluate the safety of the administered samples, the pigs were observed for 30 days after administration. Body weight was measured and blood samples were collected before and 1, 8, 15, and 29 days after surgery. Animals that showed intraoperative (e.g., sudden decrease in blood pressure, sudden heart failure) or postoperative deterioration of condition (dyspnea and cyanosis, cessation of feeding and defecation, loss of voluntary movements, signs of distress) were euthanized and autopsied immediately.
2.2 Statistical analysis
To analyze differences between two or more groups, Student's t-test, Fisher's exact test, and ANOVA were performed using GraphPad Prism ver.9.00 for Windows (GraphPad Software). A value of p < 0.05 was considered to be statistically significant.
3 RESULTS
3.1 Stability of ICG in different media types
To select a suitable medium for preparation of LPs, we first examined the stability of ICG alone stored long term (>3 weeks) in several different types of aqueous media (Figure 1A). ICG was not stable in saline and phosphate-buffered saline due to the high ionic strength of these solutions. ICG was also not stable in pure water. The highest stability was seen for 10 mM PB. Next, we determined the optimum concentration of ICG in 10 mM PB (Figure 1B). Fluorescence intensity increased linearly up to 10 μM before reaching saturation at 20 μM. Above 20 μM, the fluorescence was quenched due to aggregation or concentration-dependent quenching. ICG at 20 μM was used for the LP preparation.

3.2 Preparation of ICG-LP coated with Ca2+/phytate complex
ICG-LP was prepared using a standard hydration method with hydration medium containing 10 mM PB and 20 μM ICG. The LP composition included a small amount of phosphatidic acid (DMPA), which has a phosphate group that interacts with the Ca2+/phytate complex (Figure 2A). The prepared LP was diluted with PB containing a physiological concentration of Ca2+ (1 mM) and/or phytate. The coating of ICG-LP with the Ca2+/phytate complex was confirmed based on DLS measurement of the size of ICG-LP (Figure 2B,C; Table 1). In the presence of Ca2+, the size of LPs without DMPA (DMPA[−] LP) was essentially unaffected, but LPs containing DMPA (DMPA[+] LP) were enlarged, indicating chelating activity of Ca2+ toward the DMPA phosphate group. In contrast, the presence of phytate caused an increase in the LP size whether or not DMPA was present, which may indicate an electrostatic interaction between the phytate and cationic ammonium group of HSPC. The presence of both phytate and Ca2+ resulted in particular enlargement of DMPA-containing LP, indicating the significant contribution of DMPA to the Ca2+/phytate complex. Together, these results suggest that DMPA-containing LP and spontaneous coating with the Ca2+/phytate complex could enhance retention of the LPs in lymph nodes.
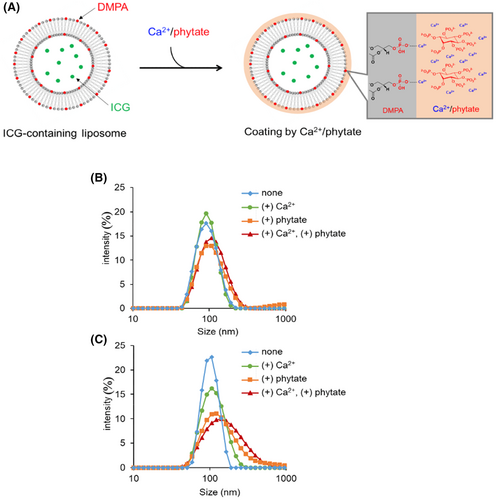
| DMPA (%) | Phytate (0.88 mM) | Ca2+ (1 mM) | Size (nm) | PdI | Z-potential (mV) |
|---|---|---|---|---|---|
| 0 | − | − | 89.7 | 0.13 | −3.8 |
| 0 | − | + | 89.4 | 0.076 | - |
| 0 | + | − | 113 | 0.28 | - |
| 0 | + | + | 106 | 0.19 | −6.7 |
| 5 | − | − | 98.5 | 0.036 | −10 |
| 5 | − | + | 109 | 0.16 | - |
| 5 | + | − | 138 | 0.31 | - |
| 5 | + | + | 146 | 0.27 | −13 |
- Note: Hyphen (-) means not measured.
- Abbreviations: DMPA, dimyristoyl-sn-glycero-3-phosphatidic acid; LP, liposome; PdI, polydispersity index.
3.3 Lymph node retention of tracers in mice
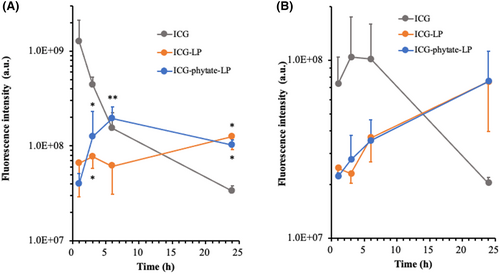
ICG, ICG-LP, and ICG-phytate-LP (20 μL of a 20 μM solution) were administered subcutaneously in the right inguinal region of mice. At the indicated time after administration, the right inguinal lymph node as a SLN and the right axillary lymph node as a secondary lymph node (SN) were resected to follow the migration of each tracer from SLN to SN (Figure S1). In the SLN of mice injected with ICG alone, the fluorescence of ICG reached maximum intensity 1 h after administration and thereafter rapidly disappeared. In the SN, ICG fluorescence intensity was observed beginning 1 h after administration and peaked between 3 and 6 h, before rapidly disappearing. These results showed the rapid migration of ICG from the injected region to the SLN and then to the SN. Meanwhile, in mice treated with ICG-LP or ICG-phytate-LP, the fluorescence intensity increased more slowly than that seen for ICG alone, and the fluorescence was retained for a longer period in the SLN. ICG-phytate-LP showed a clear peak 6 h after administration, whereas a constant weak fluorescence signal was seen for ICG-LP during the 24 h after administration. The ICG-phytate-LP group showed significantly enhanced fluorescence intensity relative to the ICG-LP group (Figure 3). Both tracers had a similar migration rate to the SN, and this rate was much slower than that for ICG alone. Based on the clear maximum in fluorescence intensity and long retention in the SLN, ICG-phytate-LP appears to be the most useful tracer among the three tested.
3.4 Imaging performance and safety of ICG tracers in pigs
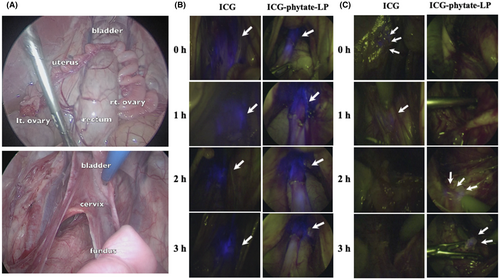
We next confirmed the safety of ICG and ICG-phytate-LP in a larger animal model involving pigs. A total of three pigs were used for each tracer. Trocars were implanted under general anesthesia and the abdominal cavity was observed. The cysto-uterine fossa peritoneum was incised and expanded, and 2 mL of tracer (ICG concentration was set at 20 μM) was administered directly from the peritoneal cavity to the uterine cervix. After tracer administration, the retroperitoneal cavity was expanded to check lymphatic flow (Figure 4A). The uterine cervix and retroperitoneum with lymphatic flow were checked with a NIR laparoscopic system immediately after drug administration, and 1, 2, and 3 h later. In the region of the cervix, no differences were observed grossly between the fluorescence of ICG and that of ICG-phytate-LP (Figure 4B). In the lymphatic flow after retroperitoneal delivery to the left PLN region, fluorescence of ICG was observed immediately after administration and 1 h later. At 2 and 3 h, fluorescent intensity has disappeared in the proximal lymph node. On the other hand, in the group treated with ICG-phytate-LP, fluorescence intensity persisted even 2 and 3 h after administration in proximal lymph node. (Figure 4C; Video S1).
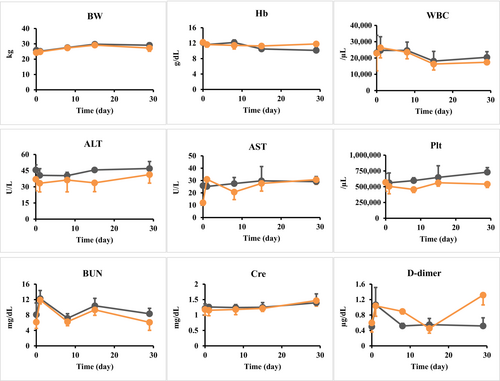
The blood test results and body weight on days 1, 8, 15, and 29 after surgery are shown in Figure 5. No weight loss or death was observed on the day after surgery, or 8, 15, and 29 days after surgery, for either tracer (Figure 5). Although results of blood tests did have some variation, differences between the ICG and ICG-phytate-LP groups were not significant and the overall safety was good.
4 DISCUSSION
Here, we designed a new tracer agent termed ICG-phytate-LP, and investigated its efficacy and safety relative to ICG. NIR fluorescence imaging measurements using wavelengths between 700 and 900 nm have been used to observe ICG, and NIR has also been used for optical imaging of the lymphatic system.25 However, ICG is unstable in solution and can rapidly enter venous capillary lymph vessels after local injection, making use of ICG alone unsuitable for some applications like quantitative lymph node imaging.24 Moreover, ICG aggregates in solution and rapidly loses fluorescence if not injected within a few hours of preparation.22 The low molecular weight of ICG allows it to quickly drain out of vessels through the collecting lymph vessels or to be taken up by venous capillaries at the injection site or high endothelial veins in the lymph nodes.23 Therefore, soon after injection into the uterine cervix, ICG may spread via lymphatic flow to the secondary and third nodes too rapidly to permit confirmation of vascular and lymphatic routes of transport or identification of more than one node. The rapid transport of ICG may also allow inadequate time to perform appropriate SLNB.
For this reason, we sought to develop an ICG-containing material that had a larger size which was comparable to that of 99mTc used in the RI method. A larger probe with a longer retention time would allow broader application of SLNB even at facilities without RI capabilities. Here, we developed a tracer that combined ICG with LPs, which are expected to be retained in the SLN for long periods. LPs are colloidal particles that can interact with or incorporate ICG and improve its spectral properties. For example, large phospholipids in LPs can decrease self-quenching of dyes and slightly increase their quantum yield.24, 26 The safety of LPs as well as their relatively small size, their stability after dilution, and range of composition and surface properties suggest that they would be promising agents for use as novel tracers for lymphatic system imaging.
Phytate is known to form colloidal aggregates with Ca2+ in biofluids in vivo.27 Coadministration of RIs such as Tc with phytate results in their incorporation into colloidal aggregates of Ca2+/phytate in situ via electrostatic interaction.28 Such aggregates promote enhanced retention of these isotopes in SLN. Ca2+/phytate complexes have previously been successfully used to prolong retention of ICG in tissues.29 Here, coadministration of ICG with phytate resulted in increased retention of ICG fluorescence in lymph nodes.
Nanoparticles containing PEGylated LPs have also been used to improve retention of ICG in lymph nodes.30 ICG aggregates in physiological saline conditions due to its hydrophobicity, and this aggregation results in quenching of its fluorescence. However, incorporation of ICG in the inner phase of PEGylated LPs avoids such aggregation by protecting ICG from the saline.24, 31, 32 Recently, the Akita group reported that the negatively charged lipid bilayers of PEGylated LPs are associated with enhanced retention in SLN.33, 34
Here we tested retention and safety of ICG-encapsulating LPs coated with a Ca2+/phytate complex, which is anchored to phosphatidic acid in the LP membrane. Thus, coadministration of these LPs with phytate will result in interactions with Ca2+ present in body fluids and spontaneous coating with Ca2+/phytate complexes. The negative charge of the complex may also promote its uptake by macrophages in the lymph nodes and in turn enhance retention in SLN. The animal studies also indicated that the tracer has good overall safety that may in part be due to its composition of only compounds that are approved for in vivo injection.
As mentioned above, ICG has been successfully used in gynecology for reduction surgery that involves SLN.14, 17, 18 Several reports described use of ICG in robot-assisted surgery not only for gynecological cancers but also for complete resection of deep endometriosis lesions while preserving pelvic nerves.35 In gynecologic cancers SLNs are located deep in the retroperitoneum, and, as has also been reported for head and neck cancers,36 can require identification of deep regional lymph nodes. The detection of SLNs can also be challenging due to their location in deep areas where tissue attenuation can limit signal transmission of fluorescent probes. Thus, ICG could flow to secondary or tertiary lymph nodes before ICG fluorescence is confirmed. In the present study, we showed that combining ICG and LPs with phytate resulted in a longer residence time in SLNs compared with ICG alone, and detectable fluorescence intensity was maintained for at least 6 h after administration. Taken together, this new tracer for SLNB for gynecological cancers, in contrast to those for breast cancer and malignant melanoma, which are close to the skin, could allow more time for identification of SLNs, particularly in the presence of retroperitoneal expansion.
This study does have several limitations. Injection of the tracer in mice and pigs could demonstrate its overall safety, but confirming the flow of ICG fluorescence and lymphatic flow over time in pigs was challenging. Moreover, at our facilities we do not have the capability to carry out RI studies in large animals. Thus, in a future study the performance of our new tracer should be compared with that of the conventional RI method.15, 16 In addition, further experiments involving more animals are needed to confirm the SLN model.
In conclusion, our results indicate that ICG-phytate-LP may be a useful tracer for gynecological cancers that require time for lymph node identification due to a retroperitoneal approach. We think that our novel tracer may contribute to the clinical application and dissemination of SLN in the future.
ACKNOWLEDGMENTS
We are grateful to Prof. Mototsugu Shimokawa, Department of Biostatistics, Graduate School of Medicine, Yamaguchi University for consultation on the statistical analyses. We also received technical assistance from the Animal Center and Research Support Center, Graduate School of Medicine, Kyushu University, and the Animal Welfare Committee of Kobe City.
FUNDING INFORMATION
Our study was supported in part by a Grant-in-Aid for Scientific Research from Kyushu University School of Medicine Obstetrics and Gynecology Research Fund (grant number: FAKF401232) and the Japan Society for the Promotion of Science (number: JP19K09804).
CONFLICT OF INTEREST STATEMENT
The corresponding author (Kiyoko Kato) is an Editorial Board Member of Cancer Science. The corresponding author has received funding from Astra Zeneca K.K. and Fuji Pharma Co., Ltd. Other authors have no conflict of interest.
ETHICS STATEMENT
The study protocol was approved by the Institutional Review Board of Kyushu University.
Animal Studies: All procedures involving mice were conducted under guidelines approved by the Animal Care and Use Committee of Kyushu University (approval reference A19-265-0, A21-127-0), and all experiments involving pigs were approved by the Intervention Technical Center Animal Welfare Committee, Kobe Japan (IVT20-142, IVT-21-15, IVT21-144). The study was conducted according to the Animal Research Reporting In Vivo Experiments (ARRIVE) guidelines.
Informed Consent: N/A.
Registry and the Registration No. of the study/trial: N/A.




