c-Src inhibitor selectively inhibits triple-negative breast cancer overexpressed Vimentin in vitro and in vivo
Funding information
Talents planning of six summit fields of Jiangsu Province (Grant/Award Number: ‘WSW-026‘), National Natural Science Foundation of China (Grant/Award Numbers: ‘81302305‘, ‘81172502‘), Maternal and child health research projects of Jiangsu Province (Grant/Award Number: ‘F201678‘), The Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD) (Grant/Award Number: ‘JX10231801‘).
Abstract
Oncogene c-Src has been found to be a potential target for the treatment of triple-negative breast cancer (TNBC). However, the therapeutic effects of the c-Src inhibitor on TNBC patients are controversial compared to those on cell lines. The molecular mechanisms of the inhibitory effects of the c-Src inhibitor on TNBC remain unclear. Herein, we showed that a specific c-Src inhibitor, PP2, was effective in inhibiting phosphorylation of c-Src in 4 cell lines: T-47D, SK-BR-3, SUM1315MO2, and MDA-MB-231, regardless of hormone receptors and human epidermal growth factor receptor 2 (HER2) expression levels. Giving PP2 preferentially reduced the S phase of cell cycles and inhibited colony formation in SUM1315MO2 and MDA-MB-231, but not in SK-BR-3 and T-47D cells. Furthermore, PP2 effectively blocked cell migration/invasion and epithelial-mesenchymal transition (EMT) in TNBC cell lines, SUM1315MO2 and MDA-MB-231. An EMT biomarker, vimentin, was highly expressed in 2 TNBC cell lines when they were compared with SK-BR-3 and T-47D cells. Further depletion of vimentin by shRNA remarkably attenuated the inhibitory effects of the c-Src inhibitor on TNBC cells in vitro and in vivo, indicating a crucial action of vimentin to affect the function of c-Src in TNBC. This study provides an important rationale for the clinic to precisely select TNBC patients who would benefit from c-Src inhibitor treatment. This finding suggests that traditional markers for TNBC are not sufficient to precisely define this aggressive type of cancer. Vimentin is identified as an important biomarker to enable categorization of TNBC.
1 INTRODUCTION
Breast cancer is the cancer with the highest incidence cancer among women and accounts for 14% of total cancer-related deaths.1 Concerning gene expression and phenotype, breast cancer has been classified into 21 distinct histological subsets and at least 4 different molecular subtypes.2, 3 Triple-negative breast cancer (TNBC) is the most intractable subgroup, because of its higher frequency of relapse and metastasis than others. It represents 15%-20% of breast carcinomas and is defined as estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) negative breast cancer.4, 5 Lack of hormone receptors (ER and PR) and HER2 means that TNBC patients cannot benefit from endocrine therapy and targeted therapy. Thus, the discovery of effective and specific medications for TNBC is urgently needed. Currently, a large number of potential targets have been suggested for cancer treatment, such as p53, mammalian target of rapamycin (mTOR), tyrosine kinases and epidermal growth factor receptor (EGFR).6-9 However, in clinical trials, none has yet been certified for efficient TNBC therapy.7
Proto-oncogene c-Src is widely overexpressed in breast cancer. It plays a vital role in multiple cellular signal transduction pathways and participates in diverse processes, such as cell growth, motility, invasion, attachment, and angiogenesis.10-12 c-Src inhibitors can actively suppress tumor cell growth in the breast, colon, ovaries, and liver.13-15 A large number of studies have indicated that TNBC shows higher sensitivity to the c-Src inhibitor than do other cancer subgroups.16-19 It has been proven that ER and HER2 expression levels affect the therapeutic effects of the c-Src inhibitor in breast cancer cell lines.16 Unfortunately, patients with TNBC receive limited benefit from c-Src inhibitor treatment.20-22 A phase II clinical trial showed only a 9.3% clinical benefit rate when a c-Src inhibitor, dasatinib, was used as a single agent.22 This suggests that ER and HER2 expression levels are not sufficient to precisely define the resistance to the c-Src inhibitor in TNBC.
TNBC is a heterogeneous subset and shares (80%-90%) genetic abnormalities and morphological similarities with basal-like breast cancer (BLBC).23 BLBC contains at least 2 major subtypes which are termed BLBC A and BLBC B (VIM positive).24 Finn et al17 reported that the BLBC B subgroup is 85.7% (6 in 7) highly sensitive to dasatinib whereas BLBC A was 0% (0 in 9). Nevertheless, further exploration is needed to clarify the molecular mechanisms of this subtype of breast cancer.
Vimentin is a primary marker of epithelial-mesenchymal transition (EMT) and is responsible for maintaining cell shape, integrity of the cytoplasm, and stabilizing cytoskeletal interactions. It functions as an important adaptor of many crucial proteins involved in adhesion, migration, invasion, and cell signaling.25, 26 In breast cancer, vimentin expression is upregulated during EMT, and is highly expressed in BLBC B with poor prognosis.27 Thus, there is a need to investigate how BLBC B is highly sensitive to the c-Src inhibitors and whether vimentin is involved.
In the present study, we sought to investigate whether vimentin expression affects the therapeutic effects of the c-Src inhibitor in a panel of breast cancer cell lines with different phenotypes. Four different subtype breast cancer cell lines were selected: SUM1315MO2 (BLBC B), MDA-MB-231 (BLBC B), SK-BR-3 (HER2 + ), and T-47D (ER+). Our results showed that the c-Src inhibitor effectively inhibits cell migration and invasion mainly through blocking EMT in SUM1315MO2 and MDA-MB-231 cells, but not in SK-BR-3 and T47D cells. Vimentin is identified as a crucial molecule to affect the function of the c-Src inhibitor. Knockdown of vimentin remarkably attenuates the inhibitory effects of the c-Src inhibitor on 2 TNBC cell lines. This study provides an important rationale for the clinic to precisely select TNBC patients who would benefit from c-Src inhibitor treatment.
2 MATERIALS AND METHODS
2.1 Reagents and antibodies
PP2 was purchased from MCE (MedChemExpress, Monmouth Junction, NJ, USA) and dissolved in DMSO (Sigma-Aldrich, St Louis, MO, USA). Anti-human Src, p-Src (Tyr416), Akt, p-Akt (Thr308), GAPDH, CDK4, cyclin D1, cyclin E1, vimentin, E-cadherin, and N-cadherin antibodies were obtained from Cell Signaling Technology (Danvers, MA, USA). Anti-human Snail antibody was purchased from Abcam (Cambridge, MA, USA). Anti-mouse and anti-rabbit antibodies were from Jackson Immunoresearch (West Grove, PA, USA).
2.2 Cell lines
Human breast cancer cell lines were obtained as described: MDA-MB-231, SK-BR-3, and T-47D from ATCC (Manassas, VA, USA) and SUM1315MO2 was kindly provided by Stephen Ethier (University of Michigan, USA). These cells were all routinely maintained in DMEM media (Gibco, Grand Island, NY, USA) supplemented with 10% heat inactivated FBS (Gibco) and penicillin-streptomycin solution at 37°C in a humidified atmosphere containing 5% CO2.
2.3 Quantitative real-time reverse transcription PCR
Cells were treated with different concentrations of PP2 (0, 2.5, and 5.0 μmol/L) for 48 hours. Cells were then harvested in Trizol (TaKaRa, Kusatsu, Japan). Total RNA isolated from cells was converted to first-strand cDNA using Primescript RT Reagent (TaKaRa) following the manufacturer's instructions. Quantitative real-time PCR assays were carried out with Fast-Start Universal SYBR Green Master (TaKaRa) and the StepOnePlus Real-Time PCR System (Applied Biosystems, Carlsbad, CA, USA). Relative quantification was calculated by the 2-ΔΔCt method. Sequence of primers is shown in Table S1. All data were normalized by GAPDH.
2.4 CCK-8 assay
Quantity of viable cells was evaluated by using a CCK-8 kit (Dojindo, Tokyo, Japan) according to the manufacturer's protocol. Cells were trypsinized and seeded at a density of 5 × 103 in 96-well plates (Corning, Corning, NY, USA). After overnight incubation, PP2 was added to the medium with different concentrations for certain times. At the end of the experiments, the medium in each well was replaced with 100 μL serum-fresh DMEM containing 10% CCK-8. Plates were incubated at 37°C for 2 hours and then read at 450 nm with a microplate reader (Tecan Austria, Grödig, Austria).
2.5 Colony formation assay
For clonogenic assay, cells were pretreated with DMSO or (2.5, 5.0 μmol/L) PP2 for 48 hours. SUM1315MO2 and MDA-MB-231 breast cancer cells were seeded at 500 per well for 14 days, SK-BR-3 and T-47D were seeded at 1000 per well for 21 days. The colonies were fixed with 70% ethanol for 3 minutes, then stained with 1% crystal violet for 15 minutes, and finally washed twice with PBS.
2.6 Cell cycle analysis
Briefly, cells were treated with vehicle control (DMSO) or PP2 (2.5 or 5.0 μmol/L) for 48 hours. Then, cells were harvested and gradually fixed with 75% ethanol (EtOH) on ice. After staining with propidium iodide (PI), cells were analyzed using a FACSort flow cytometer (BD Biosciences, San Jose, CA, USA), and the data were analyzed by CellQuest software (BD Biosciences).
2.7 Wound-healing assay
Cells were pretreated with DMSO or PP2 (2.5 and 5 μmol/L) for 48 hours. Then, cells were seeded into 6-well plates and cultured at 37°C until they reached 80%-90% confluence. The monolayers were scratched with a pipette tip (200 μL; Corning) and cell fragments were removed by washing with PBS. Next, cells were cultured in medium without FBS. Images were taken at 0 and 24 hours. Migration ratios were then calculated. Migration distance was assessed using Image-Pro Plus 6.0 software (Media Cybernetics, Rockville, MD, USA).
2.8 Cellular migration and invasion assay
Cell migration and invasion assays were carried out using a Boyden chamber (8 μm, 24-well format; Corning). Invasive chamber was coated with 150 μL ice-cold Matrigel (BD Biosciences) diluted in DMEM basal medium and incubated overnight at 37°C. Cells were pretreated with DMSO or PP2 (2.5 and 5 μmol/L) for 48 hours. Then, cells were seeded on the upper chamber in serum-free medium. The lower chamber was filled with 600 μL of 10% FBS-supplemented medium. After 20 hours, non-migrated cells were removed with a cotton swab in the top chamber, and then the migrated/invasive cells on the membrane were stained with 1% crystal violet for 15 minutes. Number of invading/migrating cells was then counted under a microscope and photographed.
2.9 Western blotting
Cells treated with PP2 for 48 hours were harvested with lysis buffer (1 mL lysis buffer with 10 μL PMSF, 10 μL phosphatase inhibitors, and 1 μL protease inhibitors). Supernatant was collected after 19 283 g centrifugation for 12 minutes. Proteins were separated by SDS-PAGE gel and transferred to a PVDF membrane. Signal pathways were probed with specific antibodies.
2.10 Construction of plasmids and stable transfected cell lines
Plasmid vectors and negative control were designed and packaged from GeneCopoeia (Guangzhou, China). Sequence of shRNAs is shown in Table S2. Cells were then transfected with these plasmids using Lipofectamine 3000 (Thermo Fisher Scientific, Waltham, MA, USA) following the manufacturer's instructions. Puromycin (Amresco, Solon, OH, USA) was used to screen stable cell lines. All of the vectors were marked by enhanced GFP.
2.11 Tumor xenograft mouse model
Animal experiments were conducted in an animal room with specific pathogen free (SPF) standards. All animal experiment protocols were reviewed and approved by the Institutional Animal Care and Use Committee of Nanjing Medical University. Female BALB/c nude mice aged 5-6 weeks used in this study were obtained from The Animal Model Research Center of Nanjing University (Nanjing, China). Mice were divided into two groups (n = 12): 1 group was s.c. injected with SUM1315MO2 cells transfected with control vector; another group was s.c. injected with SUM1315MO2 cells transfected with ShRNA1 after anesthesia by injecting 1% pentobarbital sodium. Seven days later, mice of each group were randomly divided into a treatment group and a control group (n = 6). The control group mice received 1% DMSO, and the treatment group mice received a daily i.p. injection of 10 mg/kg PP2. Mice were treated for 3 weeks. Body weight and tumor size were monitored daily. Finally, mice were killed and the tumor tissues were excised. Tumor volume was calculated using the following formula: l × b2 × 0.5, where l and b are the largest perpendicular of the tumor.
2.12 Statistical analysis
Each experiment in this study was repeated at least 3 times unless otherwise specified. All results are presented as mean ± SD. A 1-sided Student's t test was used to calculate the statistical significance between the groups in vitro whereas tumor volume was analyzed by a 2-sided Student's t test. Data were analyzed using Image-Pro Plus 6.0 software, GraphPad Prism 6.0.1 software (GraphPad, LaJolla, CA, USA) and SPSS 10.0 software (IBM, Armonk, NY, USA). P < .05 was considered statistically significant.
3 RESULTS
3.1 c-Src inhibitor effectively blocks phosphorylation of c-Src in all cell lines regardless of phenotype
In the current study, a specific c-Src inhibitor, PP2 (Figure 1A) was given to investigate the function of c-Src in a panel of breast cancer cell lines (SUM1315MO2, SK-BR-3, T47D, and MDA-MB-231). Protein levels of c-Src were similar among the 4 cell lines (Figure 1B), whereas expression levels of vimentin were much lower in SK-BR-3 and T47D than in SUM1315MO2 and MDA-MB-231 cells (Figure 1B). mRNA expression levels of c-Src and vimentin were consistent with protein levels (Figure 1C,D, respectively). Four cell lines were further treated with PP2 at different concentrations for 48 hours. c-Src inhibitor effectively inhibited phosphorylation of c-Src in all cell lines (Figure 1E). This suggests that PP2 can significantly block c-Src phosphorylation in both vimentin-positive and -negative breast cancer cell lines.
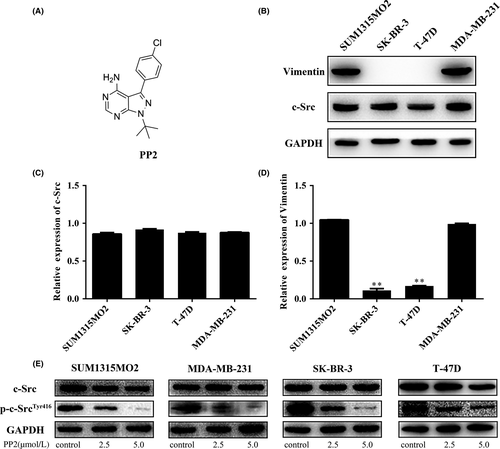
3.2 c-Src inhibitor displays different inhibitory effects on vimentin-positive and -negative breast cancer cell lines
To examine the influence of PP2 on cell proliferation, 4 cell lines were treated with different doses of PP2 for 48 hours. CCK-8 results indicated that PP2 inhibited cell growth in a dose-dependent method (Figure 2A), but IC50 of SUM1315MO2 and MDA-MB-231 was half of that in SK-BR-3 and T-47D. Then, cells were treated with 1 dose of PP2 (5.0 μmol/L) for different times (days). As shown in Figure 2B, PP2 remarkably inhibited vimentin-positive SUM1315MO2 and MDA-MB-231 cell growth, but not vimentin-negative SK-BR-3 and T-47D cells (Figure 2B). Consistently, the number of cell clones of SUM1315MO2 and MDA-MB-231 was significantly reduced when they were compared with SK-BR-3 and T-47D (Figure 2C), indicating that vimentin-positive SUM1315MO2 and MDA-MB-231 cells were more sensitive to PP2 treatment. Cell cycle analysis showed that PP2 clearly decreased S phage of cell cycles in SUM1315MO2 and MDA-MB-231 cells, but was without significant effects on the other 2 vimentin-negative cell lines (Figure 2D). Further examination of signal pathways indicated that PP2 significantly inhibited an important cell growth pathway, Akt phosphorylation, in SUM1315MO2 and MDA-MB-231 cells, but not in SK-BR-3 and T-47D cells (Figure 2E). As for another growth pathway, RAS-MAPK, PP2 significantly inhibited phosphorylation of MAPK in MDA-MB-231 cells, but not in SK-BR-3 cells (Figure S1). In line with this, cell cycle-associated signals: cyclin E1, cyclin D1, and CDK4 were reduced in SUM1315MO2 and MDA-MB-231 cells after PP2 dosage, whereas this was without significant effects on the other 2 cell lines (Figure 2E). All of these results show that PP2 predominantly inhibits vimentin-positive TNBC cell proliferation.
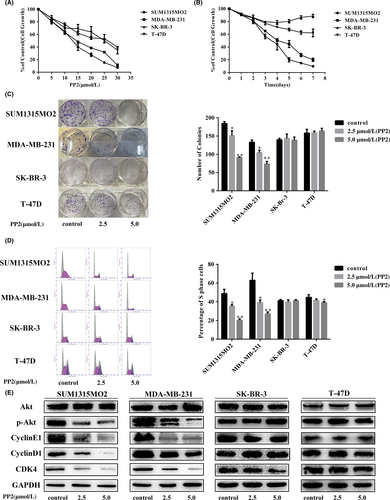
3.3 c-Src inhibitor prevents EMT in TNBC expressed high vimentin cell lines
c-Src is an important adaptor protein to mediate cellular focal adhesions.16 We first examined the effect of PP2 on cell motility in 4 cell lines. Migrated cell numbers of SUM1315MO2 and MDA-MB-231 were much higher than those in Sk-Br-3 and T47D cells (Figure 3A), and PP2 significantly blocked migration in the 4 cell lines, particularly in the 2 triple negative cell lines (Figure 3A). Further invasive assay indicated that MDA-MB-231 was aggressive with the highest invasive capacity among the 4 cell lines (Figure 3B) and PP2 completely prevented the invasion of MDA-MB-231 cells (Figure 3B). Invasive capacity of SUM1315MO2 was weaker than in MDA-MB-231 cells, but higher than in the other 2 cell lines, SK-BR-3 and T47D. PP2 effectively blocked invasion of SUM1315MO2 (Figure 3B), whereas it almost had no effects on the SK-BR-3 and T-47D cell lines. By wound-healing assay, PP2 remarkably prevented wound healing in 2 TNBC cell lines, but not in SK-BR-3 and T47D cells (Figure 3C). Further examination of EMT markers by western blotting indicated that the expression levels of mesenchymal markers (N-cadherin and vimentin) were decreased, whereas E-cadherin was increased by PP2 in SUM1315MO2 and MDA-MB-231 cells (Figure 4D). Snail was also inhibited by PP2 in TNBC cells, which serves as an induction transcription factor for EMT.28, 29 There was no clear alteration of those markers after PP2 treatment in SK-BR-3 and T47D cells (Figure 4D). To further confirm the relationship between c-Src and vimentin, SUM1315MO2 and MDA-MB-231 were treated with siRNA or another c-Src inhibitor PP1 to knock down c-Src. Our results indicated that vimentin expression was reduced by c-Src siRNA and PP1 (Figures S2,S3). These results indicate that prevention of EMT is an important mechanism for the c-Src inhibitor to inhibit TNBC cell migration and invasion.
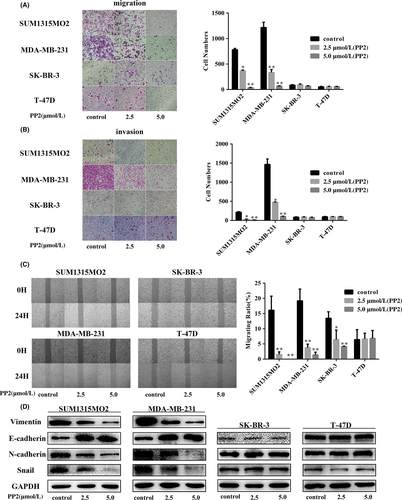
3.4 Knockdown of vimentin prevents the c-Src inhibitor from blocking colony formation in TNBC cell lines
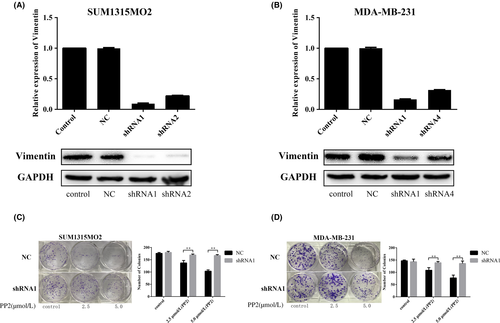
To verify that vimentin expression levels can impact the therapeutic effects of PP2, vimentin was knocked down by shRNAs in SUM1315MO2 and MDA-MB-231 cells. Four different shRNAs were used to deplete vimentin, and our results showed that shRNA1 and shRNA2 effectively downregulated vimentin in mRNA and protein levels in SUM1315MO2 cells (Figure 4A). Also, shRNA1 and shRNA4 both reduced mRNA in MDA-MB-231 cells, but only shRNA1 worked well to significantly decrease protein levels of vimentin in MDA-MB-231 (Figure 4B). Thus, shRNA1 was selected for further experiments. Colony formation by SUM1315MO2 and MDA-MB-231 cells was compared between vimentin expression and vimentin knockdown (Figure 4C,D). Our results showed that knockdown of vimentin did not affect colony formation in either cell line. It was interesting to find that depletion of vimentin remarkably prevented PP2 from inhibiting colony formation in SUM1315MO2 and MDA-MB-231. This finding clearly indicates that vimentin is an important molecule to affect the therapeutic effects of the c-Src inhibitor in TNBC cell lines.
3.5 Depletion of vimentin attenuates the inhibitory effects of PP2 on the cell cycle and related signals in TNBC cells
The c-Src inhibitor remarkably reduced the percentage of S phage of cell cycles in SUM1315MO2 and MDA-MB-231 cells transfected with control shRNA (Figure 5A,B). Knockdown of vimentin did not significantly alter the cell cycles in 2 cell lines (Figure 5A,B), but clearly attenuated the inhibitory effects of PP2 on the decreasing S phase of cell cycles (Figure 5A,B). PP2 was still effective in inhibiting the phosphorylation of c-Src in 2 cell lines after vimentin knockdown (Figure 5C,D). However, depletion of vimentin prevented PP2 from inhibiting phosphorylation of Akt and cell cycle-associated signaling pathways, such as cyclin E1, cyclin D1, and CDK4 (Figure 5C,D). These results all suggest that vimentin is closely associated with c-Src to regulate the cell cycle in TNBC cells.
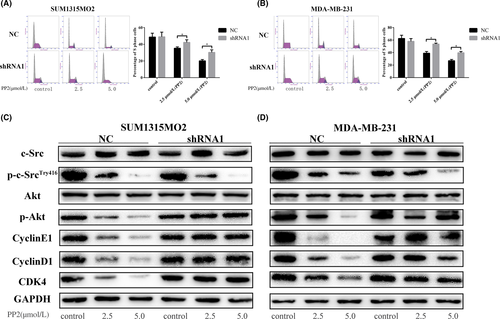
3.6 c-Src inhibitor cannot effectively prevent EMT after knockdown of vimentin in TNBC cell lines
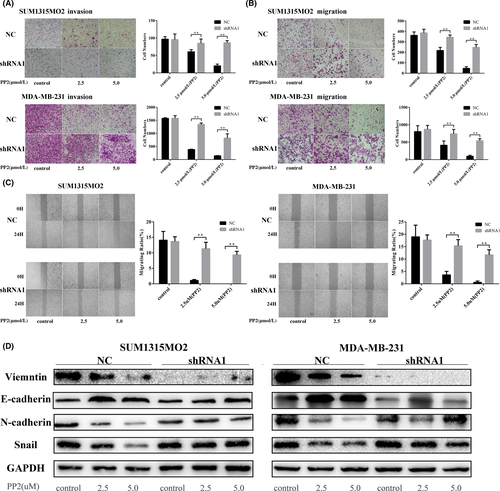
PP2 was effective at blocking cell migration and invasion in SUM1315MO2 and MDA-MB-231 cells transfected with control shRNA (Figure 6A,B). However, PP2 was unable to block either migration or invasion in these 2 cell lines after depletion of vimentin (Figure 6A,B). Similar responses were observed in that PP2 remarkably inhibited wound healing in SUM1315MO2 and MDA-MB-231 cells transfected with control shRNA (Figure 6C), but it lost the inhibitory function in these 2 cell lines after knockdown of vimentin (Figure 6C). Notably, cells became more sensitive to PP2 treatment after overexpressing vimentin in the TBNC cell line HCC1937 (Figure S4). Further examination of signaling pathways showed that the c-Src inhibitor did not increase E-cadherin nor decrease N-cadherin and Snail when vimentin was knocked down in SUM1315MO2 and MDA-MB-231 cells. All of these results indicate that vimentin is closely related with c-Src to modulate EMT in TNBC cells.
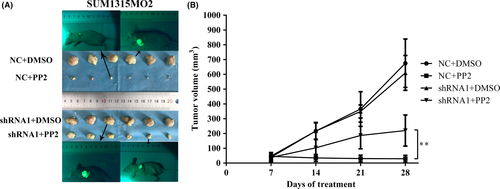
3.7 Therapeutic effects of PP2 on SUM1315MO2 xenografted mouse model
Our results show that knockdown of vimentin did not significantly change tumor size (Figure 7A,B). Although PP2 remarkably inhibited tumor growth in both vimentin normal expression and vimentin knockdown groups, the therapeutic effects of PP2 on mice xenografted with SUM1315MO2 containing normal vimentin were clearly better that those on mice xenografted with SUM1315MO2 depletion of vimentin (Figure 7A,B). This in vivo result confirmed that vimentin is a crucial molecule to affect the function of c-Src in TNBC cells.
4 DISCUSSION
Expression of high levels of ER and HER2 has been shown to be 2 indicators for resistance to c-Src inhibitor treatment in breast cancer cell lines.16 In agreement with this observation, compelling evidence indicates that TNBC cell lines show a high sensitivity to the c-Src inhibitor.16-19 However, clinical trials indicate a controversial result in TNBC patients treated with the c-Src inhibitor with a lower rate of benefit.20-22 This implies that more factors are involved in TNBC to affect the function of c-Src, in addition to the traditional biomarkers: ER/PR and HER2. Our findings show that breast cancer cells with high levels of vimentin are highly sensitive to c-Src inhibitor dosage in vitro and in vivo. Depletion of vimentin in the TNBC cell lines remarkably attenuates the inhibitory effects of the c-Src inhibitor. This suggests that vimentin is an important biomarker to predict the therapeutic effects of the c-Src inhibitor in TNBC patients.
More reports have indicated remarkable diversity in the molecular characteristics of TNBC.24, 30-32 Vimentin is identified as a mesenchymal marker for basal B TNBC, which makes epithelial cancer cells more aggressive with high motility and invasion.33, 34 SUM1315MO2 and MDA-MB-231 belong to basal B TNBC with high expression levels of vimentin, which are highly sensitive to the c-Src inhibitor. By contrast, vimentin-negative TNBC cell lines HCC1937 and MDA-MB-468 have low adherent activity and cellular invasive ability (data not shown). Further overexpressing vimentin in HCC1937 makes the cell more sensitive to PP2 treatment. These observations clearly suggest that basal B TNBC mainly relies on EMT-associated signaling pathways to progress aggressively.35 Targeting EMT is a major mechanism for the c-Src inhibitor to block invasive capacity in this subset of TNBC.
Non-receptor tyrosine kinase c-Src has been found to be an essential molecule to modulate EMT through multiple pathways.36 c-Src mediates the activation of oncogenic pathways,16, 37 including PI3K-Akt and Ras-MAPK signaling, which are associated with EMT induction.38, 39 Importantly, c-Src is a critical adaptor protein to modulate focal adhesion molecule responses related with caveolin-1, paxillin, and p130CAS.40-42 These focal adhesion signals are closely associated with metastatic potential of TNBC.36, 40, 41 How vimentin and c-Src link together to modulate EMT in TNBC remains unclear. Consistent with its role as an intermediate filament, vimentin functions as a scaffold to mediate EMT-associated signals between the cytoplasm and the nucleus.38 Our observation indicates that blockade of c-Src tyrosine kinase can significantly decrease vimentin expression in TNBC, suggesting that c-Src tyrosine kinase can modulate this structure protein. Of note, vimentin is not an independent biomarker to predict the prognosis of TNBC. Many molecules or mechanisms are involved in modulating the functions of vimentin.27, 43, 44
Collectively, multiple molecules from the nucleus to the cytoplasm are involved in the modulation of EMT in TNBC in addition to c-Src and vimentin. c-Src acts as a critical regulatory site for EMT with clinical implications for targeted therapy for TNBC patients. This finding suggests that traditional markers for TNBC are not sufficient to precisely define this aggressive type of TNBC. Vimentin is identified as an important biomarker to subdivide TNBC with different characteristics. The present study provides an important rationale for the clinic to precisely select TNBC patients who would benefit from c-Src inhibitor treatment. Further investigation in this field will find more molecules to subdivide TNBC patients for personalized therapy.
ACKNOWLEDGMENTS
We would like to thank the native English-speaking scientists of CureEdit (Houston, TX, USA) for editing our manuscript. This work was supported by Talents planning of six summit fields of Jiangsu Province (WSW-026), Maternal and child health research projects of Jiangsu Province (F201678), the National Natural Science Foundation of China (81172502, 81302305) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD, JX10231801).
CONFLICTS OF INTEREST
Authors declare no conflicts of interest for this article.




