Conditional survival and recurrence of remnant gastric cancer after surgical resection: A multi-institutional study
Abstract
The present study was designed to evaluate the dynamic survival and recurrence of remnant gastric cancer (RGC) after radical resection and to provide a reference for the development of personalized follow-up strategies. A total of 298 patients were analyzed for their 3-year conditional overall survival (COS3), 3-year conditional disease-specific survival (CDSS3), corresponding recurrence and pattern changes, and associated risk factors. The 5-year overall survival (OS) and the 5-year disease-specific survival (DSS) of the entire cohort were 41.2% and 45.8%, respectively. The COS3 and CDDS3 of RGC patients who survived for 5 years were 84.0% and 89.8%, respectively. The conditional survival in patients with unfavorable prognostic characteristics showed greater growth over time than in those with favorable prognostic characteristics (eg, COS3, ≥T3: 46.4%-83.0%, Δ36.6% vs ≤T2: 82.4%-85.7%, Δ3.3%; P < 0.001). Most recurrences (93.5%) occurred in the first 3 years after surgery. The American Joint Committee on Cancer (AJCC) stage was the only factor that affected recurrence. Time-dependent Cox regression showed that for both OS and DSS, after 4 years of survival, the common prognostic factors that were initially judged lost their ability to predict survival (P > 0.05). Time-dependent logistic regression analysis showed that the AJCC stage independently affected recurrence within 2 years after surgery (P < 0.05). A postoperative follow-up model was developed for RGC patients. In conclusion, patients with RGC usually have a high likelihood of death or recurrence within 3 years after radical surgery. We developed a postoperative follow-up model for RGC patients of different stages, which may affect the design of future clinical trials.
1 INTRODUCTION
As a rare disease, remnant gastric cancer (RGC) accounts for 1%-7% of the incidence of gastric cancer (GC).1-4 Balfour first described the disease in 1922,5 and the definition evolved as the knowledge of the disease continued to deepen. Starting from the initial gastric stump cancer, RGC refers to carcinoma of the residual stomach occurring 5 years after gastrectomy for benign ulcer disease. Until now, it was generally believed that regardless of the nature of the first surgical gastric disease, or the absence of a specific time interval, cancer that occurs in the remnant stomach is RGC.6, 7 RGC is considered to have a different disease mechanism than primary gastric cancer (PGC).3, 4, 8-10 Moreover, because RGC is generally diagnosed at a relatively advanced stage, its prognosis is often worse than that of PGC.2, 3 However, due to the rarity of RGC, there is still no specialized follow-up strategy for RGC nearly a century after it was first described. Therefore, determining how to conduct effective prognostic risk stratification for such rare patients and develop a personalized follow-up strategy has become an urgent problem in current research.
In the initial stages of treatment for any malignant disease, a reliable prognostic assessment can help clinicians make decisions about adjuvant therapy, and frequency of follow up. Currently, the American Joint Committee on Cancer (AJCC) TNM staging system is the most widely used standard for assessing the prognosis of patients with RGC.11, 12 However, Survival estimates based on traditional survival curves may not provide accurate information for long-term outcomes as most tumor recurrences or deaths occur in the first few years after surgery.13-15 Therefore, the joint evaluation of dynamic changes in recurrence and survival is not only an important indicator for evaluating prognosis but is also an important reference for clinicians to develop follow-up strategies. Unlike static survival estimates, conditional survival (CS) can explain the dynamic changes in survival probability over time. CS is, therefore, considered to be a more clinically significant evaluation criterion for predicting the long-term prognosis of cancer patients who survive for a period after surgery.16-19 To the best of our knowledge, no previous study has assessed CS among patients who have undergone curative intent surgery for RGC. The aim of the present study was to evaluate the dynamic survival and recurrence of RGC after curative surgical resection using multi-center data and to provide a reference for the development of personalized follow-up strategies.
2 METHODS
2.1 Population and covariates
Remnant gastric cancer was defined as all new diagnoses of gastric carcinomas in the remnant stomach after partial gastrectomy regardless of the previous disease. From January 2004 to January 2017, 379 patients with RGC were retrospectively identified from seven centers in China (Fujian Medical University Union Hospital, the First Affiliated Hospital of Fujian Medical University, Zhangzhou Affiliated Hospital of Fujian Medical University, Longyan First Affiliated Hospital of Fujian Medical University, the First Affiliated Hospital of Xiamen University, the Affiliated Hospital of Putian University and Shanxi Provincial Cancer Hospital). The inclusion criteria for patients were defined as follows: the presence of remnant gastric carcinoma, no combined malignancy, no distant metastasis, no neoadjuvant chemotherapy and/or radiotherapy, complete basic information and complete survival data. The remaining 298 patients who underwent curative intent resection were included in the present study. The institutional review boards of all participating institutions approved the study (approval number: 2019KY021).
Well-known clinicopathological data were collected routinely. T stages and N stages were classified according to the criteria described in the AJCC staging manual (8th edition).12 The surgical procedure begins with separation of adhesion. D2 lymphadenectomy is performed, followed by resection of the remnant stomach. The reconstruction approach is performed using the Roux-en-Y method. Fluorouracil-based adjuvant chemotherapy was recommended for patients with advanced RGC in all participating institutions. All patients received standard postoperative follow-ups, including visits every 3-6 months for the first 2 years, every 6-12 months from the 3rd to 5th year, and annually thereafter. Most routine follow-up appointments included a physical examination, laboratory testing, chest radiography, and abdominopelvic ultrasonography or computed tomography. All patients were observed until death or the final follow-up date in January 2019. Overall survival (OS) was defined as the time from surgery to death from any cause. Disease-specific survival (DSS) was defined as the time from surgery to death from gastric cancer. In this study, disease-specific death events included death from recurrence and distant metastasis at a specific site, and death after tumor marker elevation but no specific recurrence site was found. In addition, we classified death within 6 months as disease-specific death.
2.2 Conditional survival concept
Conditional survival, the origin of which is rooted in conditional probability from biostatistics, can be calculated using the life-table method.16 The 3-year conditional survival (CS3) at x years indicates the likelihood of an additional 3-year survivorship for an individual who has already survived for x years after the initial treatment and is calculated as follows: CS3 = S(x + 3)/S(x).20 In this study, the data for overall survival and disease-specific survival were used to calculate the 3-year conditional overall survival (COS3) and 3-year conditional disease-specific survival (CDSS3), respectively.
2.3 Definition and categorization of recurrence
Recurrence was defined as the presence of a biopsy-proven tumor showing adenocarcinoma cells or the presence of imaging features highly suspicious of tumor recurrence. Recurrences were categorized by the site involved, as follows: locoregional, peritoneal, distant or multiple. The presence of recurrent disease in two or more sites was defined as multiple. Multiple recurrences in the same site were not categorized as “multiple” sites of recurrence.
2.4 Statistical analysis
The associations between variables and OS or DSS were assessed using a Cox proportional hazards model. CS analysis was then used to assess the possible changes in the prognostic impact of the clinicopathologic variables over time after surgery. The differences in CS between groups were compared via the standardized differences (d) method, which was first described by Cucchetti et al21 and has been employed by several groups,14, 22 where |d| < 0.1 indicates very small differences between groups, 0.1 ≤ |d| < 0.3 indicates small differences, 0.3 ≤ |d| < 0.5 indicates moderate differences and |d| ≥ 0.5 indicates obvious differences. The associations of relevant clinicopathological variables with recurrence were examined using logistic regression. In addition, we applied a second multivariable analysis at later time points (time-dependent multivariable analysis) to assess the independent predictors of survival or recurrence among patients who were alive after a certain number of years.23
All data were processed using SPSS 22.0 (SPSS) and R software (version 3.5.2). All tests were two-sided with a significance level set to P < 0.05.
3 RESULTS
3.1 Demographic and clinicopathological characteristics
Table S1 lists the general clinicopathological data of 298 patients with RCG who underwent radical surgery; 80.5% of patients were diagnosed with cancer in the remnant stomach more than 5 years after the first gastrectomy. The mean age of diagnosis for RCG patients was 64.9 years (range 37-89), and the ratio of male to female patients was 7.3:1. In total, 158 (53.0%) RGC were in the anastomotic area, and 140 (47.0%) RGC were in the non–anastomotic area. A total of 51.7% of the 298 patients were diagnosed with pathological stage III disease. The average number of lymph nodes (LN) retrieved was 16.0 (range 1-59), and the number of LN retrieved in 54.0% of patients was no more than 15.
3.2 Actual survival
After a median follow-up time of 61 months, 107 patients (35.9%) had recurrence, 157 patients (52.7%) died, of which 138 patients (87.9%) experienced disease-specific death. The 5-year OS of the whole group was 41.2%, and the 5-year DSS was 45.8% (Figure S1A,B). The risk of death after RGC radical surgery was not constant, with most RGC patients experiencing all-cause death or disease-specific death in the first 3 years after surgery (Figure S1C,D). When assessed over time, the likelihood of all-cause death and disease-specific death peaked at 12 months after surgery and diminished thereafter.
We also evaluated the probability of RGC patients surviving for a certain period after surgery (Figure 1A) and showed that with the prolongation of postoperative survival, the probability of disease-specific survival improved. Correspondingly, with the prolongation of postoperative survival time, the probability of recurrence in RGC patients decreased (Figure 1B).
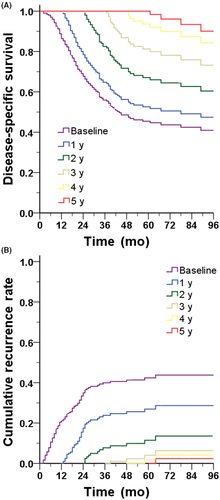
3.3 Comparing actual survival with conditional survival
Figure 2A shows the actual OS of the whole group of patients and the annual COS3 changes within 5 years after surgery. The results showed that for the entire cohort of RGC patients, the 3-year actual OS and the COS3 at baseline were equal; however, contrary to the declining trend of the actual OS curve, the COS3 increased with prolonged postoperative survival. After 2 years of survival, the COS3 was 59.3%, which was different from the actual 5-year OS (41.2%). When the patient survived for 5 years, the COS3 reached 84.0%, which was significantly higher than the actual 8-year OS (34.6%). A similar trend can be observed for DSS (Figure 2B). For example, when a patient survives for 5 years after surgery, the CDSS3 is 89.8%, which is significantly higher than the actual 8-year DSS of 41.2%.
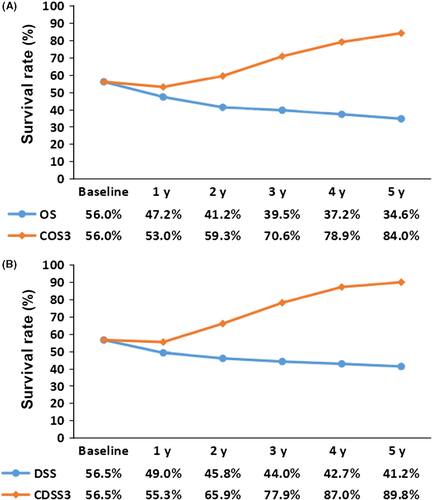
3.4 Univariate and multivariate cox regression analysis at baseline
Univariate Cox regression analysis (Table S2) showed that for the 298 patients with RGC, age, sex, lymphovascular invasion, combined resection, histological differentiation, macroscopic type, tumor size, T stage, and N stage were associated with OS at the time of surgery (P for all < 0.05). Previous disease, the interval between the first operation and the RGC diagnosis, and whether the number of LN retrieved was greater than 15 were not associated with OS at baseline (P for all > 0.05). Further multivariate Cox regression analysis showed that age (≥65 years: hazard ratio [HR] = 1.41, P = 0.038), poorly differentiated adenocarcinoma (undifferentiated: HR = 1.50, P = 0.018), large tumor size (≥4 cm: HR = 2.28, P < 0.001), advanced T stage (≥T3: HR = 3.40, P < 0.001) and lymph node metastasis (N+: HR = 2.42, P < 0.001) were independent risk factors for OS at baseline. After adjusting for the potential confounding factors for DSS, multivariate analysis showed that histological differentiation, tumor size, T stage and N stage were independent prognostic factors that initially affected DSS.
3.5 Stratified analysis of 3-year conditional survival changes
Figure 3 shows the temporal trends of OS and COS3 for each independent prognostic factor in RGC patients. The stratified analysis found that for any subgroup, COS3 was higher than the actual OS, and this gap showed an increasing trend over time. It is worth noting that in RGC patients who initially had unfavorable prognostic features, the gap between the actual OS and COS3 was more pronounced over time. For example, for patients with ≥T3 stage disease, although their 8-year OS was only 25.4%, their COS3 reached 83.0% (Δ57.6%) after 5 years of survival. However, the trend corresponding for patients with the initial relatively favorable prognostic features was not obvious, such as ≤T2 patients who had an 8-year OS of 60.1% and a COS3 of 85.7% (Δ25.6%) 5 years after surgery. Subgroup analyses (Table S3) revealed that the initial unfavorable prognosis factors (large tumors, undifferentiated cancer, advanced T stage and advanced N stage) corresponded to a rapid increase in COS3. We also found that the difference in COS3 between the patients with initial unfavorable prognostic features and those with favorable prognostic features gradually decreased over time. For example, the d values between ≥T3 patients and ≤T2 patients gradually decreased from 0.72 (significant difference) at baseline to 0.37 (moderate difference) at 3 years postoperatively and 0.07 (very small difference) at 5 years after surgery.
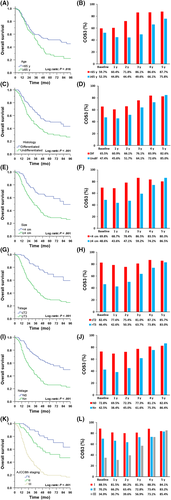
Similarly, patients with initially unfavorable tumor characteristics had the most significant changes in CDSS3 compared to patients with initially favorable tumor characteristics; differences in long-term prognosis between the calculated CDSS3 and actuarial DSS were again most pronounced among patients who were initially predicted to have the worst DSS (Figure S2, Table S4).
3.6 Timing and pattern of recurrence
Most recurrences (more than 90.0%) occurred within 3 years after surgery (Table S5). Figure 4A shows the distribution of initial recurrence sites in all patients. Most patients (74.8%) had initial recurrence involving only one site; the remaining 27 (25.2%) patients had multiple sites of recurrence. Figure 4B-D depicts the recurrence pattern in each staged RGC patient; the pattern showed that patients with RGC are more prone to recurrence at multiple sites as the disease stage progresses (Phase I: 10.0%, Phase II: 14.3%, Phase III: 30.2%; P < 0.001). There was a significant difference in recurrence rates between stages (P < 0.001), with a recurrence rate of 15.4% in stage I, 26.6% in stage II, and 49.4% in stage III. The logistics regression showed that only the AJCC stage was an independent factor that affected postoperative recurrence in RGC patients (Table S6). Detailed analysis of the AJCC staging showed that advanced T stage (T4: OR = 3.58) and advanced N stage (N3: OR = 2.99) were independent risk factors for postoperative recurrence in RGC patients (Table S7). Among patients who experienced a recurrence, the median post–recurrence survival was only 8.0 months. There was no difference in survival after relapse in each stage (P = .700; Figure S3).
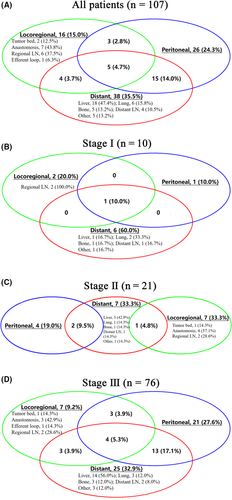
3.7 Dynamic changes in risk factors for survival and recurrence
Time-dependent multivariate Cox analysis (Table 1) showed that at baseline, age, histological differentiation, tumor size, T stage and N stage were independent prognostic factors for OS. After 3 years of survival, only age and tumor size were independent prognostic factors for OS (P for all < 0.05). Notably, after 4 years of survival, age and tumor size no longer independently affected OS in RGC patients (P for all > 0.05). At baseline, histological differentiation, tumor size, T stage and N stage significantly affected DSS, but after 3 years of survival, only tumor size remained a significant predictor of DSS (P < 0.05). However, after 4 years of survival, the common prognostic factors could no longer independently affect DSS (P for all > 0.05).
| Overall survival | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Baseline | 1 year | 2 year | 3 year | 4 year | ||||||||||
| N = 298, E = 155 | N = 240, E = 116 | N = 150, E = 69 | N = 111, E = 43 | N = 83, E = 25 | |||||||||||
| HR | 95%CI | P | HR | 95%CI | P | HR | 95%CI | P | HR | 95%CI | P | HR | 95%CI | P | |
| Age | |||||||||||||||
| <65 y | Ref | Ref | Ref | Ref | Ref | ||||||||||
| ≥65 y | 1.41 | 1.02-1.95 | .038 | 1.55 | 1.07-2.26 | .021 | 1.75 | 1.07-2.86 | .025 | 2.34 | 1.25-4.38 | .008 | 2.00 | 0.87-4.63 | .104 |
| Histology | |||||||||||||||
| Differentiated | Ref | Ref | Ref | Ref | Ref | ||||||||||
| Undifferentiated | 1.50 | 1.07-2.10 | .018 | 1.58 | 1.08-2.33 | .02 | 1.44 | 0.88-2.37 | .146 | 1.66 | 0.87-3.16 | .124 | 2.11 | 0.86-5.15 | .102 |
| Size | |||||||||||||||
| <4 cm | Ref | Ref | Ref | Ref | Ref | ||||||||||
| ≥4 cm | 1.51 | 1.01-2.26 | .047 | 1.25 | 0.80-1.95 | .319 | 1.67 | 0.91-3.06 | .099 | 2.38 | 1.03-5.48 | .042 | 1.66 | 0.58-4.81 | .347 |
| T stage | |||||||||||||||
| ≤T2 | Ref | Ref | Ref | Ref | Ref | ||||||||||
| >T3 | 2.02 | 1.20-3.42 | .008 | 1.34 | 1.02-1.78 | .038 | 1.21 | 0.86-1.71 | .265 | 1.14 | 0.75-1.75 | .532 | 1.00 | 0.56-1.79 | .988 |
| N stage | |||||||||||||||
| N0 | Ref | Ref | Ref | Ref | Ref | ||||||||||
| N+ | 1.52 | 1.04-2.22 | .032 | 1.42 | 0.92-2.19 | .113 | 1.21 | 0.69-2.12 | .513 | 0.96 | 0.47-1.97 | .906 | 0.99 | 0.36-2.69 | .983 |
| Disease-specific survival | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Baseline | 1 Year | 2 Year | 3 Year | 4 Year | ||||||||||
| N = 298, E = 138 | N = 240, E = 98 | N = 150, E = 52 | N = 111, E = 27 | N = 83, E = 13 | |||||||||||
| HR | 95%CI | P | HR | 95%CI | P | HR | 95%CI | P | HR | 95%CI | P | HR | 95%CI | P | |
| Histology | |||||||||||||||
| Differentiated | Ref | Ref | Ref | Ref | Ref | ||||||||||
| Undifferentiated | 1.50 | 1.05-2.13 | .026 | 1.66 | 1.09-2.54 | .018 | 1.64 | 0.93-2.89 | .090 | 2.04 | 0.9-4.62 | .088 | 3.31 | 0.86-12.74 | .082 |
| Size | |||||||||||||||
| <4 cm | Ref | Ref | Ref | Ref | Ref | ||||||||||
| ≥4 cm | 1.58 | 1.02-2.45 | .039 | 1.35 | 0.83-2.20 | .23 | 2.01 | 0.98-4.14 | .058 | 3.31 | 1.06-10.4 | .04 | 3.02 | 0.57-15.96 | .193 |
| T stage | |||||||||||||||
| ≤T2 | Ref | Ref | Ref | Ref | Ref | ||||||||||
| >T3 | 2.32 | 1.29-4.16 | .005 | 1.42 | 1.04-1.94 | .029 | 1.21 | 0.82-1.79 | .340 | 1.17 | 0.68-2.01 | .575 | 0.98 | 0.42-2.29 | .960 |
| N stage | |||||||||||||||
| N0 | Ref | Ref | Ref | Ref | Ref | ||||||||||
| N+ | 1.61 | 1.07-2.42 | .022 | 1.49 | 0.93-2.39 | .100 | 1.27 | 0.66-2.45 | .468 | 1.06 | 0.43-2.64 | .899 | 1.25 | 0.3-5.27 | .757 |
- E, number of events; N, sample size of each cohort.
If the AJCC stage was included in the time-dependent multivariate regression analysis, then only the AJCC stage and age were independent prognostic factors that affected OS at baseline (Table S8). The AJCC stage can continuously affect OS in the first 3 years after surgery (P < 0.05). However, after 4 years of survival, the AJCC stage no longer independently affected the OS of RGC patients. For DSS, after a stepwise backward variable removal, only the AJCC stage independently affected the OS postoperatively up to 3 years after surgery (P < 0.05), but after 4 years of survival, the AJCC stage lost its prognostic significance (P > 0.05).
Further time-dependent logistic regression analysis (Table S9) showed that the AJCC stage independently affected recurrence in RGC patients within 2 years after surgery (P < 0.05). In contrast, for patients who survived disease-free for more than 2 years, there was no significant difference in the probability of recurrence among the three stages (P for all >0.05).
3.8 Follow-up models
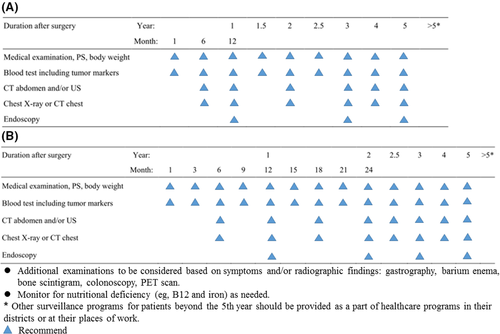
Based on the analyses of conditional survival and recurrence, we developed a postoperative follow-up model for RGC patients of different stages (Figure 5). For patients with stage I disease, follow-up visits are recommended every 6 months for the first 3 years after surgery. In the 4th and 5th years after surgery, follow-up visits are recommended once annually. For patients with stage II-III disease, follow-up visits are recommended every 3 months in the first 2 years after surgery, every 6 months in the 3rd year after surgery, and annually in the 4th and 5th years after surgery. For RGC patients who have survived for more than 5 years after surgery, the visits should be based on routine healthcare programs and supplemented by specialist examinations related to GC. At the same time, during the follow-up period, clinicians can adjust the corresponding follow-up strategy according to the patient’s symptoms and imaging findings.
4 DISCUSSION
Remnant gastric cancer is a rare and complicated cancer. Although the number of patients who undergo surgery for benign gastric diseases has been reduced, the early detection of PGC and improvements in the prognosis of PGC patients lead to an increase in the incidence of RGC.1-3 While the standardization of RGC surgery and the development of multiple adjuvant treatment therapies have improved the survival rate of RGC patients, the 5-year survival rate of RGC patients is still 10%-90% for those who have different tumor features24, 25, and as high as 27%-36% of RGC patients relapse after surgery.26-28 To compare the survival of remnant gastric cancer (RGC) with primary gastric cancer, a systematic literature search was performed using PubMed with the keywords “remnant,” ‘‘stomach” and “cancer,” revealing 1065 relevant reports published up to the end of December 2018. The 5-year survival rate of RGC and primary upper third gastric cancer (PUGC) was compared in 21 eligible studies (Table S10).1, 29-48 In total, 6611 patients (947 RGC and 5664 PUGC) were included in this meta-analysis. According to the heterogeneity test, there was statistical significance in the heterogeneity among 21 studies (I2 = 72.0%, P < 0.001). The funnel plot indicates that there is a certain publication bias (Figure S4). Therefore, a random-effect model was used. Overall, the 5-year survival rate of RGC (Figure S5) was lower than that of PUGC (risk ratio = 1.13, 95%CI: 1.01-1.26, P = 0.04). We found that the prognosis of patients with RGC was worse than that of patients with PUGC.
Traditional assessments of prognosis for patients who have malignant tumors would usually be reported as survival rates for certain time points (eg, the 5-year survival rate). However, these estimates are constant, and this simple information does not provide accurate prognostic assessments for oncologists and patients, especially when patients have survived for a long period after surgery. Many clinicians believe that RGC patients should reduce the frequency of their follow-ups 2-3 years after surgery, but there are no data or models to provide an accurate time point, so these recommendations are often only based on experience. CS, a new survival assessment indicator, can dynamically assess changes in the risk of postoperative death based on the patient’s survival time. In this study, we evaluated the actual survival and CS3 of RGC patients. The results showed that with the prolongation of survival time, the CS3 of patients initially diagnosed with unfavorable tumor characteristics gradually approached or even surpassed that of patients with favorable tumor characteristics (Figures 3 and S1). We found that the postoperative survival of RGC patients was also consistent with the “natural selection effect” hypothesis proposed by Zamboni et al.17 Most patients with a high risk of cancer-related death will die soon after surgery. In turn, the gradual death of high-risk patients promotes the natural selection of low-risk patients, making the prognosis of the surviving cohort more favorable. Understanding the increased likelihood of continued survival over time will help alleviate the anxiety of RGC survivors, especially those who were initially judged to have an unfavorable prognosis. More importantly, the optimal follow-up frequency and duration can be determined based on the patient’s actual risk of death.
Our study showed that for resectable RGC, 51.7% of patients were diagnosed at stage III; despite the development of adjuvant therapy, nearly 50% of stage III patients relapsed after surgery. The median survival time of patients after recurrence was only 8 months. The primary goal of postoperative follow ups was to improve survival by promoting early detection of recurrence in asymptomatic stages.49 This reflects the need for regular follow ups and early diagnosis of recurrence for more targeted treatment. Our results show that the AJCC stage was an independent factor that affected recurrence, and patients with more advanced RGC were more likely to relapse than those with early RGC. The exploration of optimized adjuvant chemotherapy for patients with advanced RGC is an area that needs to be addressed in future clinical research. Moreover, patients with stage III disease are more likely to have multiple recurrences and metastases after surgery. In addition, we found that 57.4% of RGC patients had distant metastatic components at recurrence, suggesting that imaging of all possible sites (eg, liver and lung) is important at follow up. Data on specific recurrence patterns and time to recurrence can not only provide information about the risk of recurrence but also provide evidence of a postoperative follow-up program. For patients with certain adverse factors, more frequent and rigorous monitoring may be needed to detect early recurrence. Our study showed that after 2 years of disease-free survival, there was no significant difference in the probability of recurrence between patients with different pathological stages. However, more than 90% of RGC patients relapse within 3 years after surgery. When RGC patients survived for more than 3 years after surgery, the probability of recurrence was greatly reduced, suggesting that the frequency of follow up for detecting recurrence can be reduced to achieve the best cost-benefit balance.
Currently, most GC guidelines only mention the definition of RGC and the location of RGC. 6, 50-52 There is no specialized consensus on the follow-up strategy for RGC. RGC are often excluded from clinical trials of surgical procedures and chemotherapy for GC due to changes in lymphatic drainage associated with RGC and potentially different tumorigenesis mechanisms from PGC. The development of an individualized postoperative follow-up model for RGC can not only benefit clinical practice but also help design future clinical trials. Our results showed that the CDSS3 of patients with RGC who survived 5 years after radical surgery was close to 90%, indicating that most survivors have achieved a “tumor cure” status after 5 years of survival. Time-dependent multivariate Cox regression analysis showed that only the AJCC stage affected OS and DSS continuously within 3 years after surgery (P < .05). To a certain extent, this reflects that the AJCC stage has a relatively stable effect on survival; it is notable that the AJCC stage also lost its ability to affect patient OS and DSS after 4 years of survival, which was reflected regardless of the clinical status at the time of initial diagnosis because tumor-specific death rarely occurs after 4-5 years of survival. Based on previous literature reports for PGC 3, 50-54 and the results of this study, we developed a follow-up model for patients with RGC. We recommend focusing on postoperative follow up for the first 3 years after RGC diagnosis and setting different follow-up intensities and frequencies (3-6 months) according to different stages. We also found that patients with stage II and III disease have a high recurrence rate, especially for peritoneal metastasis and liver metastasis, which reflects the importance of imaging examinations such as abdominal CT scanning or ultrasonography for the early diagnosis of abdominal organ metastasis in the early follow up. In the 4th and 5th years after surgery, regardless of the pathological stage, we recommended reducing the intensity and frequency of follow ups and adopting a medium-intensity follow-up strategy to avoid discomfort caused by too-frequent examinations and to reduce unnecessary medical costs. After 5 years of survival, due to the low risk of recurrence and tumor-specific death, we recommend follow-up monitoring of RGC as part of a regional general healthcare program with routine physical examinations (1-2 years).
Most of the research on RGC has been through single-center retrospective studies. The evidence level is low, and the detailed prognosis of RGC has been a knowledge blind spot. This study not only considered the CS and recurrence of RGC patients for the first time but also analyzed the effect of dynamic changes of prognostic factors on postoperative survival in patients with RGC. The present study has some limitations. First, because this study is a multi-center retrospective study, some potential biases are inevitable. Furthermore, although the results obtained by incorporating data from multiple centers in China are highly prevalent and applicable, the number of cases is still relatively small, and some stratified analyses may be limited. At present, East Asian and the USA are still referring to guidelines for advanced PGC in the treatment of advanced RGC. In East Asian countries, including China, radical gastrectomy and postoperative adjuvant chemotherapy have been used as standard treatments for advanced GC for many years. European and American countries have more choices for preoperative neoadjuvant therapy, radical surgery and postoperative adjuvant therapy as treatment for advanced GC. In this study, patients with neoadjuvant therapy were excluded. Due to the differences in treatment patterns between East Asian and the USA, it is impossible to deny that neoadjuvant therapy may affect the postoperative recurrence patterns of RGC patients. In addition, lack of validation of Western data is another limitation of this study. It is clearly inadvisable to generalize the results of this study to patients in Western countries. However, these results can at least provide a reference for similar research in Western countries.
In conclusion, RGC is a rare and unique disease, and future comprehensive cooperation is needed. Most patients with RGC experience disease-specific death and recurrence within 3 years after radical surgery, and the death and recurrence rates change dynamically over time. The evaluation of CS and recurrence can provide more valuable information to determine follow-up strategies. Based on multi-center data, we developed a follow-up model for patients with RGC, which may provide some reference for the development of guidelines and future clinical research for RGC.
ACKNOWLEDGMENTS
We thank Shisheng Wang and his “Wu Kong” platform for the help with statistical analysis methods. This work was supported by Scientific and Technological Innovation Joint Capital Projects of Fujian Province (2017Y9011), the Construction Project of Fujian Province Minimally Invasive Medical Center (No. [2017]171), the Second Batch of Special Support Funds for Fujian Province Innovation and Entrepreneurship Talents (2016B013), the Fujian Science and Technology Innovation Joint Fund Project (2017Y9004) and the Youth Project of Fujian Provincial Health and Family Planning Commission (2016-1-41).
DISCLOSURE
None of the authors have any competing interests in the manuscript.




