Adipogenesis induces growth inhibition of dedifferentiated liposarcoma
Abstract
Well-differentiated liposarcoma (WDLPS) and dedifferentiated liposarcoma (DDLPS) are the most common types of liposarcoma. Although WDLPS and DDLPS patients receive intensive treatment including radical surgery and systemic therapy, their overall 5-year survival rates are 90% and 30%, respectively, indicating that DDLPS is clinically more aggressive. We examined whether adipogenic stimulation induces adipogenesis in human WDLPS/DDLPS cells by using dexamethasone, indomethacin, insulin, and 3-isobutyl-1-methylxanthine (IBMX), all putative medications or drugs. Functional in vitro experiments showed that treatment with these four compounds induced adipogenic potency by transcriptional and translational upregulation of genes related to the maintenance of stemness and adipogenic differentiation. Using in vivo xenograft models, we found that the induction of stemness and adipogenesis inhibited the tumorigenic potency of DDLPS. This study suggests a potential application of drug repositioning in which adipogenesis-inducing compounds could be used to treat DDLPS patients in a clinical setting.
Abbreviations
-
- DDLPS
-
- dedifferentiated liposarcoma
-
- LPS
-
- liposarcoma
-
- MDM2
-
- mouse double minute 2 homolog
-
- PPAR
-
- peroxisome proliferator-activated receptor
-
- WDLPS
-
- well-differentiated liposarcoma
1 INTRODUCTION
Liposarcoma is one of the most common soft tissue sarcomas, accounting for approximately 25% of all soft tissue sarcoma cases.1 LPS is classified into four subtypes: WDLPS, DDLPS, myxoid/round cell LPS, and pleomorphic LPS.1 WDLPS and DDLPS are the most common types of LPS (88%) and contain supernumerary ring and/or giant rod chromosomes formed by the amplification of chromosome 12q13-15, which contains several hundred genes including MDM2 and CDK4.2 Overall 5-year survival rates of WDLPS and DDLPS patients are 90% and 30%, respectively, suggesting that DDLPS is clinically more aggressive than WDLPS.3
Radical surgery remains the predominant treatment for most forms of LPS, whereas more advanced or metastatic diseases are treated using systemic therapy. Recently, systemic therapy for WDLPS/DDLPS patients has included the following:4, 5 (i) conventional cytotoxic chemotherapy; (ii) marine-derived drugs; (iii) tyrosine kinase receptor inhibitors; (iv) MDM2 or CDK4 antagonists; (v) PPAR-γ agonists; and (vi) immunotherapy. Although cytotoxic-based chemotherapy tends to elicit a higher response rate in various sarcomas (45%), the overall objective response rate is low (12%) in the majority of advanced WDLPS/DDLPS cases.6, 7 Eribulin, a marine-derived drug, has been approved for use as a second-line LPS treatment.8 Pazopanib, a multi-targeted tyrosine kinase receptor inhibitor, has been approved for the treatment of advanced non-adipocytic soft tissue sarcomas.9 Although the MDM2 antagonist, Nutlin 3A, was shown to inhibit the interaction between TP53 and MDM2 in preclinical LPS models, it showed toxicity in a neoadjuvant setting; clinical trials for several CDK4 antagonists are still incomplete.10, 11 Numerous PPAR-γ agonists such as thiazolidinedione, rosiglitazone, and efatutazone showed promising safety and tolerability, but no antitumor activity or clinical response; thus, further clinical trials for these therapies are ongoing.12-14 Finally, NY-ESO-1 has been successfully used as a target for immunotherapy in malignant tumors; however, its use is restricted to myxoid/round cell LPS not WDLPS/DDLPS.15 Therefore, novel systemic therapy is urgently needed for WDLPS/DDLPS based on the unique molecular features of these diseases.
Stemness is roughly defined as the self-renewal potency and transdifferentiation ability of a cell. Cancer progression involves a gradual weakening of differentiated cellular characteristics.16 In general, undifferentiated tumors are more likely to be associated with disease progression and poor prognosis than differentiated tumors.17 Interestingly, Zhang et al18 reported that small cell lung cancer NCI-H446 cells maintained their stemness and potency for multilineage differentiation, and the growth of their corresponding xenograft tumors was inhibited by osteogenic differentiation therapy. Therefore, we examined whether adipogenic differentiation is a useful systemic treatment for WDLPS/DDLPS.
2 MATERIALS AND METHODS
2.1 Cell lines and reagents
LIPO-246 and LIPO-863B, LP6, and MLS-402 and MLS-1765 cell lines were kindly provided by Dr Dina Lev, Dr Jonathan A. Fletcher, and Dr Pierre Aman, respectively. The cells were cultured in DMEM (Thermo Fisher Scientific, Waltham, MA, USA) containing 10% FBS (Gibco) and 1% antibiotic-antimycotic (Gibco) at 37°C and in a 5% CO2 incubator. Cell lines were validated for human cell line authentication (STR DNA profiling, Figure S1) using an AmpFLSTR Identifiler PCR Amplification Kit (Thermo Fisher Scientific). Mycoplasma contamination was not detected in any cells.
2.2 Adipogenic differentiation assay
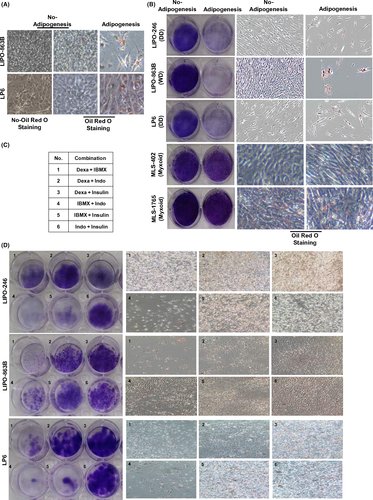
Cells were seeded into a 6-well plate in DMEM medium, which was then replaced with an adipogenic differentiation medium (StemPro Adipogenic Differentiation Kit; Invitrogen, Carlsbad, CA, USA), all four components, or the indicated combination (Figure 1C) of inducing adipogenic differentiation reagents with dexamethasone, IBMX, indomethacin, or insulin (Sigma, St Louis, MO, USA) in complete DMEM medium every 3–4 days. After 21 days, the cells were stained with an oil Red O staining kit (Lifeline Cell Technology, Carlsbad, Ca, USA) according to the manufacturer's instructions.
A more detailed version of materials and methods is included in Data S1.
3 RESULTS
3.1 Growth inhibition of human WDLPS/DDLPS cells by inducing adipogenesis in vitro
To examine whether adipogenic stimulation induces adipogenesis in human LPS cells, we carried out oil Red O staining after the cells had been cultured in commercial adipogenic induction medium. LIPO-863B (WDLPS) and LP6 (DDLPS) cells showed numerous lipid droplets compared to the corresponding control cells (cells not treated with adipogenic induction medium) (Figure 1A). Next, we reviewed the literature to identify methods of pharmacologically inducing adipogenic differentiation in human cells. Many research groups have reported that the four compounds, dexamethasone, IBMX, indomethacin, and insulin, can induce adipogenic differentiation of human bone marrow stem cells.19 Currently, dexamethasone, indomethacin, and insulin are used to treat immune disorders and attenuate uncontrolled blood sugar levels, whereas IBMX is considered a potential drug for treating inflammation. Based on this information, we examined whether these four compounds could induce adipogenic differentiation of WDLPS/DDLPS cells. WDLPS (LIPO-863B) and DDLPS (LIPO-246 and LP6) cells showed an increased level of oil Red O staining positivity, whereas myxoid LPS cells (MLS-402 and MLS-1765) did not (Figure 1B). Interestingly, treatment with these compounds inhibited the growth of WDLPS/DDLPS cells but not myxoid LPS cells (Figure 1B). These results indicate that adipogenic differentiation can inhibit the growth of WDLPS/DDLPS but not myxoid LPS cells.
To determine which of these compounds induces adipogenic differentiation and growth inhibition, WDLPS/DDLPS cells were treated with combinations of one, two, three, or four of the compounds (data not shown). We found that some of the two-compound combinations showed induction of adipogenic differentiation and inhibition of growth similar to those shown by the four-compound combination. Therefore, we compared oil Red O staining positivity and growth inhibition for each two-compound combination (Figure 1C). Compared to that in the corresponding control cells (Figure 1B, No-Adipogenesis), several combinations showed increased oil Red O staining positivity and reduced growth in WDLPS/DDLPS cells. LIPO-246, LIPO-863B, and LP6 cells showed the greatest response to treatment combinations 4, 1, and 4, and were also more responsive to treatments 5, 4, and 1, respectively (Figure 1D).
We monitored the expression level of NANOG, OCT-4, and SOX2, genes involved in maintaining stemness (Figure 2A). LIPO-246 cells treated with combination 4 showed upregulated expression of all three genes compared to those treated with combination 5, and showed a higher level of OCT-4 expression than cells treated with all four compounds. LIPO-863B cells treated with combination 1 showed upregulated OCT-4 expression compared to those treated with combination 4, whereas their NANOG and SOX2 expression levels were similar to those of cells treated with all four compounds. LP6 cells treated with combination 4 showed upregulated NANOG expression compared to those treated with combination 1; however, their OCT-4 and SOX2 expression levels were similar to those of cells treated with all four compounds.
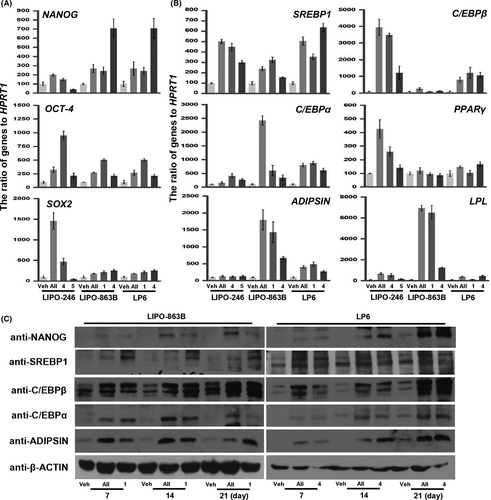
We also compared the expression level of genes serially induced during the transcriptional regulation of adipogenesis: SREBP1 (all stages), C/EBPβ (early stage), C/EBPα and PPAR-γ (middle to late stages), and ADIPSIN and LPL (late stage), for each of the treatment combinations (Figure 2B).20 LIPO-246 cells treated with combination 4 showed upregulated expression of all genes except ADIPSIN compared to those treated with combination 5, and the expression levels of all genes except PPAR-γ were similar to those in the cells treated with all four compounds. Expression of C/EBPβ was higher after treatment with all four compounds. LIPO-863B cells treated with combination 1 showed higher expression levels of all genes except C/EBPβ compared to those treated with combination 4, and their levels of PPAR-γ, ADIPSIN, and LPL expression were similar to those following treatment with all four compounds. LP6 cells treated with combination 4 showed higher SREBP1, PPAR-γ, and ADIPSIN expression levels compared to those treated with combination 1, whereas their levels of C/EBPβ, PPAR-γ, and LPL expression were similar to those following treatment with all four compounds.
Next, we examined the translational upregulation of these genes during induction with the selected combinations for 7, 14, and 21 days (Figure 2C). LIPO-863B cells treated with all four compounds showed higher expression levels of all proteins except SREBP1 compared to those treated with combination 1, and their level of C/EBPβ expression was similar to that following treatment with combination 1. LP6 cells treated with combination 4 showed similar expression levels of all proteins compared to those following treatment with all four compounds. Taken together, these results suggest that treatment with specific combinations (combination 4 for LP6 and LIPO-246, and combination 1 for LIPO-863B) induces adipogenic potency by transcriptional and translational upregulation of genes related to stemness and early-late adipogenic differentiation.
3.2 Inhibition of human DDLPS growth by inducing adipogenesis in vivo
To evaluate the antitumorigenic effect of treatment with adipogenesis-inducing compounds in vivo, LIPO-246, LIPO-863B, and LP6 cells were s.c. inoculated into nude mice and randomly treated with vehicle, two-compound combinations, or all four compounds. In the pilot trial, LIPO-863B cells formed tumors with a high penetrance (3/3); however, their volume reached only approximately 200 mm3 after monitoring for 32 days post-injection (Figure S2). Therefore, a full-scale trial could not be carried out using LIPO-863B xenografts even though reduced tumor growth (ie, weight and volume) was observed following treatment with all four compounds and combination 1 (Figure S2). LIPO-246 xenografts showed reduced tumor growth following treatment with all four compounds, but not combination 4 (Figure 3A). Tumor growth was reduced in the LP6 xenografts treated with combination 4 and all four compounds in a method dependent on the number of compounds (Figure 3B). We then carried out histological analysis on the xenograft model-derived tumors (Figure S3). Cells from the compound-derived tumors showed fewer proliferative features and had low cellularity compared to those from the vehicle-derived tumors (Figure S3). Tumors treated with all four compounds showed increased oil Red O staining positivity compared to those treated with combination 4 (Figure 3C).

In parallel with our in vitro experiments, we examined whether treatment with the compounds enhanced the transcriptional and translational regulation of the genes involved in stemness and adipogenesis in vivo (Figure 3D-F). LP6 xenografts treated with combination 4 showed upregulated expression of all genes except OCT-4 compared to those treated with all four compounds (Figure 3D, left panel). Treatment with all four compounds caused the expression of all genes except SREBP1 and ADIPSIN to be upregulated compared to treatment with combination 4 (Figure 3D, middle and right panels). However, the expression levels following treatment with combination 4 were considerably higher than in those without treatment (Veh). LP6 xenografts treated with all four compounds showed upregulated expression of all proteins except NANOG compared to that by treatment with combination 4 (Figure 3E). Tumors treated with all four compounds showed increased expression and nuclear localization of SREBP1 than those treated with combination 4. (Figure 3F). These findings indicate that the induction of adipogenesis and stemness inhibits the potency of DDLPS tumors in vivo.
4 DISCUSSION
Although DDLPS patients receive intensive treatment such as radical surgery and systemic therapy, their overall 5-year survival rate is only 30%.3 It has been shown that in vitro, PPAR-γ plays a role in the mechanism that reverts the DDLPS subtype to a well-differentiated LPS subtype associated with a potentially milder disease course; therefore, several clinical trials of PPAR-γ agonists have been conducted in LPS patients.12, 21 However, these studies have shown that antitumor activity only occurs in myxoid and pleomorphic LPS subtypes and that the agonists have mixed effects in LPS patients.12-14 Therefore, the DDLPS subtype is currently very challenging to treat and a novel systemic DDLPS therapy is needed.
Differentiation therapy emerged from several reports in the 1980s that stated that hormones and cytokines may enhance differentiation ex vivo in acute myeloid leukemia (AML). Currently, this concept is successfully used in AML treatment by combining retinoic acid and arsenic.22 Recently, drugs have been discovered which promote differentiation in nasopharyngeal carcinoma, thyroid cancer, melanoma, glioma, cholangiocarcinoma, and sarcoma, and it has been suggested that they are worthy of clinical trials.23 In the present study, we examined whether WDLPS/DDLPS cells can be converted into a more differentiated phenotype by adipogenic differentiation. It is well known that the four compounds, dexamethasone, IBMX, indomethacin, and insulin, can induce adipogenic differentiation of human bone marrow stem cells.19 Importantly, these compounds have been used to treat conditions such as immune system disorders. We first found that these four compounds can induce adipogenic differentiation of DDLPS cells by transcriptional and translational upregulation of genes involved in the maintenance of stemness and adipogenic differentiation, leading to growth inhibition both in vitro and in vivo. Many studies similar to ours reported the uncoupling of differentiation and self-renewal in an AML model under various conditions.23 Ablain et al24 reported that high levels of retinoic acid strongly induced differential potency together with loss of self-renewal, and thus caused tumor clearance. Therefore, we hypothesize that terminal adipogenic differentiation inhibits self-renewal and cell growth in DDLPS. Additionally, cells treated with specific two-compound combinations generally showed similar effects to those shown by cells treated with all four compounds, depending on the cell line used. These findings suggest that optimizing the essential components from all four compounds would help reduce the number of compounds required for further preclinical trials.
Based on their utility as treatments and the findings of our in vitro and in vivo experiments, we suggest that these adipogenesis-inducing drugs could be repositioned to treat DDLPS patients in a clinical setting.
ACKNOWLEDGMENTS
We would like to thank Dr Dina Lev, Dr Jonathan A. Fletcher, and Dr Pierre Aman for their generous gifts and Minjung Sung for technical support. This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean Government (NRF-2013R1A1A2011536 and 2016R1A5A2945889).
DISCLOSURE
Authors declare no conflicts of interest for this article.




